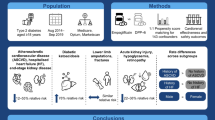
Abstract
Background
The association between antihypertensive medication and schizophrenia has received increasing attention; however, evidence of the impact of antihypertensive medication on subsequent schizophrenia based on large-scale observational studies is limited. We aimed to compare the schizophrenia risk in large claims-based US and Korea cohort of patients with hypertension using angiotensin-converting enzyme (ACE) inhibitors versus those using angiotensin receptor blockers (ARBs) or thiazide diuretics.
Methods
Adults aged 18 years who were newly diagnosed with hypertension and received ACE inhibitors, ARBs, or thiazide diuretics as first-line antihypertensive medications were included. The study population was sub-grouped based on age (> 45 years). The comparison groups were matched using a large-scale propensity score (PS)-matching algorithm. The primary endpoint was incidence of schizophrenia.
Results
5,907,522; 2,923,423; and 1,971,549 patients used ACE inhibitors, ARBs, and thiazide diuretics, respectively. After PS matching, the risk of schizophrenia was not significantly different among the groups (ACE inhibitor vs. ARB: summary hazard ratio [HR] 1.15 [95% confidence interval, CI, 0.99–1.33]; ACE inhibitor vs. thiazide diuretics: summary HR 0.91 [95% CI, 0.78–1.07]). In the older subgroup, there was no significant difference between ACE inhibitors and thiazide diuretics (summary HR, 0.91 [95% CI, 0.71–1.16]). The risk for schizophrenia was significantly higher in the ACE inhibitor group than in the ARB group (summary HR, 1.23 [95% CI, 1.05–1.43]).
Conclusions
The risk of schizophrenia was not significantly different between the ACE inhibitor vs. ARB and ACE inhibitor vs. thiazide diuretic groups. Further investigations are needed to determine the risk of schizophrenia associated with antihypertensive drugs, especially in people aged > 45 years.
Similar content being viewed by others
Background
Schizophrenia is a mental disorder affecting approximately 1% of the world’s population and is a severe disorder that leads to functional deterioration [1]. Despite cardinal features of schizophrenia, it remains the least understood psychiatric disorder owing to the lack of pathological hallmarks [2, 3]. With the identification of schizophrenia susceptibility genes [4], genetic traits have been considered to play important roles in schizophrenia occurrence [5]. The relative contribution of genetic factors in schizophrenia is estimated to be up to 80% [6].
Recently, the target genes of antihypertensive medications were reported to be associated with the risk of schizophrenia. Specifically, low angiotensin-converting enzyme (ACE) messenger RNA and protein levels, which are targets of ACE inhibitors, are associated with an increased risk of schizophrenia [7]. In addition, according to Fan et al., genetically proxied ACE inhibitors were reported to be associated with an increased risk of SCZ in Europeans and East Asians [8]. Contrary to ACE inhibitors, other antihypertensive medications such as BB and CCB were found to have no association. Animal experiments demonstrated that the brain RAS targeted by ACE inhibitors can regulate various brain functions such as sensory information processing, learning, memory, and emotional responses [9]. However, it remains unclear whether this potential biological association translates into clinically significant difference of the schizophrenia occurrence in real-world scenarios. Given the widespread use of ACE inhibitors in hypertensive patients and their potential biological implications for schizophrenia risk, investigating this association using real-world data is essential.
Therefore, comparing the effects of antihypertensive drugs on schizophrenia may be a way to identify potential risk factors for schizophrenia occurrence. We aimed to conduct a head-to-head study comparing the occurrence of schizophrenia between antihypertensive drugs in patients with hypertension. Specifically, we investigated whether the use of ACE inhibitors increased the risk of schizophrenia compared with the use of angiotensin receptor blockers (ARBs) or thiazide diuretics in the US and Korea across the Observational Health Data Sciences and Informatics (OHDSI) network [10].
Methods
Data source
We performed a population-based, retrospective cohort study using two claims databases in the US and South Korea: US Open Claims and Health Insurance Review and Assessment Service National Claims (HIRA) (see eMethod 1 in Supplement 1 for database details). These databases were standardized using the Observational Medical Outcomes Partnership Common Data Model, version 5.3 [11].
