Abstract
Objectives
Invasive group A Streptococcus (iGAS) disease is serious and sometimes life-threatening. The Paediatric Active Enhanced Disease Surveillance (PAEDS) Network collects voluntary notifications from seven major Australian paediatric hospitals on patients with certain conditions, including iGAS disease. Our aims were to: 1) Describe the epidemiological distribution of paediatric iGAS disease in Australia and correlate this with influenza notifications, 2) Identify GAS strains commonly associated with invasive disease in children.
Methods
IGAS and influenza notification data were obtained (from the PAEDS Network and the Australian Institute of Health and Welfare, respectively, for the period 1 July 2016 to 30 June 2018). Included iGAS patients had GAS isolated from a normally sterile body site. Data were described according to selected clinical and demographic characteristics, including by age group and Australian State, with proportions and minimum incidence rates estimated.
Results
A total of 181 patients were identified, with most (115, 63.5%) <5 years old. The mean annual minimum incidence rate was 1.6 (95% confidence interval: 1.1–2.3) per 100,000 children across the study period. An epidemiological correlation with the seasonal burden of influenza was noted. Contact prophylaxis was not consistently offered. Of 96 patients with emm-typing results available, 72.9% showed emm-1, −4 or − 12.
Conclusions
Robust surveillance systems and cohesive patient management guidelines are needed. Making iGAS disease nationally notifiable would help facilitate this. Influenza vaccination may contribute to reducing seasonal increases in iGAS incidence. The burden of disease emphasises the need for ongoing progress in GAS vaccine development.
Similar content being viewed by others
Background
Group A Streptococcus (GAS) produces a wide range of illnesses in humans. Invasive GAS (iGAS) diseases are associated with acute mortality and considerable morbidity, including permanent disability. IGAS disease is defined by the isolation of GAS from a normally sterile bodily site [1]. Examples of iGAS disease include: septic arthritis, osteomyelitis, meningitis, bacteremia/septiceamia, pneumonia, and necrotizing fasciitis. Even in high-income countries, these conditions are often associated with case fatality rates (CFR) of approximately 10–15%; with the CRF higher still for necrotizing fasciitis (CFR approximately 20%) and streptococcal toxic shock syndrome (STSS; recent CFRs reported as ≤28%) [2,3,4,5]. A global 2005 review estimated that over 660,000 people develop iGAS disease worldwide each year, with over 160,000 resulting deaths [6]. Much of the disease burden is concentrated in low/middle-income countries [6]. People with the highest risk of iGAS disease include young children, elderly people, injecting drug users, and patients with certain comorbidities such as diabetes, influenza and immunosuppression. Patients’ close contacts have an approximately 2000-times increased risk of developing iGAS disease themselves; termed ‘secondary disease’ [7, 8]. This increased risk is especially pronounced for mother-neonate pairs and co-habiting couples aged > 74 years old [8]. Despite this, there are no official Australian guidelines concerning the prevention of secondary disease using contact prophylaxis. Disparate clinical guidelines from other high-income countries and some Australian States (Victoria, Queensland, New South Wales, Northern Territory) exist [1, 9,10,11,12,13,14,15].
The incidence of iGAS disease has increased across several high-income regions for unclear reasons, with 2017/2018 rates of 7–10 per 100,000 across the general population reported in the United States (US) and Canada [16,17,18,19,20]. One possible explanation may involve the increasing diversity in emm-types [20, 21]. Socioeconomic drivers may have an important role in promoting iGAS disease, particularly excessive drug and alcohol use [20, 22]. The incidence of iGAS disease among First Nations peoples of Canada exceeded 30/100,000 during 2009–2014, [23, 24] which was comparable to rates observed for Indigenous populations in Australia and New Zealand [25,26,27,28]. Inequitably high rates of iGAS disease have also been documented among US First Nations peoples [29]. A study set in the Northern Territory of Australia study estimated an iGAS incidence rate of 70/100,000 for the Indigenous population as a whole, almost 8-fold higher than that reported for the non-Indigenous population [25]. An Australian study in Queensland noted high ethnic inequities amongst children aged < 19 years-old, with an annualised incidence of 13.2/100,000 for Indigenous children, nearly 4-fold higher than for non-Indigenous children [26].
