Abstract
Background
While effective physical activity referral schemes (PARSs) and related structures for promoting physical activity (PA) already exist in several countries, in Germany, PARSs have not yet been implemented systematically and nationwide. Through a co-production approach with relevant actors in the German healthcare system, a PARS was developed, and an implementation plan was created (e.g. financing). This study protocol aims to evaluate the developed PARS for people with non-communicable diseases (NCDs) in Germany regarding its potential effectiveness and implementation success.
Methods
To evaluate the effectiveness and implementation success of the PARS, we will apply a pragmatic cluster-randomised controlled trial (cRCT) in Hybrid II design by comparing two intervention groups (PARS vs PA advice [PAA]). The trial will take place in the Nürnberg metropolitan region, with 24 physician practices recruiting 567 people with NCDs. Both groups will receive brief PA advice from a physician to initially increase the participants’ motivation to change their activity level. Subsequently, the PARS group will be given individualised support from an exercise professional to increase their PA levels and be transferred to local exercise opportunities. In contrast, participants in the PAA group will receive only the brief PA advice as well as information and an overview of regional PA offerings to become more active at their own initiative. After 12 and 24 weeks, changes in moderate to vigorous PA and in physical activity-related health competence (movement competence, control competence, self-regulation competence) will be measured as primary outcomes. Secondary outcomes will include changes in quality of life. To measure implementation success, we refer to the RE-AIM framework and draw on patient documentation, interviews, focus groups and surveys of the participating actors (physicians, exercise professionals).
Discussion
Through a between-group comparison, we will investigate whether additional individual support by an exercise professional compared to brief PA advice alone leads to higher PA levels in people with NCDs. The acceptance and feasibility of both interventions in routine care in the German healthcare system will also be evaluated.
Trial registration
ClinicalTrials.gov, NCT04947787. Registered 01 June 2021.
Similar content being viewed by others
Background
In Germany, only approximately 45% of adults are adequately physically active and meet the national and international physical activity (PA) recommendations of at least 150 min/week of moderate to vigorous activity or 75 min/week of intensive activity [1]. The worldwide increase in physical inactivity has led not only to an increase in non-communicable diseases (NCDs) and mortality rates but also to rising medical costs [2]. Regular PA has been associated with comprehensive positive physical and mental health effects, as scientifically demonstrated for more than 25 NCDs, including obesity, type 2 diabetes mellitus and cardiovascular diseases [3]. Despite these benefits, persons with chronic diseases in particular show considerably lower PA levels compared to healthy adults [4].
Physicians have been prescribing PA for over 2,500 years. The Indian physician Susruta, for example, recommended PA for his patients as early as 600 BC, and Hippocrates (460 – 370 BC) wrote the first-known exercise prescription for patients to manage chronic diseases [5]. Today, physician-initiated exercise is recommended in the European Union (EU) PA Strategy, the Global Action Plan on Physical Activity 2018–2030 (WHO) and German guidelines for PA and PA promotion (NEBB) as an effective PA measure [6, 7]. Accordingly, there are already established structures for physician-initiated PA promotion, such as ‘Exercise on referral’ (England) [8], ‘Physical activity on prescription’ (Sweden) [9] and ‘Green prescription’ (New Zealand) [10]. In Germany, physicians already make PA recommendations as part of routine care but without referring to an existing PA service or receiving financial compensation for the services provided [11].
The project BewegtVersorgt, funded by the German Federal Ministry of Health, aims to develop structures to promote PA for people with NCDs and to equip individuals with the competences necessary to lead a healthy, physically active lifestyle [12]. Based on a co-production process with relevant actors in the German healthcare system, a physical activity referral scheme (PARS) was developed, as detailed in Weissenfels et al. [13]. Following other promising referral schemes, key constituent elements, such as PA screening, assessment, counselling, referral form/prescription, feedback and follow-up [14], were identified and adapted to the structures of the German healthcare system. International studies have shown that these referral schemes have a meaningful impact, but the results vary widely, and merely transferring a specific concept to other healthcare systems is typically not feasible [15, 16].
Internationally, there are also simpler healthcare structures than PARS for PA promotion, such as PA advice (PAA), that provide good evidence for individuals’ initial motivation to change their PA behaviour but remain questionable regarding long-term PA promotion [17]. Therefore, the current study aims to compare a PARS and PAA intervention group to examine whether the individual support of an exercise professional using behaviour change techniques in the PARS group produces a more significant change in PA behaviour than in the PAA group.