Each data partner executes the package locally inside the firewall. The pre-designated statistical results (without patient-level information) were shared for interpretation and database-level meta-analyses. All partners received Institutional Review Board approval or exemption (IRB number: AJIRB-MED-MDB-21-274).
Study design
Active-comparator new-user designs were applied in our study to mitigate the methodological limitations of observational studies [12]. For new-user design, we identified patients who had newly initiated antihypertensive medications. For the active-comparator design, ACE inhibitors were compared with ARBs and thiazide diuretics (thiazide or thiazide-like diuretics), which are commonly used for the same indication and reported to be unrelated to the occurrence of schizophrenia [13]. We compared the incidence of outcomes between the two groups (ACE inhibitor vs. ARB and ACE inhibitor vs. thiazide diuretics).
We conducted distributed network analyses similar to previous studies [14, 15]. The statistical analytical protocol (see Supplement 2) was pre-specified before execution. According to this protocol, the study package for the entire process was built using the OHDSI Health Analytics Data-to-Evidence Suite in R; detailed study codes are available online at https://github.com/ohdsi-studies/Ceeamos. The study protocol was registered with the EU Post-Authorization Studies register under EUPAS42783.
Study population and exposure
We identified adult (aged ≥18 years) patients who were exposed to antihypertensive drugs (ACE inhibitors, ARBs, or thiazide diuretics) for the first time according to their medical history. Combination products of each ingredient were not included in this study. The index date was defined as the date of the first exposure to antihypertensive drugs. To avoid left censoring (i.e., incomplete data on patients who were already on antihypertensive treatment before entering the study), we excluded patients who were enrolled in the database for < 1 year before the index date. We excluded patients without a diagnosis 1 year before the index date. The other exclusion criteria were as follows: (1) a history of exposure to any hypertension treatment (prevalent user), (2) schizophrenia diagnosis and heart failure diagnosis at any time before the index date, (3) prescription of other blood pressure lowering medications (non-thiazide diuretics, beta blockers, and calcium channel blockers) and (4) prescription of the opposite drug (ARBs or thiazide diuretics for the ACE inhibitor group and vice versa) during the 7 days after the index date for ascertaining first-line treatment. Further details on cohort definitions are presented in Supplement 2.
Outcomes and follow-up
The primary outcome was a diagnosis of schizophrenia for the first time. To increase the specificity of the diagnosis, we applied a restricted definition of outcome, which included at least one diagnosis of schizophrenia, at least two prescriptions of antipsychotics, or at least two psychiatric procedures (electroconvulsive therapy and psychotherapy) at any time after the first diagnosis of schizophrenia. The secondary outcome was a specific definition of schizophrenia at the emergency department visit. Further details of the outcome definitions are provided in Supplement 2.
Our analysis considered the time-to-first event and was followed up to the earliest date among last date of assigned treatment, date of last observation in the database, date of occurrence of the endpoint, and date of censoring (as-treated [AT] approach). Each treatment was considered to be continued if the patient received a new prescription for the same treatment within 30 days of the last date of the previous prescription. Treatment discontinuation was defined as the last prescription with no further prescription within 30 days. Censoring events were defined as events in which patients were no longer under the observation due to another antihypertensive medications. (i.e., patients in the ACE inhibitor group were considered censored if they were exposed to ARBs or thiazide diuretics).