Local Australian surveillance data indicates that the incidence rate of iGAS disease is increasing in Victoria, with a new high level reached in 2017, of 3.6/100,000 (95% confidence interval, CI: 3.2–4.1/100,000; i.e. 220 new cases that year) [30, 31]. A 2017 outbreak among Victorian children is especially concerning, and was associated with a high burden of seasonal influenza [31]. As iGAS disease is currently notifiable only in two Australian jurisdictions (Northern Territory & Queensland), it is difficult to accurately ascertain the burden of disease [10, 12]. During 2017–2018, 143 patients were notified in the Northern Territory [32]. In Queensland, a new high number of 381 patients were notified in 2017 (and 355 patients were notified in 2018). Of the 2017–2018 Queensland total, 16.2% of patients were aged <20 years [33].
The Paediatric Active Enhanced Disease Surveillance (PAEDS) Network compiles detailed information on selected serious childhood conditions treated in seven major paediatric hospitals across Australia [34]. IGAS disease was included as a condition of interest in July 2016, however not all states commenced data collection at the same time and retrospective recruitment was frequent (Additional file 2: Figure S1). This national surveillance followed a pilot study at one of two participating PAEDS sites in Melbourne, Victoria over 2014–2016 (the Royal Children’s Hospital, RCH) [30]. A key goal of the PAEDS Network was to enhance the understanding the disease burden and provide data to support targeted control and prevention activities, including vaccination [34]. The major limitation of the PAEDS Network is that data collected are limited to patients treated in participating hospitals. Patients treated at non-notifying hospitals are missed by this system.
Currently there is no vaccine to prevent iGAS disease. However, vaccine development is underway in the US, Brazil, Europe and in Australia, with endorsement from the World Health Organization (WHO). Important steps in vaccine development include establishing the burden of disease and associated financial costs, as well as identifying GAS strains causing invasive disease [35,36,37].
Accordingly, this research utilises PAEDS Network data with aims to: 1) Describe the epidemiological distribution of paediatric iGAS disease and correlate this with the seasonal burden of influenza, and 2) Identify any GAS strains that are particularly likely to be associated with invasive disease in children.
Methods
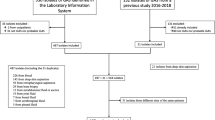
Surveillance of iGAS disease was conducted over 24 months from July 1, 2016 to June 30, 2018 at seven major paediatric centres in Australia; Royal Children’s Hospital (RCH) and Monash Children’s Hospital (MCH; Victoria), Queensland Children’s Hospital (QCH; Queensland), Children’s Hospital at Westmead (CHW; New South Wales), Royal Darwin Hospital (RDH; Northern Territory), Perth Children’s Hospital (PCH; Western Australia), Women’s and Children’s Hospital (WCH; South Australia; Additional file 3: Figure S2). Children with iGAS disease who were seen in settings other that those hospitals listed above were not able to be included in this analysis.
Data sources
Surveillance data were collected under the auspices of the national PAEDS Network. (http://www.paeds.edu.au/) [34]. RCH and MCH conducted prospective recruitment throughout the entire 24 month study period. The other five centres initiated prospective surveillance at various times during the study period, with retrospective recruitment to detect patients diagnosed earlier during the study period (the prospective surveillance period ranged from 13 to 20 months) because of varied timing for ethics approvals (Additional file 2: Figure S1).
In Australia, influenza is legally notifiable to the Australian National Notifiable Diseases Surveillance System (NNDSS), overseen by the Australian Institute of Health and Welfare. Data on all laboratory confirmed influenza patient notifications were obtained from NNDSS for the study period.
Study procedures
When conducting prospective recruitment, diagnostic laboratory staff at each hospital informed the study team when GAS was isolated from a normally sterile site in a patient. The study team then approached the patient and their family to invite them to participate. Once written informed consent was obtained, demographic and clinical data were collected and entered onto a RedCap database, including data on clinical outcomes from a follow up survey conducted 6 months post-discharge. When conducting retrospective recruitment, patients were identified in clinical records following a waiver of consent, with relevant data extracted and entered onto the database.
When available, GAS isolates underwent emm-gene typing for strain identification using standard laboratory protocols [38].
Case definitions
All children aged <18 years admitted to a participating hospital during the study period with laboratory confirmed iGAS disease were eligible for inclusion. We defined iGAS disease as the isolation of GAS from a normally sterile bodily site using standard diagnostic microbiological laboratory procedures. ‘Severe disease’ occurred when a patient was admitted to the intensive care unit (ICU), or was treated with (any of) inotropes, haemofiltration, vasopressors, mechanical ventilation or extracorporeal membrane oxygenation (ECMO).