Objectives
Based on a Hybrid II design [18], the objectives of the pragmatic trial are twofold. The first objective is to evaluate the effectiveness of the developed PA interventions (PARS vs PAA) in terms of a) increasing moderate to vigorous PA, b) changes in physical activity-related health competence (PAHCO) (movement competence, control competence, self-regulation competence, see Carl et al. [19, 20]) and c) influencing the quality of life of people with NCDs. The second objective is (d) to test the success of the implementation plan. The following study protocol focuses particularly on evaluating effectiveness as more details on the success of the implementation plan will be presented in another article.
Primary hypotheses
The key elements of the PA interventions focus on the improvement of PAHCO and long-term changes in PA levels, so both outcomes are considered equally important as primary endpoints.
-
1)
The PARS group members increases their (subjective) PA significantly after 12 and 24 weeks compared to the PAA group.
-
2)
The PARS group members report significant improvements in PAHCO after 12 and 24 weeks compared to the PAA group.
Secondary hypothesis
-
3)
The PARS group shows a significant increase in quality of life after 12 and 24 weeks compared to the PAA group.
Further hypothesis
-
4)
Participating actors (physicians, exercise professions) are able to implement the developed PA interventions in routine care and evaluate them positively.
Trial design
The study described in this protocol is a cluster-randomised controlled trial (cRCT) with two intervention arms (PARS vs PAA) and is designed to be pragmatic using the PRECIS-2 tool [21]. Based on the pragmatic application, the trial is guided by a Hybrid II design, following Curran et al. [18], which simultaneously tests the effectiveness of the interventions and the implementation plan. The study will be conducted as a pilot project in the Nürnberg metropolitan region, Germany, and will be scientifically supported by the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Germany.
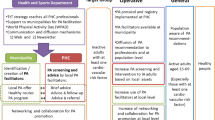
This protocol is based on the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [22]. An overview of schedule of enrolment, both interventions, and assessments is provided in Fig. 1. The pragmatic trial will be conducted and reported in accordance with the reporting guidelines provided in the CONSORT 2010 statement [23], taking into account the CONSORT extension for pragmatic trials [24] and cRCTs [25].
Schedule of enrolment, interventions, and assessments following SPIRIT. PA physical activity; PARS physical activity referral scheme; PAA physical activity advice; EQ-5D-5L European Quality of Life – 5 Dimensions – 5 Level; SSA self-efficacy towards physical activity; HCCQ-D Health Care Climate Questionnaire; SSK sport- and movement-related self-concordance; PREMs patient-reported outcome measures
Methods
Study setting and participants
The PARS was developed using a co-production approach that involved all relevant actors in the healthcare system (e.g. healthcare service providers, healthcare insurance providers, representatives of patient associations; n = 12 organisations). More information about the co-production process can be retrieved from the study protocol covering all project phases (development, implementation, evaluation, scaling-up) [13]. The pragmatic trial will take place in the German primary healthcare setting. As part of a routine examination, suitable patients will be recruited by physicians (general practitioners and medical specialists) and screened for eligibility. Participants are eligible for the study if they (a) are at least 18 years old and live in the Nürnberg metropolitan region; (b) have at least one of the following NCDs: controlled diabetes mellitus type 2, chronic cardiovascular disease, obesity (Body Mass Index [BMI] ≥ 30 kg/m2) or arthrosis in knee and/or hip; (c) do not meet official German PA recommendations (i.e. performing less than 150 min of moderate to vigorous aerobic activity per week); (d) are insured with health insurance companies cooperating with the project and (e) can safely participate in PAs based on medical judgement. Individuals will be excluded if they (a) plan to leave the Nürnberg metropolitan region during the study period, (b) are participating in another study, (c) plan to be absent for more than four weeks during the 12-week intervention, (d) have cognitive impairments that prevent an effective communication with the physician and the exercise professional, (e) have a mental illness, such as psychosis, substance abuse or mood and personality disorders, (f) have an unstable clinical situation or serious health impairments that prevent them from undertaking PA safely (e.g. acute myocardial infarction, unstable angina pectoris, fever, terminal tumour diseases).
Recruitment procedures
Physicians
The recruitment of participants will take place via trained physicians in the Nürnberg metropolitan region. Participating general practitioners and medical specialists will be recruited through ‘quality circles’ (a continuing education programme for physicians), medical associations, a regional Bavarian science journal and the homepages of the project stakeholders. The project stakeholders consist of 12 organisations from the German healthcare system and include representatives from patients, the medical profession, the exercise professions, and insurance providers.
Inclusion criteria will be that the physicians (a) are located in the Nürnberg metropolitan region, (b) work in general medicine or specialise in internal medicine (cardiology, diabetology or endocrinology), orthopaedics, physical and rehabilitative medicine or geriatrics, (c) have expressed their willingness and commitment to participate in the study, (d) have adequate resources to manage the study and comply with the protocol, (e) have adequate patient volume according to participants’ inclusion criteria (given diseases, healthcare insurance) and (f) participate in a 90-min digital training session (introduction to motivational interviewing and interview guide for PA advice). Exclusion criteria will be (a) participation in conflicting studies and (b) practice relocation or retirement planned during the study period.