Statistical analysis
A large-scale propensity score (PS) adjustment [16] was performed using L1 penalized logistic regression, which used > 10 000 baseline patient characteristics between each of the two cohorts, including all available demographic characteristics, as well as the diagnosis, medication, and procedure history in each database. All variables were dichotomized, and missing variables were considered absent. The study populations were matched using variable-ratio PS matching with a maximum ratio of 10 (caliper = 0.2). Differences between the two matched cohorts were considered negligible when the absolute standardized mean differences (aSMDs) of all covariates were < 0.1 [17]. The incidence rates (IRs) per 1000 person-years (PY) were estimated. Cox proportional hazard models were used to estimate the association between exposure and outcomes. Next, we performed empirical calibration of all hazard ratio (HR) estimates, their 95% confidence intervals (CIs), and their 2-sided P values by fitting an empirical null distribution to point estimates of falsification end points [18]. We identified a total of 26 falsification endpoints to quantify systematic error (eTable 1 in Supplement 1) [19, 20]. These outcomes are not known to cause differences between antihypertensive drugs, such as ingrowing nails and fractures of the upper limb. Using calibrated estimates, we performed a random-effects meta-analysis to calculate the summary HR and 95% CI of the pooling effect estimates across the databases. The Kaplan–Meier method and log-rank tests were used to derive the cumulative incidence and comparative risk between-group differences. Statistical significance was set at a pre-specified two-sided P value < 0.05. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Sensitivity analyses
Multiple sensitivity analyses were conducted using different definitions of the study population, outcomes, and follow-up strategies. To examine the association with late-onset schizophrenia as previously described [21], we sub-grouped the study population according to age over 45 years. We also varied our follow-up strategy to intention-to-treat (ITT) to estimate the effect of being assigned to a given treatment regardless of non-adherence. Overall, 16 different analyses (two cohort definitions (by age) × two outcome definitions × two follow-up strategies × two comparison pairs) were performed.
Results
Cohort characteristics
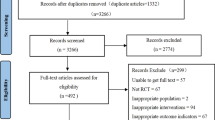
In total, 5,907,522; 2,923,423; and 1,971,549 patients across the two databases for the three study populations (ACE inhibitor, ARB, and thiazide groups, respectively) were included in the analysis (Fig. 1). The number of matched patients in the ACE inhibitor versus ARB comparison was 21,410 and 577,637 pairs from the HIRA and 2,130,393 and 2,151,531 pairs from the US Open Claims database, respectively. In the ACE inhibitor versus thiazide diuretic comparison, there were 9,852 and 70,491 pairs from the HIRA and 1,777,108 and 1,864,047 pairs from the US Open Claims database, respectively. The baseline characteristics of the study populations before and after PS matching for the three target-comparator combinations are presented in eTable 2 (Supplement 1) and Table 1. After PS matching, the aSMD for all baseline patient characteristics between the two drug users was < 0.1 within each data source (eFigure 1 in Supplement 1).
Primary outcome assessment
The cumulative incidence curves for schizophrenia for all comparisons are shown in Fig. 2. Figure 3 shows the results of the meta-analyses, including the calibrated HR and 95% CI. The detailed numbers of events, PY, and IRs are presented in Table 2. In the US and South Korea, the comparison between the ACE inhibitor and ARB groups for the risk of schizophrenia occurrence was not significant (US: IR 0.43/1 000 PY, 0.37/1 000 PY, calibrated HR 1.14 [95% CI, 0.98–1.32]; Korea: IR 0.44/1 000 PY, 0.22/1 000 PY, HR 1.47 [95% CI, 0.70–3.10]) (Fig. 3and Table 2). Overall, the meta-analysis result showed no significant difference in schizophrenia occurrence between the ACE inhibitor and ARB groups (IR 0.43/1 000 PY, 0.33/1 000 PY, summary HR 1.15 [95% CI, 0.99–1.33], P =.06) (Fig. 3).
The forest plots for the risk of schizophrenia. Forest plots showing the calibrated HRs and 95% CIs for the occurrence of schizophrenia for each dataset. Summary HRs were calculated using a random-effects model. An HR of > 1 indicated a higher risk in the ACE inhibitor group. The size of the data marker indicates the weight of the study. Error bars represent 95% CIs. HR, hazard ratio; CI, confidence intervals; ACE, angiotensin-converting enzyme
In the ACE inhibitor group, compared with the thiazide group, the risk was not significantly different between the US and South Korea (US: IR 0.54/1 000 PY, 0.65/1 000 PY, calibrated HR 0.91 [95% CI, 0.78–1.07]; Korea: IR 0.57/1 000 PY, 0.53/1 000 PY, calibrated HR 0.88 [95% CI, 0.22–3.46]) (Fig. 3and Table 2). Additionally, the meta-analysis result showed no significant difference in schizophrenia occurrence between the ACE inhibitor and thiazide groups (IR 0.54/1 000 PY, 0.64/1 000 PY, summary HR 0.91 [95% CI, 0.78–1.07], P =.26) (Fig. 3).