Statistical analysis
Descriptive epidemiological analyses were performed according to specified demographic patient features, clinical aspects and GAS emm-type strain, as reported in the PAEDS database. When calculating iGAS disease and influenza notification incidence rates, census estimate data for the age group and jurisdiction/s of interest were obtained from the Australia Bureau of Statistics website and used as denominator data [39]. Due to the likelihood of missing iGAS patient data due to children not being seen in notifying hospitals, our incidence estimates are described as ‘minimum incidence rates’. Rates were annualised to adjust for data collection occurring over partial years (i.e. 2016 and 2018) using a factor of 2. When calculating annualised rates by year quarter, the adjustment was made using a factor of 4. Where prophylaxis was offered to family or household contact/s of patients, this was recorded in the PAEDS database. Risk ratio (RR) calculations with 95% CI were produced to describe the likelihood of patients’ contacts being offered antibiotics as GAS prophylaxis. The reference group was usually selected on the basis that it included the highest number of patients.
Results
Key characteristics of notified patients
A total of 192 patients were notified over the study period, of whom 181 met our criteria for laboratory confirmed iGAS disease and were included. The hospitals that contributed most data on included patients were QCH (43 patients, 23.8% total patients) and RCH (37 patients, 20.4%). Most children, 121 (69.6%) had GAS isolated from blood. IGAS disease was more common in males (107 patients, 59.1%). The majority of patients were less than 5 years old (115, 63.5%), including 32 (17.7%) aged < 1 year old.
Of the 181 patients, 21 (11.6%) identified as having Aboriginal Australian or Torres Strait Islander (ATSI) ethnicity. A total of 74 patients (40.9%) had severe disease and 26 patients (14.4%) had STSS. Although the majority, (122 patients, 67.4%) made a full recovery, 5 children died and the remainder were left with physical deficits and/or ongoing disability at 6 months post-discharge (Table 1).
Retrospective reviews identified 79 (43.6%) patients, with others recruited prospectively (Additional file 1: Table S1).
Antibiotic prophylaxis was offered to family or other household contacts of 85 patients (47.0%, Table 2).
Of patients with severe disease, 37 of 74 (50.0%) had antibiotic prophylaxis offered to contacts, compared to 48 of 107 patients (44.9%) with non-severe disease. There appeared to be a wide range of practices, with antibiotic prophylaxis offered to 0.0–81.1% of patients by hospital. The likelihood of household contacts being offered secondary prophylaxis was higher when the patient had household contact/s aged <10 years old (RR: 2.42, 95% CI: 1.54–3.79).
Annualised minimum incidence rate of paediatric iGAS disease
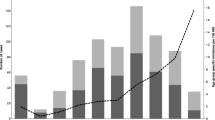
The annualised minimum incidence rate of paediatric iGAS disease for all children aged <18 years old is shown in Figs. 1, 2a. Rates are displayed for the whole country and by jurisdiction. The mean minimum incidence rate was 1.6 (95% CI: 1.1–2.3)/100,000 children for the whole study period. This rate was highest during the third quarter of 2017; 3.4 (95% CI: 2.5–4.5)/100,000 children; 47 patients, and lowest during the first quarter of 2017; 0.8 (95% CI: 0.4–1.4)/100,000 children, 11 patients. This pattern followed trends in the national incidence rate of influenza notifications for the total population, with a low burden during the first quarter of 2017; 135.8 notifications (95% CI: 132.9–138.7)/100,000 population, which preceded a sharply increased peak rate in the third quarter of that year; 3376.1 notifications (95% CI: 3391.0-3362.2)/100,000 population, Fig. 1.
The mean annualised minimum incidence rate for ATSI children (<18 years old) across the study period was 3.4 (95% CI: 2.1–5.7)/100,000, 2.1-fold higher than for all children (1.6, 95% CI: 1.1–2.3/100,000).
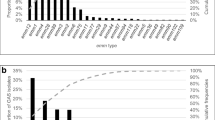
The NT had the highest mean annualised minimum incidence rate; 4.0, (95% CI: 1.5–7.9)/100,000 children, although with small patient numbers (5 patients in total), followed by SA; 3.0, (95% CI: 1.2–6.0)/100,000, WA; 2.3 (95% CI: 1.1–4.9)/100,000, VIC; 2.2 (95% CI: 1.2–3.8)/100,000, QLD; 1.9 (95% CI: 1.0–3.5)/100,000, and NSW; 0.7 (95% CI: 0.2–1.3)/100,000 (Fig. 2a).