Participants
Suitable patients from the cooperating health insurance companies will be included in the study as part of their routine care (pragmatic trial). Eligible patients will be identified by the physicians or medical staff in advance (see inclusion and exclusion criteria above). Selected patients will receive information about the study and a screening assessment at their routine appointment (see Fig. 2), which will ask about their PA levels and contraindications. After a brief conversation about the benefits of PA with the physician, the patient can decide whether to participate in the programme and is referred to the next level of each intervention arm.
Exercise professionals (PARS group only)
Exercise professionals (e.g. physiotherapists, sport and exercise therapists) will be recruited using various strategies. An overview of all facilities related to PA promotion (physiotherapy practices, rehabilitation sports, prevention sports and fitness facilities related to health promotion) in the Nürnberg metropolitan region has already been compiled. The study team will systematically contact the responsible staff of each facility via telephone to present the study as well as general conditions and ascertain levels of interest. If the facility staff express interest, the responsible exercise professionals will participate in a 1.5-day digital training course in which they are familiarised with the intervention (individual PA promotion and assessments) and will have the opportunity to try out motivational interviewing. The study team will also advertise the project through project stakeholders’ websites, newsletters and information letters to the stakeholders’ members.
Sample size
To calculate the number of participants, we focus on the primary endpoints of ‘moderate to vigorous PA (min/week)’ and ‘PAHCO’, including cluster adjustment. As the primary outcomes lead to four statistical tests, a Bonferroni correction is used accordingly with α = 0.05/4. With an assumed drop-out rate of 10%, a sample size of 567 participants from 24 medical practices (α = 0.0125, power = 80%) is required to detect differences between both intervention groups of low to moderate effect size (f = 0.175). To account for the effects of clustering, an intraclass correlation (ICC) of 0.02 is considered, estimated based on previous studies for cRCTs in primary care [26, 27]. We consider this ICC to be realistic in our trial as the participating practices are similarly structured (professional background, qualification level, treating patient group size and region) and were trained by the research team.
Randomisation, allocation and blinding
A cRCT with randomisation at the physician practice level will be conducted for organisational reasons and to avoid the risk of mixing participants from both groups within a medical facility (contamination). The research team will recruit the physicians and provide them with detailed information about the study or answer questions. After physicians express interest in participating in the study and meet the inclusion criteria, they must provide written informed consent, which needs to be supported by the cooperating health insurance company. Each medical practice will be randomly allocated to the PARS or PAA group using computer-based block randomisation (Excel 2016). Due to the local density of physician practices in the Nürnberg metropolitan region, six blocks for randomisation will be defined (Nürnberg city [n = 10], Nürnberg surrounding area [n = 2], Fürth city [n = 10], Fürth surrounding area [n = 2], Erlangen city [n = 10] and Erlangen surrounding area [n = 2]). The practices will then be informed about further procedures, and physicians, as well as medical staff working in the facility, will undergo specific training according to the assigned intervention arms.
Because of group-specific procedures and the corresponding training, blinding the participating actors (physicians, exercise professionals) is not possible in this study. However, blinding participants is possible due to randomisation on the physician practices’ level. The participants will only be informed about the intervention in which they are involved and will not come into contact with the other intervention arm during the study. The statistician will also be blinded to the group allocation until completion of the statistical analysis.
Interventions
The intervention period will last 24 weeks for the individual participant and will take place in the primary care setting of the German healthcare system. Both groups (PARS and PAA) will receive brief PA advice about increasing their activity levels. The study design is shown in Fig. 2. All trained actors in both groups will be asked to adhere to the intervention guidelines. For this, there are checklists to be completed and all participating actors as well as the participants are subsequently questioned about compliance. Participants are asked to maintain their lifestyle and not to start any other activities besides the study. Participants of both groups can quit the intervention at any time without giving any reason.
Physical activity referral scheme (PARS)
The PARS pathway is based on a conventional prescription for physical therapy in Germany and includes key actors, such as physicians, exercise professionals and exercise organisations. A detailed description of the pathway according to the PARS reporting checklist by Hanson et al. [28] can be found in Additional file 1.
