Abstract
Background
Significant long-term reduction in health-related quality of life (HRQoL) is often observed in survivors of the acute respiratory distress syndrome (ARDS), and return to work (RtW) is limited. There is a paucity of data regarding the relationship between the quality of care (QoC) in the intensive care unit (ICU) and both HRQoL and RtW in ARDS survivors. Therefore, the aim of our study was to investigate associations between indicators of QoC and HRQoL and RtW in a cohort of survivors of ARDS.
Methods
To determine the influence of QoC on HRQoL and RtW 1 year after ICU-discharge, ARDS patients were recruited into a prospective multi-centre patient cohort study and followed up regularly after discharge. Patients were asked to complete self-report questionnaires on HRQoL (Short Form 12 physical component scale (PCS) and mental component scale (MCS)) and RtW. Indicators of QoC pertaining to volume, structural and process quality, and general characteristics were recorded on ICU level. Associations between QoC indicators and HrQoL and RtW were investigated by multivariable linear and Cox regression modelling, respectively. B values and hazard ratios (HRs) are reported with corresponding 95% confidence intervals (CIs).
Results
877 (of initially 1225 enrolled) people with ARDS formed the DACAPO survivor cohort, 396 were finally followed up to 1 year after discharge. The twelve-month survivors were characterized by a reduced HRQoL with a greater impairment in the physical component (Md 41.2 IQR [34–52]) compared to the mental component (Md 47.3 IQR [33–57]). Overall, 50% of the patients returned to work. The proportion of ventilated ICU patients showed significant negative associations with both 12 months PCS (B = − 11.22, CI −20.71; − 1,74) and RtW (HR = 0,18, CI 0,04;0,80). All other QoC indicators were not significantly related to outcome.
Conclusions
Associations between ICU QoC and long-term HrQoL and RtW were weak and largely non-significant. Residual confounding by case mix, treatment variables before or during ICU stay and variables pertaining to the post intensive care period (e.g. rehabilitation) cannot be ruled out.
Trial registration
Clinicaltrials.govNCT02637011.
(December 22, 2015, retrospectively registered)
Similar content being viewed by others
Background
Acute respiratory syndrome (ARDS) is characterised by respiratory failure with either a direct pulmonary (e.g. pneumonia) or extra-pulmonary cause (e.g. sepsis) that requires treatment in intensive care including mechanical ventilation [1]. Approximately 10% of all patients admitted to the ICU develop ARDS [2]. This represents a considerable amount of patients. Further, cost associated with ARDS are very high. For instance, a study from the UK estimated mean societal cost over 1 year including initial ICU treatment cost at £ 44.077 [3]. A US study estimated the median cost due to hospitalization post-discharge at $ 18.756 [4].
With mortality estimated up to 45%, the focus of ARDS research has been on mortality for a long time. With decreasing mortality rates [5] however, the interest in long-term outcomes such as mental health, return to work (RtW) and health-related quality of life (HRQoL) of ARDS patients increased [6,7,8,9,10].
A multi-centre national study in the U.S. showed that nearly half of previously employed ARDS survivors were jobless at 12 months after ARDS and that this was accompanied by substantial lost earnings [11]. If one aims at improving long-term outcome for these patients, it is important to shed light on possible determinants of outcomes such as HRQoL and RtW. We performed a systematic literature review [12] summarising the existing evidence regarding the determinants of HRQoL or RtW in ARDS patients, including 24 highly heterogeneous observational studies. One of the main findings was that the core focus of published research was on clinical and care-related determinants (performance in pulmonary function testing, duration of ICU treatment etc.) which mainly showed small, non-significant effects on HRQoL and RtW. Despite the evident role of the care provided to patients with ARDS in the ICU, surprisingly, the role of quality of care (QoC) for long-term HRQoL and RtW has not been investigated thus far while ARDS mortality has been investigated in relation to university level of care [13].
Against this background, the hypothesis underlying the study presented in this paper was that better QoC (defined by quality indicators) received during the acute ICU stay was associated with better HRQoL and a higher rate of RtW in survivors of ARDS. Thus, our aim was to identify QoC indicators predictive of HRQoL and RtW.