Figure 2b shows the mean annualised minimum incidence rate of paediatric iGAS disease by age group and the mean annual number of patients. The minimum incidence was consistently highest for infants <1 year old; 5.1 (95% CI: 1.6–12.2)/100,000, 32 patients, with rates decreasing for older age groups (Fig. 2b). The minimum incidence for all age groups was highest during the third quarter of 2017, with the exception of 15–17 year old children.
emm-type distribution of patient isolates
There were 96 patients (53.0%) with GAS strain data available (from RCH, MCH, QCH, PCH). A total of 20 different emm-types were identified. The most common was emm-1 (36 isolates, 37.5% of those with strain data), followed by emm-4 (20 isolates, 20.8%) and emm-12 (14 isolates, 14.6%, Additional file 1: Table S2). Most patients with strain data (53, 55.2%) were hospitalised in Victoria. Fig. 3 shows the distribution of emm-types by Australian jurisdiction.
Discussion
Data collected through the national PAEDS Network has permitted the epidemiological distribution of paediatric iGAS disease and associated emm-types to be clearly described over the two-year study period. A concerning burden of disease was documented, concentrated among young children and infants, including a high proportion (12%) of Australian Aboriginal and Torres Strait Islander people, who comprise 3% of the national Australian population. As indicated by previous research in Victoria, Australia, the rate of paediatric iGAS disease among our cohort appeared to broadly correlate with national notifications for influenza, although on a greatly reduced scale [31]. Cases of influenza and iGAS co-infection have been frequently reported in the international literature [40]. Increases in iGAS disease have been correlated with high rates of influenza in several overseas settings, including the UK, Sweden, and Israel [41,42,43]. While the pathogenic pathways mediating an increased risk of iGAS disease with concurrent/antecedent influenza are not fully understood, certain influenza proteins (such as haemagglutinin) might enhance GAS virulence and/or suppress the immune response [40, 44, 45]. Our findings support the hypothesis that influenza vaccination may offer some protection against iGAS disease, as indicated by a study of US military recruits [44].
We have shown a high morbidity and mortality burden associated with iGAS in Australian children. Our national minimum incidence estimate of 1.6 iGAS patients per 100,000 children is comparable to the rate reported for 5–17 year-old children in Alaska (1.8/100,000 during 2001–2013), [29] and for 5–9 year-old children in the UK during 2017–2018 [21]. Our minimum incidence estimate for children aged < 1 year (5.1/100,000) is close to the reported rate for this age group in Canada (4.8/100,000 during 2001) [15] and in the US (5.3/100,000 during 2000–2004) [5]. Over 40% of the iGAS patients identified by our study were categorised as having severe disease. Furthermore, five children died and nearly 30% were left with a physical deficit and/or disability following hospital discharge. All Australian states that contributed data to the PAEDS Network were affected by paediatric iGAS disease. The national mean annualised minimum incidence rate (1.6/100,000 children) for paediatric iGAS disease is similar to the rate of Meningococcal infection (1.5/100,000 total population in 2017), which is a nationally notifiable condition in Australia [46] and exerts a similar high morbidity and mortality burden to iGAS disease [47]. Further, the risk of secondary iGAS disease among household/family contacts (a 2000-times increased risk) is even higher than the risk of secondary Meningococcal infection (500–800-times increased risk). These findings support the need for iGAS patient notification and evaluation of contact prophylaxis/education for the prevention of secondary disease [7, 8, 48].
Geographic disparities in contact prophylaxis for iGAS patients were apparent. The 2.4-fold increased likelihood of contact prophylaxis being offered when a patient had household contacts aged <10 years old may indicate clinical recognition of the need to protect young children cohabiting with patients from GAS transmission. The geographic variation in offering contact prophylaxis between hospitals, however, illustrates the need for a consensus national recommendation. Despite infants consistently demonstrating the highest rate of iGAS disease, [8] household contacts of infant patients were no more likely to be offered prophylaxis than older patients’ household contacts. Any national recommendation should also take into consideration the especially pronounced risk of infection in mother-neonate pairs [8].
Twenty different emm-types were associated with iGAS disease, with the three most prevalent strains (emm-1, −4, and − 12) accounting for nearly three-quarters of patients (for whom strain data was available). This strain diversity is much less than observed among the general population of Victoria or of Sydney, NSW [49, 50]. A GAS vaccine would need to possess broad strain coverage in order to effectively prevent invasive disease - but should a vaccine incorporate the three most prevalent strains observed in this study, it would potentially prevent nearly three-quarters of disease in paediatric patients.