The referral scheme begins with the physician’s screening. During a routine medical visit, the patient’s eligibility for participation in the study will be assessed. For this purpose, in addition to the inclusion and exclusion criteria described earlier, a short screening questionnaire will be used to determine average weekly PA. The questionnaire is based on three validated questionnaires for evaluating activity levels subjectively (Single-item [29], Physical Activity Vital Sign (PAVS) [30], Kaiser Permanente Southern California Exercise Vital Sign [31]) and is adapted to the trial setting. If the questionnaire reveals suitability – that is, the patient is inactive (< 150 min/week of moderate to vigorous PA) – brief PA advice (10 min) follows. The main goal is to initially motivate patients to change their behaviour and participate in the following intervention. The referral form can then be used to transfer the patient to a participating exercise professional.
After a 45-min initial assessment by the exercise professional, which is also part of the intervention, individually targeted PA promotion takes place over six sessions of 60 min each. The focus is on working out PA preferences with the patient, trying them and planning their subsequent implementation (e.g. in a regional exercise programme). For this purpose, exercise professionals can use a set of behavioural change techniques (BCTs), following Mitchie et al. [32], which has been identified as effective for PA behavioural change [33]. The BCTs are described in detail for both groups in Additional file 2. Exercise professionals in this study will focus particularly on the techniques of ‘goals and planning’, ‘feedback and monitoring’, ‘social support’, ‘shaping knowledge’ and ‘self-belief’. During the fourth session, a transfer to existing exercise or PA offers in the region will occur. Via a comprehensive brochure (more than 1000 offers from approximately 300 exercise providers), sorted by postal codes and types of offer, the participants will receive an overview of health-related PA opportunities and find the contact details for the respective exercise providers. After 12 weeks, a 45-min final assessment will take place to reflect on changes in the participant’s PA level and PAHCO and to formulate new goals for the next 12 weeks. The attending physician will receive feedback on the patient’s development. The transfer to existing PA programmes or independent activities will enable long-term maintenance of an active lifestyle, assessed in a follow-up assessment after a further 12 weeks. Before each assessment, participants will receive baseline or follow-up questionnaires to evaluate the effectiveness of the intervention and success of its implementation from their perspective. The questionnaires can be completed independently, although the treating exercise professional can assist with any questions.
Physical activity advice (PAA)
After being screened for eligibility, the PAA group will receive similar brief PA advicePAA to the PARS group. However, instead of being referred to exercise professionals for a more intensive intervention, the PAA participants will then receive a brochure with PA information, steps to start PA and adopt a more active lifestyle, specific information about the diseases targeted in this study and concrete tasks. The brochure also contains materials on BCTs, in which the BCTs ‘goals and planning’, ‘feedback and monitoring’ and ‘shaping knowledge’ are used but to a lesser extent than in the PARS group. The major difference between the two groups is the social support from an exercise professional, which is not available in the PAA group. An overview of the integrated BCTs for the PAA group is included in Additional file 2.
Like the PARS group, the PAA group will also receive the overview of local PA offers. A baseline questionnaire and follow-up questionnaire to be completed after 12 and 24 weeks will be sent by mail with stamped envelopes for their return to the project team. If the questionnaire has not been answered after 14 days, participants will receive a reminder by mail. If participants in this intervention arm have any questions about the brochure or completing the questionnaires, they can contact the research team by phone or email in specific time slots.
Outcome measures
Except for participant characteristics, all data will be collected at the following three time points: after brief PA advice by the physician (basal), at 12 and at 24 weeks.
Participant characteristics
Participants’ characteristics will be captured through questions about age, gender, birthplace, marital status, social status, education level and income in the baseline questionnaire. Medical health status (type of disease) and medication intake will also be queried. Further specific medical characteristics prone to PA interventions, such as HbA1c, cannot be captured via questionnaires. For organisational reasons, these data cannot be retrieved systematically from all participating practitioners.
Primary outcomes
Physical activity (PA) level
PA level will be measured by the Physical Activity, Exercise and Sport Questionnaire (Bewegungs- und Sportaktivität Fragebogen; BSA-F 3.0 [34]) – a validated German questionnaire that assesses the respondent’s amount of PA during the last four weeks. Based on the Frequency-Intensity-Time-and-Type (FITT) principles [35, 36], the questionnaire differentiates between leisure and transportation activities (PA score) and sport- and exercise-related activities (sport score). Participants report the frequency and duration of activities executed during the last four weeks. Minutes of leisure-time PA per week and sport-/exercise-related activity per week are calculated to gain the PA and exercise scores, which are combined to give the overall PA volume.
Physical activity (PA)-related health competence
Changes in PAHCO will be measured using the German version of the Physical Activity-Related Health Competence Questionnaire (BGK Questionnaire) [12, 37]. The questionnaire consists of 42 items that measure the following three sub-competencies necessary for health-promoting PA behaviour: movement competence (18 items; min = 0, max = 17.6), control competence (10 items; min = 0, max = 10.8) and self-regulation competence (14 items; min = 0, max = 14.8). Higher scores indicate higher competencies.