Secondary outcome assessments
The meta-analysis of secondary outcomes is shown in eFigure 3 (Supplement 1). There was no significant difference in schizophrenia occurrence between the ACE inhibitor and ARB groups (summary HR 1.19 [95% CI, 0.59–2.39], P =.62). Similarly, no significant difference in schizophrenia occurrence was observed between the ACE inhibitor and thiazide groups (summary HR 0.87 [95% CI, 0.54–1.42]; P =.59).
Falsification endpoint analyses and sensitivity analyses
In the analyses of falsification endpoints between the ACE inhibitor and ARB groups, 95.5% (21/22) in South Korea and 80.8% (21/26) in the United States had 95% CIs that covered 1.0 of the HR, suggesting that the level of systematic error was modest (eFigure 2 in Supplement 1). In another comparison between the ACE inhibitor and thiazide groups, the level of systematic error after calibration was also modest (South Korea: 77.8% [14/18]; United States: 73.1% [19/26] of the nominal 95% CIs covered 1.0) (eFigure 2 in Supplement 1).
In the subgroup analyses regarding age over 45 years to identify the relationship with late-onset schizophrenia, there was no significant difference in the schizophrenia occurrence between the ACE inhibitor and thiazide groups (summary HR 0.91 [95% CI, 0.71–1.16]; P =.44). However, the ACE inhibitor group showed a higher risk of schizophrenia occurrence than the ARB group (summary HR 1.23 [95% CI, 1.05–1.43]; P =.01, see eFigure 4 in Supplement 1). For the secondary outcome in the subgroups, there was no significant difference in the occurrence of schizophrenia in all comparisons between the target and comparator groups (eFigure 5 in Supplement 1).
The results of the additional follow-up strategy are presented in eTable 3 and eFigures 6–9 in Supplement 1. For the primary outcome at ITT follow-up, the ACE inhibitor group showed a lower risk of schizophrenia occurrence than the thiazide group (summary HR 0.92 [95% CI, 0.86–0.99]; P =.02). However, no significant difference was observed in schizophrenia occurrence in the meta-analysis for other comparisons of the target and comparator groups, including the total population and subgroups. In the US results, the ACE inhibitor group showed a higher risk of schizophrenia occurrence than the ARB group, including the total population and the subgroup (total population: calibrated HR 1.21 [95% CI, 1.13–1.29]; subgroup: calibrated HR 1.18 [95% CI, 1.09–1.27]). The ACE inhibitor group showed a lower risk of schizophrenia occurrence than the thiazide group in only the total population (total population: calibrated HR 0.91 [95% CI, 0.85–0.98]; subgroup: calibrated HR 0.96 [95% CI, 0.88–1.04]). Conversely, the results from South Korea showed no significant difference in the occurrence of schizophrenia for all comparisons between the target and comparator. eFigure 6 and eFigure 7 in Supplement 1). For the secondary outcome at ITT follow-up, the ACE inhibitor group exhibited a higher risk of schizophrenia occurrence than the ARB group (summary HR 1.27 [95% CI, 1.07–1.51]; P =.006). However, there was no significant difference in the occurrence of schizophrenia in the meta-analysis for other comparisons of the target and comparator groups, including the total population and subgroups. In the US results, the ACE inhibitor group showed a higher risk of schizophrenia occurrence than the ARB group, including the total population and the subgroup (total population: calibrated HR 1.29 [95% CI, 1.08–1.54]; subgroup: calibrated HR 1.24 [95% CI, 1.01–1.52]). However, the ACE inhibitor group showed a lower risk of schizophrenia occurrence than the thiazide group in only the total population (total population: calibrated HR 0.86 [95% CI, 0.75–0.99]; subgroup: calibrated HR 0.88 [95% CI, 0.73–1.06]). Contrary to the results from the US, those from South Korea showed no significant difference in the occurrence of schizophrenia for all comparisons between the target and comparator. (eFigure 8 and eFigure 9 in Supplement 1).