Under-notification of patients is likely to be a serious issue affecting the completeness and ultimately, the usefulness, of surveillance data, including that used in this study. Reporting to the PAEDS Network is voluntary and is limited to active surveillance at seven sentinel tertiary hospitals, so smaller regional hospitals and other tertiary hospitals will have managed iGAS disease patients without contributing data. Such under-notification will have impacted on the rate estimations and may particularly affect remote areas of Australia, including areas with significant ATSI populations. Consequently, the true extent of ATSI children’s overrepresentation in iGAS disease rates may be higher than was shown by this analysis. In addition, iGAS disease patients with more severe symptoms may require transfer to a (notifying) hospital for specialist care. Thus less severe paediatric iGAS disease patients may have been missed. Furthermore, a requirement of case notification was the identification of GAS from a normally sterile body site. Severe cases excluded from our analyses due to this requirement are likely to be very few in number, however STSS and necrotizing fasciitis can occur without GAS entering a sterile site [3, 51]. The extent to which the true rate is underestimated is unknown and will vary by jurisdiction. For completeness, national notifications should include all iGAS cases regardless of whether a GAS isolate was obtained from a normally sterile site. Other limitations include an absence of information on secondary prophylaxis compliance and incomplete strain data.
Conclusions
Various means of categorising ‘severe’ iGAS diseases have been used in the literature [52, 53], however on the basis of the frequency of iGAS disease identified here and the high disease burden, we advocate for urgent public health action to improve surveillance and optimise prevention activities. There is a clear need for a consensus national recommendation around the use of contact prophylaxis. The lack of mandatory patient notification limits the ability of public health programmes to effectively target, prevent and control this condition. Making iGAS disease notifiable at the national level would help to inform public health and research initiatives aiming to reduce the impact of this condition [49]. The momentum for GAS vaccine development is supported by increasing the awareness of iGAS disease morbidity and mortality, both nationally and internationally.
Availability of data and materials
Due to the potentially identifiable nature of the raw data, these are not publicly available and will not be shared.
Change history
03 May 2021
A Correction to this paper has been published: https://doi.org/10.1186/s12889-021-10798-6
Abbreviations
- ATSI:
-
Aboriginal Australian or Torres Strait Islander
- CHW:
-
Children’s Hospital at Westmead, New South Wales
- CI:
-
Confidence interval
- CRF:
-
Case fatality rate
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- iGAS:
-
Invasive group A Streptococcus
- MCH:
-
Monash Children’s Hospital, Victoria
- NNDSS:
-
Australian National Notifiable Diseases Surveillance System
- PAEDS:
-
Paediatric Active Enhanced Disease Surveillance
- PCH:
-
Perth Children’s Hospital, Western Australia
- QCH:
-
Queensland Children’s Hospital, Queensland
- RCH:
-
Royal Children’s Hospital Melbourne, Victoria
- RDH:
-
Royal Darwin Hospital, Northern Territory
- RR:
-
Risk Ratio
- STSS:
-
Streptococcal toxic shock syndrome
- US:
-
United States of America
- WCH:
-
Women’s and Children’s Hospital, South Australia
- WHO:
-
World Health Organization
References
Royal Children’s Hospital, Melbourne. Clinical Practice Guidelines. 2019; Available from: https://www.rch.org.au/clinicalguide/guideline_index/Invasive_group_A_streptococcal_infections__management_of_household_contacts/
Adalat S, Dawson T, Hackett SJ, Clark JE. In association with the British Paediatric surveillance U: toxic shock syndrome surveillance in UK children. Arch Dis Child. 2014;99(12):1078–82.
Chen KY, Cheung M, Burgner DP, Curtis N. Toxic shock syndrome in Australian children. Arch Dis Child. 2016;101(8):736–40.
Javouhey E, Bolze PA, Jamen C, Lina G, Badiou C, Poyart C, Portefaix A, Tristan A, Laurent F, Bes M, et al. Similarities and differences between staphylococcal and streptococcal toxic shock syndromes in children: results from a 30-case cohort. Front Pediatr. 2018;6:1.
O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, et al. The epidemiology of invasive group a streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis. 2007;45(7):853–62.
Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group a streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–94.
O’Grady KA, Kelpie L, Andrews RM, Curtis N, Nolan TM, Selvaraj G, Passmore JW, Oppedisano F, Carnie JA, Carapetis JR. The epidemiology of invasive group a streptococcal disease in Victoria, Australia. Med J Aust. 2007;186(11):565–9.