Methods
Study design and sample
Methods of the DACAPO (Surviving ARDS: the influence of quality of care and individual patient characteristics on health-related quality of life) study have been described in detail elsewhere [14, 15]. Briefly, adult patients with diagnosed ARDS according to the Berlin definition [16] were recruited in 61 intensive care units (ICUs) across Germany into a cohort study with three postal follow-ups (3, 6 and 12 months). Ethical approval was obtained from the ethics committee of the University of Regensburg (file number: 13–101-0262) and additionally from the ethics committees overseeing the respective study sites.
Measurements
Outcomes
The Short Form-12 self-report questionnaire (SF-12) was used to measure HRQoL [17]. Scores for the Physical Component (PCS-12) and the Mental Component Scales (MCS-12) range from 0 to 100 (higher values indicating better HRQoL). Fifty is the mean value for the general population (German norm values [18]). RtW was captured as self-report, asking whether and when people had returned to their previous or another job (only for persons in employment before admission to ICU). We included data from all participants who returned valid questionnaires up to 13 months after discharge from ICU. Mortality status and date of death were assessed either through reports from the patients’ caregivers or through local population registries.
Exposures
QoC was assessed at the level of the participating ICUs once during the period of the study. It was operationalized as structural quality (head of the ICU with additional training in intensive care medicine), process quality (implementation of daily multi-professional ward rounds with documentation of daily therapy goals), volume (number of ventilated patients per year), and general characteristics (membership of the hospital in the ARDS Network Germany). These pre-specified indicators showed limited statistical variance and were thus complemented by additional quality indicators comprising the proportion of physicians with completed specialized ICU training, the availability of weekly microbiological ward rounds, the proportion of ventilated patients on all patients, and general level of care (university hospital versus other hospital). Both the pre-specified and the additional quality indicators were largely based on the published list of German quality indicators in intensive care medicine [14, 19, 20] and together with generally accepted indicators such as volume and level of care have been used in the comprehensive QoC assessment in the DACAPO study.
Potentially confounding variables
Variables related to socio-demographic, clinical and care aspects were assessed as potentially confounding variables. The socio-demographic variables included age, sex, living situation (living with versus without a partner), nationality (German, other), and health insurance (statutory, private, other). An education score was derived from information on the participants’ educational and professional levels (according to Lampert et al. [21]). The score ranges between 1 and 7, with higher values indicating higher educational and/or professional achievements. General medical characteristics were captured with regard to body mass index (BMI: kg/m2), cause of ARDS (pulmonary / extra-pulmonary), Simplified Acute Physiology Score II (SAPS-II) [22], and Sequential Organ Failure Assessment (SOFA) [23] score, as well as self-reported physician-diagnosed mental disorder before treatment in the ICU (any, none). The Berlin definition of ARDS was modified in terms of the classification of the severity of hypoxemia (PaO2/FIO2 ratio [P/F ratio]) since the classification into two levels (P/F ratio ≤ 150 mmHg versus > 150 mmHg) may allow better selectivity between mild and severe cases [24, 25].
Statistical analysis
Owing to the data structure (patients are nested within different ICUs), we used hierarchical linear modeling, testing whether there was systematic variance between the second-level units (ICUs) in the primary outcome at the three follow-ups. For all outcomes, these analyses yielded an intraclass correlation coefficient (ICC) consistently close to zero on the basis of the fully unconditional model and non-significant p-values for the likelihood ratio tests indicating non-deterioration in model fit if the random intercept was restricted to zero (data not shown). For this reason, fixed-effects linear, logistic and Cox regression models were applied.
Outcome: health-related quality of life (HRQoL)
The twelve-month analysis was the primary analysis. For each follow-up (3, 6 and 12 months) and each exposure–outcome combination non-adjusted, minimally adjusted, and fully adjusted linear regression models were used. The minimally adjusted model contained sex, age, and ARDS severity as covariates; all socio-demographic and medical variables which were significantly (p-value < 0.05) associated with exposures or outcome at any follow-up period were included in the fully adjusted regression models. Non-standardized regression coefficients with 95% confidence intervals (CIs) were computed as were standardized regression coefficients.