Discussion
The potential association between ACE inhibitors and an increased risk of schizophrenia is highly relevant because of the number of affected patients and the burden of schizophrenia, warranting thorough investigation. In this study, we extensively estimated the comparative risks of ACE inhibitors and thiazide diuretics or ARBs on the occurrence of schizophrenia. No differences in risk were found between the use of ACE inhibitors versus ARB or between the use of ACE inhibitors versus thiazide diuretics. Although the use of ACE inhibitors was associated with an increased risk of schizophrenia compared with the use of ARB in the group aged > 45 years, the results were not consistent in the sensitivity analyses. Regarding the secondary outcome, no difference in risk was found among the antihypertensive drugs.
Schizophrenia imposes significant health, social, and economic burdens on individuals, families, caregivers, and society at large [22]. Unfortunately, by the time schizophrenia becomes apparent behaviorally, neural damages may already be irreversible [23]. Owing to the limited effectiveness of treatments, identifying psychosis risk factors for prevention and early detection has become crucial [24]. Additionally, regarding antihypertensive medications, 31.1% of adults worldwide are affected by hypertension [25]. ACE inhibitors are the most commonly used antihypertensive medications in the US. Previous studies on the relationship between ACE inhibitors and schizophrenia have limitations in terms of sample size or cross-sectional design [26, 27]. Therefore, we conducted this well-designed longitudinal cohort study. First, we selected the fit-for-purpose databases for the two countries. The large claims database has less fragmentation than individual electronic medical records, allowing us to conduct longitudinal cohort studies for identifying genetic relationships in schizophrenia [28]. Given the different prevalence of schizophrenia between countries [29], we analyzed more than 2 million patients in the United States and South Korea. In particular, the HIRA database contains nationwide claims data for the entire Korean population; therefore, our results are sufficiently representative. Second, many robust designs and methods were applied to infer associations between study groups. Controlling biases is critical in observational studies using routinely collected observational databases [30]. Using an active-comparator new-user design, large-scale PS methods can resolve biases arising from time-related design and comparability [31, 32]. An assessment of systematic errors using falsification endpoints also provides a more reliable statistical interpretation and minimizes the effect of residual bias [18].
Additionally, in the subgroup of individuals aged ≥ 45 years, a significant difference was observed between ACE inhibitors and ARBs. The possible biological pathway for the association between ACE inhibitors and schizophrenia is that ACE and the central RAS may play a role in inflammation and immunity [33]. Immune dysfunction due to reduced ACE activity may contribute to the development of schizophrenia [34]. Especially, according to studies on the pharmacokinetics of ACE inhibitors, the Area Under the Plasma Concentration-Time Curve has been reported to be greater in older individuals compared to younger ones, attributed to renal function decline and changes in body composition [35]. These findings suggest that the impact of ACE inhibitors is more pronounced in older individuals, as in our results. As another possible explanation, a previous study suggested the therapeutic potential of ARBs in patients with schizophrenia through the anti-inflammatory properties of gamma-aminobutyric acid [36], while thiazide diuretics had no effect on schizophrenia [13]. This could explain why ARBs are associated with a lower risk than ACE inhibitors. Nonetheless, the results were not significant in the ITT setting and require further study, making it difficult to draw definitive conclusions.
Moreover, differences in the risk of schizophrenia based on antihypertensive medication existed in the US data at the ITT follow-up. Although the differences between ACE inhibitors and thiazide diuretics were inconsistent, ACE inhibitors were consistently associated with a higher risk of schizophrenia than ARBs in both subgroup analyses and secondary outcomes. This result appears to be consistent with the results of the subgroup analysis at the AT follow-up. However, ITT can overestimate the effects of treatment in the presence of differential adherence [37].