Mearkle R, Saavedra-Campos M, Lamagni T, Usdin M, Coelho J, Chalker V, Sriskandan S, Cordery R, Rawlings C, Balasegaram S. Household transmission of invasive group A Streptococcus infections in England: a population-based study, 2009, 2011 to 2013. Euro Surveill. 2017;22:19.
Ontario Ministry of Health. Guidelines for management of contacts of cases of invasive group A streptococcal disease (GAS) including streptococcal toxic shock syndrome (STSS) and necrotizing fasciitis. Toronto; 1995. Available from: http://microbiology.mtsinai.on.ca/protocols/pdf/k5c.pdf
Queensland Health. Invasive group a streptococcal disease Queensland Health guidelines for public Health units. Brisbane; 2017. Available from: https://www.health.qld.gov.au/cdcg/index/igas
New South Wales Government. Invasive group a Streptococcus control guideline. Sydney; 2016. Available from: http://www.health.nsw.gov.au/Infectious/controlguideline/Pages/invasive-group-a-strep.aspx
Centre for Disease Control, Northern Territory of Australia. Public health management of invasive group A streptococcal infection. Darwin; 2015. Available from: https://digitallibrary.health.nt.gov.au/prodjspui/bitstream/10137/1187/1/iGAS%20guidelines%20Nov%202015.pdf
The Working Group on Prevention of Invasive Group A Streptococcal Infections. Prevention of invasive group a streptococcal disease among household contacts of case-patients: is prophylaxis warranted? JAMA. 1998;279(15):1206–10.
Interim UK guidelines for management of close community contacts of invasive group a streptococcal disease. Commun Dis Public Health. 2004;7(4):354–61.
Public Health Agency of Canada. Canada Communicable Disease Report. Guidelines for the Prevention and Control of Invasive Group A Streptococcal Disease. Ottawa: CCDR; 2006.
Public Health England. Group A streptococcal infections: third report on seasonal activity in England, 2017/18. Health Protect Rep. 2018;12(13):5–7.
Centres for Disease Control and Prevention. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Group A Streptococcus. Atlanta, US. Available from: https://www.cdc.gov/abcs/reports-findings/surv-reports.html
Stevens DL. Invasive group a streptococcal infections. Infect Dis Clin Pract (Baltim MD). 2002;11(1):16–22.
British Coloumbia Center for Disease Control. Invasive Group A Streptococcal Disease (iGAS) in British Columbia 2017 Annual Summary. 2017; Available from: http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Immunization/Coverage/BC%20iGas%202017%20Epi%20Summary.pdf
Tyrrell GJ, Fathima S, Kakulphimp J, Bell C. Increasing rates of invasive group a streptococcal disease in Alberta, Canada; 2003-2017. Open Forum Infect Dis. 2018;5(8):177.
Public Health England. Group A streptococcal infections: second report on seasonal activity in England, 2017/18. Health Protect Rep. 2018;12(8):1–8.
Bundle N, Bubba L, Coelho J, Kwiatkowska R, Cloke R, King S, Rajan-Iyer J, Courtney-Pillinger M, Beck CR, Hope V, et al. Ongoing outbreak of invasive and non-invasive disease due to group a Streptococcus (GAS) type emm66 among homeless and people who inject drugs in England and Wales, January to December 2016. Euro Surveill. 2017;22:3.
Health Canada. First nations Health status report: Alberta region 2009–2010. In: First Nations and Inuit Health-Alberta Region. Edmonton, Canada; 2011.
Bocking N, Matsumoto CL, Loewen K, Teatero S, Marchand-Austin A, Gordon J, Fittipaldi N, McGeer A. High Incidence of Invasive Group A Streptococcal Infections in Remote Indigenous Communities in Northwestern Ontario, Canada. Open Forum Infect Dis. 2017;4(1):ofw243.
Boyd R, Patel M, Currie BJ, Holt DC, Harris T, Krause V. High burden of invasive group a streptococcal disease in the Northern Territory of Australia. Epidemiol Infect. 2016;144(5):1018–27.
Whitehead BD, Smith HV, Nourse C. Invasive group a streptococcal disease in children in Queensland. Epidemiol Infect. 2011;139(4):623–8.
Safar A, Lennon D, Stewart J, Trenholme A, Drinkovic D, Peat B, Taylor S, Read K, Roberts S, Voss L. Invasive group a streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis. 2011;17(6):983–9.
Harris P, Siew DA, Proud M, Buettner P, Norton R. Bacteraemia caused by beta-haemolytic streptococci in North Queensland: changing trends over a 14-year period. Clin Microbiol Infect. 2011;17(8):1216–22.