Outcome: return to work (RtW)
We used Cox proportional hazards models to analyse RtW. The minimally adjusted models had sex, age, and ARDS-severity as covariates. The fully adjusted multivariate Cox models included all covariates that showed significant effects with either outcome or exposure in univariate Cox regression analyses. Observations were censored if RtW did not occur within 395 days following ICU discharge. Hazard ratios (HRs) with 95% CIs were computed.
Outcome: mortality
We examined the influence of the exposure variables on 1-year mortality in ICU survivors. The set of covariates for the fully adjusted logistic regression models was determined as described for HRQoL and RtW. Odds ratios (ORs) and 95% CIs were computed. A p-value of < 0.05 was considered statistically significant. Analyses were performed using Stata 13.1 (Stata Corporation, College Station, TX, U.S.A.).
Results
Descriptive results
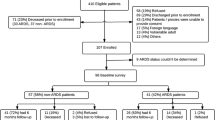
We preliminarily included 1900 ARDS patients from 61 ICUs across Germany between September 2014 and April 2016. One thousand two hundred twenty five patients with informed consent formed the initial ICU sample (Fig. 1). Eight hundred seventy-seven patients formed the DACAPO survivor cohort. Four hundred eighty-one patients (54.8%) were lost to follow up for various reasons (e.g.inability to participate). Information on death or survival could not be obtained for 66 patients. At 1 year after discharge, 19.8% had died and 396 persons were followed up.
Patient flow. a For all patients who were lost to follow-up survival was assessed via local municipal population registries. b Written informed consent and patient data were transferred to the study centre with a delay of more than 12 months; thus, follow-up measurement was not possible within the scheduled follow-up period
The socio-demographic and general medical characteristics of the respondents for the twelve-month follow-up are described in an additional file (Table 4, additional file 1). In about 60% the diagnosis was made in a DACAPO ICU, the remainder was transferred. The ARDS was predominantly caused by direct (pulmonary) conditions. The majority of patients (75%) suffered from ‘moderate-to-severe’ ARDS (P/F ratio < 150). A lifetime diagnosis of a mental disorder was reported in 15%.
The general characteristics and the organizational and structural QoC indicators of the participating ICUs were as follows (Table 1): 70% of study centres were members of the German ARDS network and 28 centres were university institutions. The median number of patients ventilated per year was 493 and the median percent of patients who were ventilated was 44%. The twelve-month ARDS survivors reported a median physical SF-12 (SF-12 PCS) of 41 (IQR 35–52) and a mental SF-12 (SF-12 MCS) of 47 (IQR 33–57) Fig. 2. No substantial increase in SF-12 values was observed between 3 and 12 months. Slightly over half of the patients returned to work after 1 year.
Analytical results
The main analysis refers to the effect of QoC on HRQoL (Table 5, additional file 2) and RtW (Table 2) after 12 months, and additional analyses were computed for three (Table 6, additional file 3) and 6 months (Table 7, additional file 4) and mortality (Table 3).
Main analytical results
There was no significant association between most QoC variables and HRQoL at 12 months (Table 5, additional file 2). However, in the fully adjusted analysis, treatment in ICUs that had a higher percentage of ventilated patients was significantly associated with a decreased PCS-12. The analysis of the effects of QoC on RtW after 12 months (Cox regression analyses, Table 2) showed a significantly decreased hazard of RtW for patients treated in institutions with a higher proportion of ventilated of patients but no significant association was found for all further variables.
Additional analytical results
In the fully adjusted analysis of the three-month follow-up (Table 6, additional file 3), a higher percentage of patients ventilated was significantly associated with a decreased PCS-12. There was a trend towards a decreased PCS-12 for patients treated in university hospitals (p = 0.054). No significant associations were observed for any QoC parameter and 6 months HRQoL (Table 7, additional file 4).
Based on a logistic regression analysis among ICU survivors twelve-month mortality risk (Table 3) was significantly elevated for patients treated in institutions with a higher number of ventilated patients per year. Additionally, patients treated in university hospitals had a significantly increased twelve-month mortality risk (OR 1.946, p = 0.032) compared to non-university institutions. A trend was seen for a reduced mortality risk in patients treated in ICUs with a higher percentage of ICU specialist physicians.