Hypertensive patients are more likely to be diagnosed with mental disorders, and hypertension increases the severity of psychological distress. On the contrary, mental disorders are independent risk factors for hypertension. In other words, there is a clinically significant bidirectional relationship between hypertension and mental disorders [38,39,40]. In these situations, it is important to clarify how and to what extent antihypertensives affect schizophrenia from a clinical perspective. Given our findings of no significant differences by antihypertensive medication, there is insufficient evidence to recommend clinically that antihypertensive medications be reduced or discontinued. From the patient’s perspective, information about hypertension medications associated with schizophrenia risk could impact treatment adherence for people with hypertension, given what has happened to them during COVID-19 [41]. Considering our results, it does not appear that people with hypertension need to consider whether to use or change their antihypertensive medication because of the risk of schizophrenia.
This study had some limitations. First, there may be unmeasured risk factors for schizophrenia. For example, the balance for hypertension status (including blood pressure values) between the two groups could not be determined due to the nature of the claims data. A family history of schizophrenia and social history, such as immigration, are related to the development of schizophrenia [42, 43]. In addition, economic variables (such as income status) may also be associated with the development of schizophrenia, but were not used in this study. However, we used large-scale PS methods that can help reduce the impact of measured confounders and balance the distribution of these variables between groups [44]. Nevertheless, given the uncontrolled confounding by the propensity score method, further studies including social and family factors are needed. Second, the number of patients varies across the databases. Although data from 50 million people in Korea were used, only approximately 22,927 patients used ACE inhibitors, while data from the United States exceeded 5 million patients using ACE inhibitors. Such discrepancies in sample sizes could potentially impact the generalizability of our findings [45]. However, it is important to note that this heterogeneity in clinical practice can also be seen as a strength of our study. By utilizing data with diverse prescribing patterns, we can generate more reliable and generalizable evidence that better reflects real-world clinical scenarios. Moreover, the use of multinational databases and studies with multiple databases of varying sizes have previously demonstrated feasible and consistent results [46]. Third, the diagnostic system for schizophrenia has limitations. Prior reviews have shown variability in schizophrenia diagnosis [47], which may be due to the complexity and heterogeneity of schizophrenia [48]. These diagnostic problems appear not only in schizophrenia but also in other psychiatric diseases such as depression and bipolar disorder [49]. For the strictness of diagnosis, we added prescriptions of antipsychotics and occurrences of psychiatry procedures. Lastly, more comprehensive analyses are still needed to generalize our findings. This study only included RAS inhibitors and thiazide diuretics among the main antihypertensive drugs, and additional analyses such as calcium channel blockers could be considered. It also excluded patients on two or more medications, which are prescribed to more than half of all patients with hypertension [50], and further research is needed on patients on such combination therapies.
Conclusions
In conclusion, there was no explicit difference in the risk of schizophrenia between ACE inhibitors, ARBs, and thiazide diuretics across the two large databases in the US and South Korea. These results are not sufficient to justify a change in current prescribing guidelines in hypertensive patients because of the risk of schizophrenia. Considering the unmeasured confounders, further investigations are needed to clarify the association between schizophrenia and antihypertensive drugs.
Data availability
Data are available from the corresponding authors upon reasonable request and with permission of HIRA and IQVIA.
References
Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, et al. Schizophrenia: manifestations, incidence and course in different cultures a World Health Organization ten-country study. Psychol Med Monogr Supplement. 1992;20:1–97.
McGlashan T. Schizophrenia in translation: is active psychosis neurotoxic? Schizophr Bull. 2006;32(4):609–13.
Yin D-M, Chen Y-J, Sathyamurthy A, Xiong W-C, Mei L. Synaptic dysfunction in schizophrenia. Synaptic Plast. 2012:493–516.
Ikeda M, Takahashi A, Kamatani Y, Momozawa Y, Saito T, Kondo K, et al. Genome-wide association study detected novel susceptibility genes for schizophrenia and shared trans-populations/diseases genetic effect. Schizophr Bull. 2019;45(4):824–34.
Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18(12):727–40.
Giegling I, Hosak L, Mössner R, Serretti A, Bellivier F, Claes S, et al. Genetics of schizophrenia: a consensus paper of the WFSBP Task Force on Genetics. World J Biol Psychiatry. 2017;18(7):492–505.
Chauquet S, Zhu Z, O’Donovan MC, Walters JT, Wray NR, Shah S. Association of Antihypertensive Drug Target genes with Psychiatric disorders: a mendelian randomization study. JAMA Psychiatry. 2021;78(6):623–31.