Secondary outcomes
The European Quality of Life – 5 Dimensions – 5 Level (EQ-5D-5L) questionnaire is a self-administered quality of life scale that consists of a descriptive system and a visual analogue scale [38]. The descriptive system contains five well-being dimensions: mobility, self-care, usual activity, pain/discomfort and anxiety/depression, each of which has five levels of severity (no problems to incapacitated/extreme problem), coded from one to five. The result is a five-digit code that represents the participant’s health profile. The visual analogue scale assesses the participant’s overall current health.
In addition to the EQ-5D-5L results, the following secondary outcomes will be collected:
-
Self-efficacy towards PA (SSA scale) [39]
-
Participant’s perceived autonomy support (HCCQ-D) [40]
-
Stage of change [41]
-
Sport- and movement-related self-concordance (SSK scale) [42]
Evaluation of the implementation plan
As well as effectiveness, the degree of implementation will be examined (see hypothesis 4) based on the five dimensions of the RE-AIM (Reach-Effectiveness-Adoption-Implementation-Maintenance) model [43], which include different aspects of implementation status and success at the individual and organisational levels. To examine the degree of implementation, a mixed method approach will be used, drawing on quantitative (e.g. characteristics of participating actors [Adoption] and patients reach [Reach]) and qualitative measurement procedures (e.g. adherence to guidelines [Implementation], adjustments during intervention [Implementation] and adoptions for long-term use [Maintenance]). For this purpose, a plan will be developed to capture various aspects in each dimension using different methods (e.g. interviews, focus groups, online surveys and document analysis) and at different moments during the intervention period. The perspectives of both the participating actors and patients will be addressed. Focusing on Patient-Reported Experience Measures (PREMs) [44], the patients’ perspectives will be retrieved with questionnaire surveys after 12 and 24 weeks, after which interviews or focus groups with patients may also be conducted. The actors will be asked for their perspectives during and after the interventions.
Data collection and management
Within the pragmatic trial, participating actors (physicians and exercise professionals) will assist with data collection for the effectiveness evaluation in the PARS group by handing out the questionnaires. For the PAA group, the questionnaire will be delivered by mail. The questionnaire comprises a compilation of reliable and validated survey instruments (see ‘Outcome measures’ above) and will be completed independently by the study participants. To ensure standardised data collection, the participating actors will prepare for this task in a special training session. Due to the personal supervision of the PARS group participants, the data will be collected by the same exercise professional at all three measurement time points. In the PARS group, participants will receive the questionnaires prior to the baseline and at the 12- and 24-weeks follow-up assessments, while PAA group participants will receive them by mail and are expected to return them to the study team with a prepaid envelope. The study team will periodically collect the documents of the PARS group from the participating medical and therapeutic practices. Adverse events will be assessed in both groups via questionnaires at both 12 and 24 weeks. In the case of a study dropout, the exercise professional in the PARS group can also report specific reasons.
The data for evaluating the implementation plan will be collected by the study team during and after the intervention period. For example, data on the characteristics of the participating actors (e.g. physicians and exercise professionals) will be collected during the intervention, while information about the success of the interventions will only be collected after their completion. Planned interviews or focus groups will be conducted by trained researchers from the FAU study team.
All data will be transcribed, pseudonymised, recorded, archived and evaluated by the research team using quantitative methods (descriptive and inferential statistics via, e.g. SPSS, R) and qualitative social research methods (e.g. transcription and coding of structured interviews via MAXQDA). The data will be stored securely, and only direct project members of the FAU will have access to them. The deletion of all collected data is planned after the legal retention period of 10 years, beginning with the end of the project duration (30.11.2022).
Data analysis
A linear modelling approach will be used to analyse the effectiveness of the intervention. The group information (PARS vs PAA) will constitute the decisive independent variable to be modelled on participants’ outcomes after the intervention. In this context, participants’ baseline values will be integrated into the calculations to control for potential differences before the intervention. Given the clustered design, it is necessary to account for the nested character of the data (level 2: physicians; level 1: patients). Depending on the final number of clusters included (level 2), participants recruited (level 1) and the power achieved, either physicians will be included as a control variable or the hierarchical structure will be considered through multilevel structural equation modelling (applying the Kenward-Roger correction for small cluster sizes [45]).
Adopting an intention-to-treat paradigm, we will include all participants who have been randomised in the analysis. To avoid underestimation of treatment effects through conservative replacement strategies (such as baseline observation carried forward), we will apply imputation techniques to this trial. Accordingly, dropouts and missing data will be treated by applying full information maximum likelihood (FIML) imputation. The Mardia test, with its inspection of statistical skewness and kurtosis, will determine multivariate normality. If the assumptions of normality are violated, the calculations will be based on robust maximum likelihood (MLR) procedures.