Discussion
Key findings
The main finding of our study was that of all the quality indicators investigated – taking important confounding factors into account – only the proportion of ventilated patients on all patients showed a significant (negative) association with both twelve-months physical HRQoL (PCS-12) and RtW. Secondary findings were a negative significant association of the proportion of ventilated patients on all patients and positive significant associations between the number of ventilated patients per year and university hospital level of care with post ICU mortality risk. No other quality indicator was significantly associated with the outcomes of interest. The DACAPO survivor cohort was characterized by a reduced HRQoL compared to the general population, with a greater impairment in the physical component compared to the mental component. 50% of the surviving patients who were in employment before ARDS had returned to work one year after transferred from the ICU.
Interpretation, in relation to literature
Our results need careful interpretation. A study by Raymondos et al. [13] showed that the hospital mortality risk of ARDS patients was considerably higher in patients who were treated in non-university hospitals compared to university institutions. However, the present study did not demonstrate a similar effect for HRQoL or RtW 1 year after discharge. A systematic review [26] demonstrated that critically ill patients generally benefit from institutions with high volume regarding mortality with more substantial effects in high risk patients, and there was evidence that this relationship is in part mediated by key hospital or ICU organizational factors. We could not confirm this evidence.
It must be noted though that in contrast to mortality, HRQoL is a complex construct containing individual aspects (multiple dimensions, often operationalized as social, somatic and psychological variables [27]. Our above mentioned systematic review [12] demonstrated significant associations with HRQoL after ARDS only for determinants which were closely related to the scales of the HRQoL instruments and which were measured at the same time as HRQoL.
We were unable to consider variables pertaining to the period after discharge although the post ICU period may play an important role in terms of HRQoL. For instance, a prospective one-year follow-up of 126 patients who received prolonged mechanical ventilation by Unroe et al. [28] showed that these experienced multiple ‘trajectories’ after their transfer from the ICU, resulting in frequent readmissions or transitions to various healthcare institutions.
It might be argued that we did not find strong associations between our exposures of interest (i.e. parameters of QoC) and the different outcomes because 1) the exposures showed little variability and 2) effects may have been seen if intermediary outcomes such as length of ventilation or prevention of multiorgan failure would have been considered. However, when it became clear that the quality indicators that were chosen initially did not show sufficient variability, we considered further quality indicators which were related to those that were chosen initially. These indicators did indeed show variability. Second, our research interest was precisely to study modifiable institutional-level indicators of quality of care in relation to patient-level outcomes. We are coming from a public health/health care research perspective in which we can improve health or achieve better disease outcomes, even if we do not know the mechanisms linking exposure to outcome. In light of this background, we did not attempt to explore pathways between QIs, HRQoL, RtW or mortality through intermediary outcomes. Treatment-related exposures not assessed on the institutional but on the individual patient level (parameters of the intensity of acute care management and critical events) have been investigated in a separate paper [29].
Strenghts and limitations
We were successful in the conduct of a prospective patient cohort study with regular follow-ups. Unfortunately, the number of people lost to follow-up was considerable which may have introduced attrition bias. ICU mortality was not even used as outcome in secondary analysis because the recruitment strategies used focused on the survivor cohort as baseline. For instance, only surviving patients or patients with a legal guardian providing informed consent could be included at some sites, while consent by next-of-kin was acceptable at other sites. Any analysis of factors predictive of ICU mortality would therefore be seriously biased.
Although we adjusted for ARDS severity in our minimally adjusted models, and further corrected for SAPS-II and SOFA scores in the fully adjusted regression models, residual confounding may still be present in relation to ARDS severity/case-mix which might explain our findings. Treatment received before or after ICU care was also not corrected for which might have further contributed to residual confounding.