Fan B, Zhao JV. Genetic proxies for antihypertensive drugs and mental disorders: mendelian randomization study in European and east Asian populations. BMC Med. 2024;22(1):6.
Carnovale C, Perrotta C, Baldelli S, Cattaneo D, Montrasio C, Barbieri SS, et al. Antihypertensive drugs and brain function: mechanisms underlying therapeutically beneficial and harmful neuropsychiatric effects. Cardiovascular Res. 2023;119(3):647–67.
Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Studies in health technology and informatics. 2015;216:574.
Overhage JM, Ryan PB, Reich CG, Hartzema AG, Stang PE. Validation of a common data model for active safety surveillance research. J Am Med Inform Assoc. 2012;19(1):54–60.
Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–41.
Lintunen J, Lähteenvuo M, Tiihonen J, Tanskanen A, Taipale H. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NPJ Schizophrenia. 2021;7(1):1–7.
Bate A, Chuang-Stein C, Roddam A, Jones B. Lessons from meta‐analyses of randomized clinical trials for analysis of distributed networks of observational databases. Pharm Stat. 2019;18(1):65–77.
You SC, Rho Y, Bikdeli B, Kim J, Siapos A, Weaver J, et al. Association of Ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. 2020;324(16):1640–50.
Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol. 2018;47(6):2005–14.
Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46(3):399–424.
Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med. 2014;33(2):209–18.
Lipsitch M, Tchetgen ET, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiol (Cambridge Mass). 2010;21(3):383.
Voss EA, Boyce RD, Ryan PB, van der Lei J, Rijnbeek PR, Schuemie MJ. Accuracy of an automated knowledge base for identifying drug adverse reactions. J Biomed Inform. 2017;66:72–81.
Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R. Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am J Psychiatry. 1995;152(5):722–30.
Chong HY, Teoh SL, Wu DB-C, Kotirum S, Chiou C-F, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatric disease and treatment. 2016:357–73.
Moghaddam B. A mechanistic approach to preventing schizophrenia in at-risk individuals. Neuron. 2013;78(1):1–3.
Alvarez-Jimenez M, Priede A, Hetrick S, Bendall S, Killackey E, Parker A, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1–3):116–28.
Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37.
Mohite S, de Campos-Carli SM, Rocha NP, Sharma S, Miranda AS, Barbosa IG, et al. Lower circulating levels of angiotensin-converting enzyme (ACE) in patients with schizophrenia. Schizophr Res. 2018;202:50–4.
Gadelha A, Yonamine CM, Nering M, Rizzo LB, Noto C, Cogo-Moreira H, et al. Angiotensin converting enzyme activity is positively associated with IL-17a levels in patients with schizophrenia. Psychiatry Res. 2015;229(3):702–7.
Ripke S, Neale B, Corvin A, JTR W, Farh K, Schizophrenia Working Group of the Psychiatric Genomics C. Biological insights from 108 schizophrenia--associated genetic loci. Nature. 2014;511:421.
Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. 2018;44(6):1195–203.
Franklin JM, Eddings W, Austin PC, Stuart EA, Schneeweiss S. Comparing the performance of propensity score methods in healthcare database studies with rare outcomes. Stat Med. 2017;36(12):1946–63.
Bykov K, He M, Franklin JM, Garry EM, Seeger JD, Patorno E. Glucose-lowering medications and the risk of cancer: a methodological review of studies based on real‐world data. Diabetes Obes Metabolism. 2019;21(9):2029–38.
Sendor R, Stürmer T. Core concepts in pharmacoepidemiology: confounding by indication and the role of active comparators. Pharmacoepidemiol Drug Saf. 2022;31(3):261–9.
Bernstein KE, Khan Z, Giani JF, Cao D-Y, Bernstein EA, Shen XZ. Angiotensin-converting enzyme in innate and adaptive immunity. Nat Rev Nephrol. 2018;14(5):325–36.
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry. 2015;2(3):258–70.
Hockings N, Ajayi A, Reid J. Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol. 1986;21(4):341–8.