Ethics and dissemination
The ethics application for BewegtVersorgt was submitted in written form to and approved by the responsible FAU institution (committee reference number 110_21 B). Any changes made to the study after the review will be reported immediately. The pragmatic trial adheres to the Declaration of Helsinki, and the data will only be published in pseudo-anonymised form to ensure that no assignment to persons can take place. Data protection rules will be respected throughout the project’s duration, and all participating actors and patients will be informed in advance about the study and required to give their written informed consent to participate. They will have the option of revoking this consent at any time without giving reasons. The study results will be published in peer-reviewed journals and presented at national and international congresses. Regarding dissemination and scaling-up strategies, the transfer and scaling-up of the referral scheme (national level, other target regions and groups) will be discussed in a working group of national experts. However, as the planning of the scaling-up process depends on the results of the effectiveness analysis and the implementation success, it can only be concretised at a later stage in the project.
Discussion
The study aims to improve PA, PAHCO and quality of life among inactive people with NCDs via physician-initiated PA promotion. It will also examine whether the implementation of such PA interventions, especially the referral scheme, is successful in the German healthcare system or whether adjustments have to be made.
We expect that individuals in the PARS group, who receive PA support from exercise professionals, will show significantly higher increases in their PA levels and PAHCO than those in the PAA group. We will also evaluate the participants’ adherence to selected activities at the 24-week follow-up assessment. The largest difference between the interventions that may influence individual behaviour change is the extent to which BCTs, and the ‘social support’ category in particular, are used. If the PAA group participants also succeed in making positive changes, it will be necessary to discuss which intervention can be used most efficiently to produce the desired health effects.
The execution of the comprehensive evaluation concept in this pragmatic trial is largely dependent on the recruitment rate in the physicians’ practices and the implementation success of the PA interventions in routine care. Thus, an assessment of the results and an adjustment of the evaluation concept will only be possible once the pragmatic study is in progress. While we also acknowledge that a longer follow-up period would be desirable, it is not possible due to the funding amount and period of the project (including the co-production approach and planning).
Through the results and process of co-productive development with stakeholders in the German healthcare system, we hope to facilitate a potential transfer to the national level and to other target regions or groups. As a result of the co-production process, the intervention’s adaptation to the legal and financial conditions of the healthcare system has already laid a foundation for such a transfer and may ease the subsequent transfer to routine care.
In summary, the results of this study will make it possible to evaluate the PA-promoting effects of PARS in primary healthcare in Germany, laying the foundation for nationwide dissemination/scaling up.
Trial status
NCT04947787 (Status: Recruiting).
Availability of data and materials
Data will be available upon reasonable request from Dr Anja Weissenfels; Email: anja.weissenfels@fau.de.
Abbreviations
- CONSORT:
-
Consolidated Standards of Reporting Trials
- BCT:
-
Behaviour change techniques
- BMI:
-
Body mass index
- EQ-5D-5L:
-
European Quality of Life – 5 Dimensions – 5 Level
- HCCQ-D:
-
Health Care Climate Questionnaire
- ICC:
-
Intraclass correlation
- NEBB:
-
German guidelines for PA and PA promotion
- PAHCO:
-
Physical activity-related health competence
- PAVS:
-
Physical Activity Vital Sign
- SSA:
-
Self-efficacy towards physical activity
- SSK:
-
Sport- and movement-related self-concordance
- cRCT:
-
Cluster-randomised controlled trial
- FAU:
-
Friedrich-Alexander Universität Erlangen-Nürnberg
- FIML:
-
Full information maximum likelihood
- FITT:
-
Frequency-Intensity-Time-and-Type
- MLR:
-
Robust maximum likelihood
- NCD:
-
Non-communicable disease
- PA:
-
Physical activity
- PAA:
-
Physical activity advice
- PARS:
-
Physical activity referral scheme
- PREM:
-
Patient-reported experience measure
- SPIRIT:
-
Standard Protocol Items: Recommendations for Interventional Trials
- WHO:
-
World Health Organization
References
Finger JD, Mensink GBM, Lange C, et al. Gesundheitsfördernde körperliche Aktivität in der Freizeit bei Erwachsenen in Deutschland. J Health Monitoring. 2017;2(2):37–44.
Ding D, Lawson KD, Kolbe-Alexander TL, et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. The Lancet. 2016;388(10051):1311–24 (http://www.sciencedirect.com/science/article/pii/S014067361630383X).
Pedersen BK, Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports. 2015;25(Suppl 3):1–72.
Sudeck G, Geidl W, Abu-Omar K, et al. Do adults with non-communicable diseases meet the German physical activity recommendations?. Ger J Exerc Sport Res. 2021;51:183–93.