Measuring QoC in the critical care setting is challenging. In a review by Flaatten et al. [30] 63 quality indicators (QI) measuring quality of structure, process and outcome of care were identified, which are in use with a large variation between countries and no single QI was common for all. QIs for structure predominantly refer to the qualification and quantity of health care professionals, as well as the number of ventilated patients per year [31] process quality indicators are more complex, numerous and they refer to actual recommendations of guidelines (ventilation strategies, nutrition, transfusion strategy etc.). These indicators must be defined by experts or Delphi rounds and they are difficult to be monitored continuously in clinical practice. Usually QIs for outcome include standardized mortality (ICU, hospital, 60- or 90-days) or the incidence of decubiti [31]. No previous investigation used QIs for an assessment of the association with HRQoL. In our study we had to use established QIs only assuming that they have value to describe sufficiently the effect of care on patient-reported outcomes.
Future research
Further, in this paper, we only selected some QIs from the full set. One idea for future research might be to use the full set of QIs to develop a scoring system predictive of patient-level outcome. Such an attempt however is beyond the scope of this paper.
Given the importance of next of kin / family during ICU stay [32], it would be interesting to additionally look at patient-family satisfaction and patient-family engagement in future research.
Conclusions
In conclusion, most indicators of acute QoC were not significantly associated with one-year HRQoL or RtW in ARDS survivors. Post-ICU exposure of ARDS survivors may have attenuated the assumed effects of high-volume care. Overall, we cannot rule out residual confounding by case mix, treatment variables before or during ICU stay and variables pertaining to the post ICU period.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to confidentiality of patient data but are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CRF:
-
Case report form
- ICU:
-
Intensive care Unit
- IQR:
-
Interquartile range
- HRQoL:
-
Health-related quality of life
- MCS-12:
-
Mental component summary
- Md:
-
Median
- PCS-12:
-
Physical component summary
- PTSD:
-
Post-traumatic stress disorder
- QoC:
-
Quality of care
- QoL:
-
Quality of life
- RtW:
-
Return to work
- SAPS-II:
-
Simplified acute physiology score II
- SF-12:
-
Short form 12 survey
- SOFA:
-
Sequential organ failure assessment
References
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012;38(10):1573–82.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Marti J, Hall P, Hamilton P, Lamb S, McCabe C, Lall R, et al. One-year resource utilisation, costs and quality of life in patients with acute respiratory distress syndrome (ARDS): secondary analysis of a randomised controlled trial. J Intensive Care. 2016;4:56.
Ruhl AP, Huang M, Colantuoni E, Karmarkar T, Dinglas VD, Hopkins RO, et al. Healthcare utilization and costs in ARDS survivors: a 1-year longitudinal national US multicenter study. Intensive Care Med. 2017;43(7):980–91.
Maca J, Jor O, Holub M, Sklienka P, Bursa F, Burda M, et al. Past and Present ARDS Mortality Rates: A Systematic Review. Respir Care. 2017;62(1):113–22.
Myhren H, Ekeberg O, Stokland O. Health-related quality of life and return to work after critical illness in general intensive care unit patients: a 1-year follow-up study. Crit Care Med. 2010;38(7):1554–61.
Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304.
Hashem MD, Nallagangula A, Nalamalapu S, Nunna K, Nausran U, Robinson KA, et al. Patient outcomes after critical illness: a systematic review of qualitative studies following hospital discharge. Crit Care. 2016;20:345.
Jutte JE, Needham DM, Pfoh ER, Bienvenu OJ. Psychometric evaluation of the hospital anxiety and depression scale 3 months after acute lung injury. J Crit Care. 2015;30(4):793–8.
Biehl M, Kashyap R, Ahmed AH, Reriani MK, Ofoma UR, Wilson GA, et al. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study. Crit Care. 2015;19:356.
Kamdar BB, Huang M, Dinglas VD, Colantuoni E, von Wachter TM, Hopkins RO, et al. Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year National Multicenter Study. Am J Respir Crit Care Med. 2017;196(8):1012–20.
Dodoo-Schittko F, Brandstetter S, Blecha S, Thomann-Hackner K, Brandl M, Knuttel H, et al. Determinants of quality of life and return to work following acute respiratory distress syndrome. Deutsches Arzteblatt Int. 2017;114(7):103–9.
Raymondos K, Dirks T, Quintel M, Molitoris U, Ahrens J, Dieck T, et al. Outcome of acute respiratory distress syndrome in university and non-university hospitals in Germany. Crit Care. 2017;21:122.