Oh SJ, Fan X. The possible role of the angiotensin system in the pathophysiology of schizophrenia: implications for pharmacotherapy. CNS Drugs. 2019;33(6):539–47.
Hernán MA, Hernández-Díaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trails. 2012;9(1):48–55.
Zou S, Hu J, Zou S, Zou S, Zou S, Zou S. Mental illness and hypertension. Secondary hypertension: screening, diagnosis and treatment. Springer; 2019. pp. 389–402.
Graham N, Smith DJ. Comorbidity of depression and anxiety disorders in patients with hypertension. J Hypertens. 2016;34(3):397–8.
Sumner JA, Kubzansky LD, Roberts AL, Gilsanz P, Chen Q, Winning A, et al. Post-traumatic stress disorder symptoms and risk of hypertension over 22 years in a large cohort of younger and middle-aged women. Psychol Med. 2016;46(15):3105–16.
Haberer JE, van der Straten A, Safren SA, Johnson MO, Amico KR, Del Rio C, et al. Individual health behaviours to combat the COVID-19 pandemic: lessons from HIV socio‐behavioural science. J Int AIDS Soc. 2021;24(8):e25771.
Mortensen P, Pedersen M, Pedersen C. Psychiatric family history and schizophrenia risk in Denmark: which mental disorders are relevant? Psychol Med. 2010;40(2):201–10.
Cantor-Graae E. The contribution of social factors to the development of schizophrenia: a review of recent findings. Can J Psychiatry. 2007;52(5):277–86.
Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394(10211):1816–26.
Kaplan S, Toussi M, Evans A, Dhanda S, Roy D, Lass A. Real world utilization of 91 day extended levonorgestrel-containing combined oral contraceptives in Europe: a multinational database study. Curr Med Res Opin. 2021;37(3):515–22.
Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V et al. Characterizing treatment pathways at scale using the OHDSI network. Proceedings of the National Academy of Sciences. 2016;113(27):7329–36.
Goldner EM, Hsu L, Waraich P, Somers JM. Prevalence and incidence studies of schizophrenic disorders: a systematic review of the literature. Can J Psychiatry. 2002;47(9):833–43.
Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990 2013: a systematic literature review. BMC Psychiatry. 2015;15(1):1–14.
McCoy TH Jr, Yu S, Hart KL, Castro VM, Brown HE, Rosenquist JN, et al. High throughput phenotyping for dimensional psychopathology in electronic health records. Biol Psychiatry. 2018;83(12):997–1004.
Jung M, Choo E, Lee S. Comprehensive trends and patterns of antihypertensive prescriptions using a nationwide claims database in Korea. Clinical Epidemiology. 2020:963–75.
Acknowledgements
The analysis is based in part on work from the Observational Health Sciences and Informatics collaborative. OHDSI (http://ohdsi.org) is a multi-stakeholder, interdisciplinary collaborative to create open-source solutions that reveal the value of observational health data through large-scale analytics. This work was supported by the Health Insurance Review and Assessment Service (HIRA). The views expressed are those of the authors and not necessarily those of the HIRA.
Funding
This research was supported by a grant of the project for Infectious Disease Medical Safety, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HG22C0024). Also, this research was supported by a grant (22213MFDS486) from Ministry of Food and Drug Safety in 2022 and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR16C0001).
Author information
Authors and Affiliations
Contributions
D.Y.L., C.K., and J.K. contributed equally as co-first authors. S.C.Y. and R.W.P. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors substantially contributed to the conception and design of the work and interpretation of the data. D.Y.L drafted the manuscript and all other authors gave critical revision for important intellectual content. All authors gave final approval to the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ajou University Medical Center Institutional Review Board (IRB number: AJIRB-MED-MDB-21-274). Participant informed consent was waived for retrospective studies using de-identified data according to the Ajou University Medical Center Institutional Review Board regulations and decision.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lee, D.Y., Kim, C., Kim, J. et al. Comparative estimation of the effects of antihypertensive medications on schizophrenia occurrence: a multinational observational cohort study. BMC Psychiatry 24, 128 (2024). https://doi.org/10.1186/s12888-024-05578-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-024-05578-6