Tipton CM. The history of “Exercise Is Medicine” in ancient civilizations. Adv Physiol Educ. 2014;38(2):109–17.
Pfeifer K, Rütten A. Nationale Empfehlungen für Bewegung und Bewegungsförderung: National Recommendations for Physical Activity and Physical Activity Promotion. Gesundheitswesen. 2017;79(S 01):S2–3.
World Health Organization. Global action plan on physical activity 2018–2030: more active people for a healtier world. Geneva: World Health Organization; 2018.
NICE. Physical activity: exercise referral schemes. Public health guideline 54. Available at: https://www.nice.org.uk/guidance/ph54. Published September 2014.
Kallings L. The Swedish PAP Physical activity on prescription(FaR®). 2018.
Elley CR, Kerse N, Aroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomized controlled trial. BMJ. 2003;326:793.
Curbach J, Apfelbacher C, Knoll A, et al. Physicians’ perspectives on implementing the prevention scheme “Physical Activity on Prescription”: Results of a survey in Bavaria. Z Evid Fortbild Qual Gesundhwes. 2018;131–132:66–72.
Sudeck G, Pfeifer K. Physical activity-related health competence as an integrative objective in exercise therapy and health sports – conception and validation of a short questionnaire. German Jo Exerc Sport Res. 2016;46:74–87.
Weissenfels A, Geidl W, Mino E, et al. Development, implementation, evaluation and scaling-up of physical activity referral schemes in Germany: protocol for a study using a co-production approach. BMJ Open. 2021;11(3):e045563. https://doi.org/10.1136/bmjopen-2020-045563 [published Online First: 22 March 2021].
Mino E, Geidl W, Naber I, et al. Physical activity referral scheme components: a study protocol for systematic review and meta-regression. BMJ Open. 2021;11(6):e049549. https://doi.org/10.1136/bmjopen-2021-049549 [published Online First: 18 June 2021].
Arsenijevic J, Groot W. Physical activity on prescription schemes (PARS): do programme characteristics influence effectiveness? Results of a systematic review and meta-analyses. BMJ Open. 2017;7(2):e012156.
Pavey TG, Taylor AH, Fox KR, et al. Effect of exercise referral schemes in primary care on physical activity and improving health outcomes: systematic review and meta-analysis. BMJ. 2011;343:d6462.
Grandes G, Sanchez A, Sanchez-Pinilla RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169(7):694–701.
Curran GM, Bauer M, Mittman B, et al. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26.
Carl J, Sudeck G, Pfeifer K. Competencies for a Healthy Physically Active Lifestyle-Reflections on the Model of Physical Activity-Related Health Competence. J Phys Act Health. 2020;17(7):688–97. https://doi.org/10.1123/jpah.2019-0442 [published Online First: 29 May 2020].
Carl J, Sudeck G, Geidl W, et al. Competencies for a Healthy Physically Active Lifestyle-Validation of an Integrative Model. Res Q Exerc Sport. 2020;2020:1–15. https://doi.org/10.1080/02701367.2020.1752885[publishedOnlineFirst:7July.
Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147.
Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. 2008;337:a2390.
Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. https://doi.org/10.1136/bmj.e5661 [published Online First: 4 September 2012].
Stuart B, Becque T, Moore M, et al. Clustering of continuous and binary outcomes at the general practice level in individually randomised studies in primary care - a review of 10 years of primary care trials. BMC Med Res Methodol. 2020;20(1):83. https://doi.org/10.1186/s12874-020-00971-7 [published Online First: 15 April 2020].
Adams G, Gulliford MC, Ukoumunne OC, et al. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol. 2004;57(8):785–94.
Hanson CL, Oliver EJ, Dodd-Reynolds CJ, et al. A modified Delphi study to gain consensus for a taxonomy to report and classify physical activity referral schemes (PARS). Int J Behav Nutr Phys Act. 2020;17(1):158.
Milton K, Bull FC, Bauman A. Reliability and validity testing of a single-item physical activity measure. Br J Sports Med 2011;45(3). https://pubmed.ncbi.nlm.nih.gov/20484314/.
Greenwood JLJ, Joy EA, Stanford JB. The Physical Activity Vital Sign: a primary care tool to guide counseling for obesity. J Phys Act Health. 2010;7(5):571–6.
Coleman KJ, Ngor E, Reynolds K, et al. Initial validation of an exercise “vital sign” in electronic medical records. Med Sci Sports Exerc. 2012;44(11):2071–6.
Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Geidl W, Semrau J, Pfeifer K. Health behaviour change theories: contributions to an ICF-based behavioural exercise therapy for individuals with chronic diseases. Disabil Rehabil. 2014;36(24):2091–100.