Brandstetter S, Dodoo-Schittko F, Blecha S, Sebok P, Thomann-Hackner K, Quintel M, et al. Influence of quality of care and individual patient characteristics on quality of life and return to work in survivors of the acute respiratory distress syndrome: protocol for a prospective, observational, multi-Centre patient cohort study (DACAPO). BMC Health Serv Res. 2015;15:563.
Dodoo-Schittko F, Brandstetter S, Brandl M, Blecha S, Quintel M, Weber-Carstens S, et al. German-wide prospective DACAPO cohort of survivors of the acute respiratory distress syndrome (ARDS): a cohort profile. BMJ Open. 2018;8(4):e019342.
Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–33.
Ware JE Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33.
Bullinger M, Kirchberger I. Der SF-36 Fragebogen zum Gesundheitszustand: Handbuch für die deutschsprachige Fragebogenversion. Boston: Medical Outcomes Trust; 1995.
Braun JP, Kumpf O, Deja M, Brinkmann A, Marx G, Bloos F, et al. The German quality indicators in intensive care medicine 2013--second edition. German Med Sci. 2013;11:Doc09.
Kumpf O, Braun JP, Brinkmann A, Bause H, Bellgardt M, Bloos F, et al. Quality indicators in intensive care medicine for Germany - third edition 2017. German Med Sci. 2017;15:Doc10.
Lampert T, Kroll L, Muters S, Stolzenberg H. Measurement of socioeconomic status in the German health interview and examination survey for adults (DEGS1). Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2013;56(5–6):631–6.
Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–63.
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710.
Maiolo G, Collino F, Vasques F, Rapetti F, Tonetti T, Romitti F, et al. Reclassifying acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197(12):1586–95.
Nguyen YL, Wallace DJ, Yordanov Y, Trinquart L, Blomkvist J, Angus DC, et al. The volume-outcome relationship in critical care: a systematic review and meta-analysis. Chest. 2015;148(1):79–92.
Gaudry S, Messika J, Ricard JD, Guillo S, Pasquet B, Dubief E, et al. Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review. Ann Intensive Care. 2017;7(1):28.
Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. 2010;153(3):167–75.
Bein T, Weber-Carstens S, Apfelbacher C, Brandstetter S, Blecha S, Dodoo-Schittko F, et al. The quality of acute intensive care and the incidence of critical events have an impact on health-related quality of life in survivors of the acute respiratory distress syndrome - a nationwide prospective multicenter observational study. German Med Sci. 2020;18:Doc01.
Flaatten H. The present use of quality indicators in the intensive care unit. Acta Anaesthesiol Scand. 2012;56(9):1078–83.
de Vos M, Graafmans W, Keesman E, Westert G, van der Voort PH. Quality measurement at intensive care units: which indicators should we use? J Crit Care. 2007;22(4):267–74.
Schneeberger A, Brandstetter S, Bein T, Blecha S, Apfelbacher C. Stressors and strains of next of kin of patients with ARDS in intensive care: a qualitative interview study using a stress-strain approach. Intensive Crit Care Nurs. 2019;24:102783.