Rudolph K, Bruce MG, Bruden D, Zulz T, Reasonover A, Hurlburt D, Hennessy T. Epidemiology of invasive group a streptococcal disease in Alaska, 2001 to 2013. J Clin Microbiol. 2016;54(1):134–41.
Ching NS, Crawford N, McMinn A, Baker C, Azzopardi K, Brownlee K, Lee D, Gibson M, Smeesters P, Gonis G, et al. Prospective surveillance of pediatric invasive group a Streptococcus infection. J Pediatric Infect Dis Soc. 2017;8(1):46.
Wong NX, Crawford N, Oliver J, McMinn A, Ching N, Baker C, Daley A, Steer AC. A cluster of paediatric invasive group a streptococcus disease in Melbourne, Australia coinciding with a high burden influenza season. J Pediatr Infect Dis. 2018. https://doi.org/10.1055/s-0038-1677456.
Centre for Disease Control. Northern Territory of Australia: Northern Territory Notifications of Diseases by Onset Date & Districts. Northern Territory Dis Control Bull. 2019;26(1):27.
Queendland Health. Vaccine preventable and invasive diseases in Queensland. 1 Jan - 31 Dec 2018. 2019; Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0024/832443/vpd-quarterly-surveillance-2018.pdf
The Australian Paediatric Surveillance Unit. Paediatric Active Enhaned Disease Surveillance. 2019; Available from: http://www.paeds.edu.au/surveillance-and-research/.
Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group a streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9(10):611–6.
Steer AC, Dale JB, Carapetis JR. Progress toward a global group a streptococcal vaccine. Pediatr Infect Dis J. 2013;32(2):180–2.
Moreland NJ, Waddington CS, Williamson DA, Sriskandan S, Smeesters PR, Proft T, Steer AC, et al. Working towards a group a streptococcal vaccine: report of a collaborative trans-Tasman workshop. Vaccine. 2014;32(30):3713.
Centers for Disease Control and Prevention. Protocol for emm typing. 2018; Atlanta, US. Available from: https://www.cdc.gov/streplab/groupa-strep/emm-typing-protocol.html
Australian Bureau of Statistics. 3101.0 - Australian demographic statistics, Jun 2018. 2018; Canberra, Australia. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202018?OpenDocument
Herrera AL, Huber VC, Chaussee MS. The Association between Invasive Group A Streptococcal Diseases and Viral Respiratory Tract Infections. Front Microbiol. 2016;7:342.
Zakikhany K, Degail MA, Lamagni T, Waight P, Guy R, Zhao H, Efstratiou A, Pebody R, George R, Ramsay M. Increase in invasive Streptococcus pyogenes and Streptococcus pneumoniae infections in England, December 2010 to January 2011. Euro Surveillance. 2011;16(5):03.
Darenberg J, Henriques-Normark B, Lepp T, Tegmark-Wisell K, Tegnell A, Widgren K. Increased incidence of invasive group a streptococcal infections in Sweden, January 2012-February 2013. Euro Surveill. 2013;18(14):20443.
Tasher D, Stein M, Simoes EA, Shohat T, Bromberg M, Somekh E. Invasive bacterial infections in relation to influenza outbreaks, 2006-2010. Clin Infect Dis. 2011;53(12):1199–207.
Lee SE, Eick A, Bloom MS, Brundage JF. Influenza immunization and subsequent diagnoses of group a streptococcus-illnesses among US Army trainees, 2002-2006. Vaccine. 2008;26(27–28):3383–6.
Okamoto S, Nagase S. Pathogenic mechanisms of invasive group a Streptococcus infections by influenza virus–group a Streptococcus superinfection. Microbiol Immunol. 2018;62(3):141–9.
Australian Government Department of Health. Meningococcal Disease (Invasive) Canberra, Australia. 2019; Available from: https://www.health.gov.au/internet/main/publishing.nsf/Content/ohp-meningococcal-W.htm
Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2 Suppl):S3–S11.
De Wals P, Hertoghe L, Borlee-Grimee I, De Maeyer-Cleempoel S, Reginster-Haneuse G, Dachy A, Bouckaert A, Lechat MF. Meningococcal disease in Belgium. Secondary attack rate among household, day-care nursery and pre-elementary school contacts. J Inf Secur. 1981;3(1 Suppl):53–61.
Oliver J, Wilmot M, Strachan J, St George S, Lane CR, Ballard SA, Sait M, Gibney K, Howden BP, Williamson DA. Recent trends in invasive group A Streptococcus disease in Victoria. Commun Dis Intell. 2019;2018:43.