Fuchs R, Klaperski S, Gerber M, et al. Messung der Bewegungs- und Sportaktivität mit dem BSA-Fragebogen: Eine methodische Zwischenbilanz. Zeitschrift für Gesundheitspsychologie. 2015;23(2):60–76 (Accessed 6 Jul 2020).
Pescatello LS, Franklin BA, Fagard R, et al. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36(3):533–53.
Sallis JF, Owen N. Physical activity & behavioral medicine. Thousand Oaks, Calif: Sage Publications; 1999.
Carl J, Sudeck G, Pfeifer K. Competencies for a Healthy Physically Active Lifestyle: Second-Order Analysis and Multidimensional Scaling. Front Psychol. 2020;11:558850. https://doi.org/10.3389/fpsyg.2020.558850 [published Online First: 21 December 2020].
The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208.
Fuchs R, Schwarzer R. Selbstwirksamkeit zur soortlichen Aktivität: Reliabilität undValidität eines neuen Meßinstrument. Zeitschrift für Differentielle und Diagnostische Psycholog. 1994;15(3):141–54.
Schmidt K, Gensichen J, Petersen JJ, et al. Autonomy support in primary care–validation of the German version of the Health Care Climate Questionnaire. J Clin Epidemiol. 2012;65(2):206–11. https://doi.org/10.1016/j.jclinepi.2011.06.003 [published Online First: 8 September 2011].
Lippke S, Ziegelmann JP, Schwarzer R, et al. Validity of stage assessment in the adoption and maintenance of physical activity and fruit and vegetable consumption. Health Psychol. 2009;28(2):183–93 (https://pubmed.ncbi.nlm.nih.gov/19290710).
Seelig H, Fuchs R. Messung der sport- und bewegungsbezogenen Selbstkonkordanz. Z Sportpsychol. 2006;13(4):121–39.
Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health. 1999;89(9):1322–7.
Kingsley C, Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Education. 2017;17(4):137–44.
Kenward MG, Roger JH. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics. 1997;53(3):983.
Acknowledgements
We would like to thank the following partners for their cooperation in the project BewegtVersorgt: Bayerischer Landesärztekammer (BLÄK), Bayerischer Hausärzteverband (BHÄV), Hausärzte Erlangen und Umgebung e.V., Bayerischer Sportärzteverband (BSÄV), Deutscher Verband für Gesundheitssport und Sporttherapie (DVGS e.V.), Bundesverband selbstständiger Physiotherapeuten (IFK e.V.), VDB-Physiotherapieverband, Deutscher Olympischer Sportbund (DOSB e.V.), Deutsche Diabetes-Hilfe—Menschen mit Diabetes e.V. (DDH-M e.V.), Deutsche Rheuma-Liga Landesverband Bayern e.V., AOK Bayern—Die Gesundheitskasse, DAK-Gesundheit (Landesverband Bayern) and Zentrum Patientenschulung und Gesundheitsförderung (ZePG e.V.).
Funding
Open Access funding enabled and organized by Projekt DEAL. The study is funded by the Federal Ministry of Health based on a resolution of the German ‘Bundestag’ by the federal government [grant number: ZMV I 1—2519FSB109]. The government funder has no influence in the planning of the study, the collection, management, analysis and interpretation of the data, the writing of the report and the decision to publish the report, including whether they will have ultimate authority for any of these activities.
Author information
Authors and Affiliations
Contributions
KP had the initial idea for this project and submitted the funding application for the study together with WG, KAO and PG. AW and SK coordinate the project. AW, SK, EM, IN, WG, PG, KAO and KP designed the pragmatic trial. JC supports the statistical analysis and power calculation. AW, SK, EM and IN will monitor the pragmatic trial as well as data collection and data analysis. AW, SK and JC prepared the draft of this study protocol. All authors contributed substantially to drafting the paper and its revisions. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics application for BewegtVersorgt was submitted in written form to the FAU Ethics Committee and approved by it (committee’s reference number: 110_21 B). The responsible commission gave its approval in written form and raised no objections to the study implementation. All participating actors and patients are informed about the study in advance (in writing and verbally) and asked to give their written consent to participate. They have the option to cancel the study at any time without giving reasons and will not be disadvantaged in terms of their further medical care.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary material_PARS Checklist.Physical Activity Referral Scheme (PARS) Reporting Checklist.
Additional file 2.
Supplementary material_BCT. Overview of the BCTs used in the interventions according to Michie et al. [32].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Weissenfels, A., Klamroth, S., Carl, J. et al. Effectiveness and implementation success of a co-produced physical activity referral scheme in Germany: study protocol of a pragmatic cluster randomised trial. BMC Public Health 22, 1545 (2022). https://doi.org/10.1186/s12889-022-13833-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-13833-2