Acknowledgements
We are indebted to all the intensive care specialists and study nurses throughout Germany, who, with great commitment, recruited patients for the DACAPO study:
Johannes Bickenbach, Thorben Beeker, Tobias Schürholz, Jessica Pezechk (Aachen); Jens Schloer (Amberg); Ulrich Jaschinski, Ilse Kummer (Augsburg); Oliver Kuckein (Bamberg); Steffen Weber-Carstens, Anton Goldmann, Stefan Angermair, Krista Stoycheva, Jörg Brederlau, Nadja Rieckehr, Gabriele Schreiber, Henriette Haennicke (Berlin); Friedhelm Bach, Immo Gummelt, Silke Haas, Catharina Middeke, Ina Vedder, Marion Klaproth (Bielefeld); Michael Adamzik, Jan Karlik, Stefan Martini, Luisa Robitzky (Bochum); Christian Putensen, Thomas Muders, Ute Lohmer (Bonn); Rolf Dembinski (Bremen); Petra Schäffner, Petra Wulff-Werner (Deggendorf); Elke Landsiedel-Mechenbier, Daniela Nickoleit-Bitzenberger, Ann-Kathrin Silber (Dortmund); Maximilian Ragaller, Marcello Gama de Abreu, Alin Ulbricht, Linda Reisbach (Dresden); Kai Zacharowski, Patrick Meybohm, Simone Lindau, Haitham Mutlak (Frankfurt am Main); Alexander Hötzel, Johannes Kalbhenn (Freiburg); Christoph Metz, Stefan Haschka (Freising); Stefan Rauch (Göppingen); Michael Quintel, Lars-Olav Harnisch, Sophie Baumann, Andrea Kernchen (Göttingen); Sigrun Friesecke, Sebastian Maletzki (Greifswald); Stefan Kluge, Olaf Boenisch, Daniel Frings, Birgit Füllekrug, Nils Jahn, Knut Kampe, Grit Ringeis, Brigitte Singer, Robin Wüstenberg (Hamburg); Jörg Ahrens, Heiner Ruschulte, Andre Gerdes, Matthias Groß, Olaf Wiesner, Aleksandra Bayat-Graw (Hannover); Thorsten Brenner, Felix Schmitt, Anna Lipinski (Heidelberg); Dietrich Henzler, Klaas Eickmeyer, Juliane Krebs, Iris Rodenberg (Herford); Heinrich Groesdonk, Kathrin Meiers, Karen Salm, Thomas Volk (Homburg); Stefan Fischer, Basam Redwan (Ibbenbüren); Martin Schmölz, Kathrin Schumann-Stoiber, Simone Eberl (Immenstadt); Gunther Lenz, Thomas von Wernitz-Keibel, Monika Zackel (Ingolstadt); Frank Bloos, Petra Bloos, Anke Braune, Anja Haucke, Almut Noack, Steffi Kolanos, Heike Kuhnsch, Karina Knuhr-Kohlberg (Jena); Markus Gehling (Kassel); Mathias Haller, Anne Sturm, Jannik Rossenbach (Kempten); Dirk Schädler, Stefanie D’Aria (Kiel); Christian Karagiannidis, Stephan Straßmann, Wolfram Windisch, Thorsten Annecke, Holger Herff (Köln); Michael Schütz (Langen); Sven Bercker, Hannah Reising, Mandy Dathe, Christian Schlegel (Leipzig); Katrin Lichy (Ludwigsburg); Wolfgang Zink, Jana Kötteritzsch (Ludwigshafen); Marc Bodenstein, Susanne Mauff, Peter Straub (Mainz); Christof Strang, Florian Prätsch, Thomas Hachenberg (Magdeburg); Thomas Kirschning, Thomas Friedrich, Dennis Mangold (Mannheim); Christian Arndt, Tilo Koch (Marburg); Hendrik Haake, Katrin Offermanns (Mönchengladbach); Patrick Friederich, Florian Bingold, Michael Irlbeck, Bernhard Zwissler, Ines Kaufmann, Ralph Bogdanski, Barbara Kapfer, Markus Heim, Günther Edenharter (München); Björn Ellger, Daniela Bause (Münster); Götz Gerresheim (Neumarkt i.d.OPf); Dorothea Muschner, Michael Christ, Arnim Geise (Nürnberg); Martin Beiderlinden, Thorsten Heuter (Osnabrück); Alexander Wipfel (Passau); Werner Kargl, Marion Harth, Christian Englmeier, Thomas Bein, Sebastian Blecha, Dr. Kathrin Thomann-Hackner, Marius Zeder (Regensburg); Markus Stephan (Stuttgart); Martin Glaser (Traunstein); Helene Häberle (Tübingen); Hendrik Bracht, Christian Heer, Theresa Mast (Ulm); Markus Kredel, Ralf Müllenbach (Würzburg).
Further, we are grateful to previous members of the Regensburg DACAPO study team (medical documentation: Phillip Sebök, study physician: Kathrin Thomann-Hackner), to the members of the Advisory Board of the DACAPO-Study (Julika Loss, Bernhard Graf, Michael Leitzmann, Michael Pfeifer all Regensburg) and to our student assistants (Simon Bein, Vreni Brunnthaler, Carina Forster, Stefanie Hertling, Sophie Höhne, Carolin Schimmele, Elisa Valletta, Philipp Drewitz and Chiara Eberle). We are grateful to Arthur Slutsky, Toronto, for critically reviewing the intellectual content of an earlier version of this manuscript. Medical writing support was sought from topcorrect, Germany.