Sivagnanam S, Zhou F, Lee AS, O'Sullivan MV. Epidemiology of invasive group a Streptococcus infections in Sydney, Australia. Pathology. 2015;47(4):365–71.
Carapetis JR, Jacoby P, Carville K, Ang SJ, Curtis N, Andrews R. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis. 2014;59(3):358–65.
Canadian Pediatric Society Statement. Invasive group a streptococcal infections. Paediatr Child Health. 1999;4(1):73–6.
Steer JA, Lamagni T, Healy B, Morgan M, Dryden M, Rao B, Sriskandan S, George R, Efstratiou A, Baker F, et al. Guidelines for prevention and control of group a streptococcal infection in acute healthcare and maternity settings in the UK. J Inf Secur. 2012;64(1):1–18.
Acknowledgements
We thank all the children and families included in this study, as well as the laboratory staff at the participating hospitals.
PAEDS is supported by the Australian Government Department of Health and Departments of Health in participating jurisdictions (Victoria, New South Wales, South Australia, Western Australia, Northern Territory and Queensland).
PAEDS Network members past and present include: Booy, R, Connell J, Dale R, Deverell M, Dinsmore N, Dougherty S, Finucane C, Gibson M, Gold M, Heath C, Hickie L, Hutchinson T, Jones C, Jones J, Kent J, Knight H, Kynaston A, Lee D, Lewis G, Low S, Maclean N, Macartney K, McDonald F, McLaren N, McRae J, Murphy J, Meredith K, Nissen M, Orr C, Orr K, Phillips N, Pym M, Quinn J, Richmond P, Rhind L, Roberts A, Robins C, Rost L, Royle J, Saravanos G, Snelling T, Talbott C, Tan S, Trinh L, Vidler L, Walker M, West R, Wharton C, Wood N, Zurynski Y.
Funding
This work was supported by a grant from the Shepherd Foundation (charitable trust). H Marshall acknowledges support from the National Health and Medical Research Council of Australia practitioner fellowship APP1155066. P Britton acknowledges support from National Health and Medical Research Council of Australia Early Career Fellowship. C Blyth acknowledges support from the National Health and Medical Research Council of Australia Career Development Fellowship. No funding body had any role in the design of this study, or the collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
All authors have read and approved the manuscript. JO drafted and revised the manuscript, performed the analyses and cleaned the data. ET cleaned the data, advised on data interpretation, and critically reviewed the manuscript. CB collected data, advised on data interpretation and critically reviewed the manuscript. AM provided data, advised on data interpretation and critically reviewed the manuscript. PB advised on data collection, interpretation and analysis, and critically reviewed the manuscript. JC advised on data collection, interpretation and analysis, and critically reviewed the manuscript. HS advised on data collection, interpretation and analysis, and critically reviewed the manuscript. CB advised on data collection, interpretation and analysis, and critically reviewed the manuscript. JF advised on data collection, interpretation and analysis, and critically reviewed the manuscript. JB advised on data collection, interpretation and analysis, and critically reviewed the manuscript. AS and NC conceived of the study aims, advised on data collection, interpretation and analysis, and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Royal Children’s Hospital Human Research Ethics Committee, HREC #36339 and extended to all jurisdictions through our PAEDS program of research.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised as it contained errors in the values in the second paragraph of page 4.
Supplementary information
Additional file 1: Table S1.
Recruitment method by hospital, notified iGAS patients (<18 years), Australia, July 2016–June 2018. Table S2. emm-types (N = 96) identified among notified iGAS disease patients (<18 years), Australia, July 2016–June 2018.
Additional file 2: Figure S1.
Prospective surveillance implementation periods across the seven notifying PAEDS Network sites1. 1Sites are: RDH: Royal Darwin Hospital, Northern Territory; PCH: Perth Children’s Hospital, Western Australia; WCH: Women’s and Children’s Hospital, South Australia; CHW: Children’s Hospital at Westmead, New South Wales; QCH: Queensland Children’s Hospital, Queensland; RCH: Royal Children’s Hospital Melbourne, Victoria; MCH: Monash Children’s Hospital, Victoria).
Additional file 3: Figure S2.
Location of the seven notifying PAEDS Network sites and major cities, Australia.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oliver, J., Thielemans, E., McMinn, A. et al. Invasive group A Streptococcus disease in Australian children: 2016 to 2018 – a descriptive cohort study. BMC Public Health 19, 1750 (2019). https://doi.org/10.1186/s12889-019-8085-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-019-8085-2