Funding
The DACAPO study was funded by a research grant from the German Federal Ministry of Education and Research (01GY1340). Grant holders were TB (University Hospital Regensburg, principal investigator) and CA1 (University of Regensburg, co-principal investigator). SB1, FDS, MB and SB2 were funded by this grant for parts of or the entire study period. All other authors received payments from the grant to support patient recruitment. The funding body had no role in the design of the study, nor in the collection, analysis, and interpretation of the data, nor in writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
CA1 and TB conceived the study with the help of SB1, SB2, FDS, SWC and MQ. CA1, SB1, FDS and MB were responsible for data management, quality assurance, conduct of the follow-up and statistical analyses. CA1 and TB wrote the manuscript with the help of SB1, FDS, CK and SWC. SK, CP, SB3, BE, TK, CA2, PM were involved in recruitment and practical implementation of the study. All authors critically read and approved the article.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University of Regensburg (file number: 13–101-0262) and by the ethics committees responsible for the participating hospitals. All participants provided a written informed consent form.
Consent for publication
Not applicable.
Competing interests
TB, CK, MQ, SK, CP, SB3, BE, TK, CA2, PM, and SWC are members of the German ARDS-Network. TB: received honoraria for lectures from Xenios Company, Germany. MQ: received honoraria for lectures from Maquet, Company, and Xenios Company, Germany. All other authors declare: no relationships/conditions/circumstances that present a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
Excel File. Table 4. Socio-demographic and general medical characteristics of 12 months respondents. a derived from educational and professional level22, considered for regression analyses. SAPS-II score: Simplified Acute Physiology Score II without Glasgow Coma Scale; SOFA score: Sequential Organ Failure Assessment; BMI: Body Mass Index. bas assessed at admission at the DACAPO ICU, c without consideration of Glasgow Coma Scale.
Additional file 2:
Excel File. Table 5. Linear Regression Analyses of Health-related Quality of Life after 12 months on Quality of Care. a adjusted for age, sex, severity of ARDS; b adjusted for age, sex, severity of ARDS, BMI, Education score, SAPS-II score, SOFA score, diagnosis of ARDS (participating vs. other ICU), self-reported physician-diagnosed mental disorder before ARDS diagnosis; Notes: ARDS = acute respiratory distress syndrome; MCS-12 = mental component scale of short-form 12 questionnaire; PCS-12 = physical component scale of short-form 12 questionnaire.
Additional file 3:
Excel File. Table 6. Linear Regression Analyses of Health-related Quality of Life after 3 months on Quality of Care. a adjusted for age, sex, severity of ARDS; b adjusted for age, sex, severity of ARDS, BMI, Education score, SAPS-II score, SOFA score, diagnosis of ARDS (participating vs. other ICU), self-reported physician-diagnosed mental disorder before ARDS diagnosis Notes: ARDS = acute respiratory distress syndrome; MCS-12 = mental component scale of short-form 12 questionnaire; PCS-12 = physical component scale of short-form 12 questionnaire.
Additional file 4:
Excel File. Table 7. Linear Regression Analyses of Health-related Quality of Life after 6 months on Quality of Care. a adjusted for age, sex, severity of ARDS; b adjusted for age, sex, severity of ARDS, BMI, Education score, SAPS-II score, SOFA score, diagnosis of ARDS (participating vs. other ICU), self-reported physician-diagnosed mental disorder before ARDS diagnosis. Notes: ARDS = acute respiratory distress syndrome; MCS-12 = mental component scale of short-form 12 questionnaire; PCS-12 = physical component scale of short-form 12 questionnaire.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Apfelbacher, C., Brandstetter, S., Blecha, S. et al. Influence of quality of intensive care on quality of life/return to work in survivors of the acute respiratory distress syndrome: prospective observational patient cohort study (DACAPO). BMC Public Health 20, 861 (2020). https://doi.org/10.1186/s12889-020-08943-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-020-08943-8