Abstract
Background
It has been hypothesized that the IL-6/sIL-6R/sgp130 complex, an inflammatory complex, plays a critical role in the pathogenesis of major depressive disorder (MDD). Estradiol (E2) is a sex steroid hormone involved in emotional regulation and MDD. This study aimed to investigate the relationship between E2 and IL-6/sIL-6R/sgp130 complex in patients with MDD.
Methods
Using enzyme-linked immunosorbent assay, the levels of IL-6, sIL-6Rα, and sgp130 were compared between 117 female patients with MDD and 122 healthy controls.The serum concentrations of E2 and other biomarkers were also measured.
Results
(1) The serum levels of IL-6 and sIL-6Rα in patients with MDD were significantly higher than those in the control group, while the serum levels of sgp130 and E2 were significantly lower (all P < 0.05). (2) Low levels of E2 were associated with high levels of IL-6 and low levels of sgp130 (all P < 0.01). (3) HAMD-24 score was positively correlated with the serum level of IL-6, but negatively correlated with the serum levels of sgp130 and E2(all P < 0.05). (4) IL-6 and sgp130 had certain prognostic values in MDD, and the combination of various indicators showed a significantly superior prognostic value.
Conclusions
The IL6/sIL-6R/sgp130 complex in female patients with MDD was closely related to E2 level. In addition, IL-6 and sgp130 may be valuable serum biomarkers for the diagnosis and prognosis of MDD in women.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD), a common public health problem, is the most frequent cause of disability and death [1, 2]. MDD not only brings heavy mental and economic pressure to patients but also imposes a great burden on families and society. However, the importance of MDD has not been fully understood, and most patients do not receive timely treatment. Therefore, it is important to explore the potential mechanisms and variable risk factors triggering MDD.
Inflammation, as a common feature of mental illness, is a vital factor in the pathogenesis and exacerbation of MDD [3]. Increased levels of peripheral inflammatory markers are common findings in MDD and inflammation. Interleukin 6 (IL-6) is the most common inflammatory cytokine, which plays a significant role in the pathogenesis of MDD. Recently, several studies highlighted that elevated levels of IL-6 are closely related to MDD [4, 5]. IL-6 binds to the soluble IL-6 receptor (sIL-6R) to form an IL-6/sIL-6R complex, which then binds to the membrane binding glycoprotein 130 (gp130) subunit, transduces trans-signaling, and triggers a pro-inflammatory response [6]. It has been hypothesized that IL-6 trans-signaling is closely associated with MDD, cancer, and chronic inflammation [6, 7]. Ferencova et al. [8] reported that MDD is a chronic inflammatory disease that can activate the inflammatory response system (IRS) and elevate the serum levels of IL-6 and sIL-6R levels. Animal studies have also shown that IL-6 trans-signaling is associated with “disease behavior” similar to depression, and inhibition of IL-6 trans-signaling alleviated pathogenic behavioral symptoms [7]. In addition, it has been found that soluble glycoprotein 130 (sgp130) can competitively bind to the IL-6/sIL-6R complex, thereby preventing trans-signaling [9, 10]. Sgp130 has been extensively studied in pancreatic cancer [11], inflammatory acute lung injury [12], atherosclerosis [13], and other diseases, while its role in mental diseases, such as depression, is poorly understood. Therefore, this study measured the serum levels of sgp130 in patients with MDD.
MDD is an emotional disorder related to sex hormones.Changes in estrogen levels are particularly important in female patients with MDD. Estrogen, a steroid hormone with neuroprotective effects, and its receptors can protect the brain against neurodegenerative diseases, emotional disorders, and cognitive decline [14, 15]. It has been demonstrated that estrogen deficiency in postmenopausal women may increase IL-6 production, and estrogen can inhibit the inflammatory response of astrocytes and microglia and downregulate inflammatory factors such as IL-6 and TNF-α [16]. Similarly, animal studiesfound that low serum levels of estradiol cause hippocampal inflammation, anxiety, and depression-like behaviors in mice, which were alleviated by estrogen supplementation [17]. Estrogen can also affect the IL-6/gp130 signaling pathway. Similarly, it has been observed that estradiol can inhibit IL-6 production and downregulate the expression of gp130 in secretory cells [18]. However, most of the previous studies only focused on the role of inflammation in MDD or emphasized the relationship between estrogen and MDD, with few studies exploring the mechanisms by which estrogen regulates inflammation and affects MDD. This study provides a new horizon in the field of MDD. Rebalancing estrogen levels may regulate IL-6 and sgp130 production as a new strategy for treating MDD. Considering the relationship between estrogen, inflammation, and depression, we hypothesized that estradiol can regulate the IL-6/sIL-6R/sgp130 signaling pathway and improve MDD.
Methods
Participants
This study was a retrospective study with 131 inpatients who visited the Department of Psychiatry and Clinical Psychology of Renmin Hospital of Wuhan University from November 2021 to February 2023. Of 131 femal patients with MDD, 14 were excluded, 8 could not confirm whether they had a family history of mental illness, and 6 refused to participate. Finally, 117 female patients with MDD aged 42 (20–57) years were included in the analysis. At the same time, 122 healthy subjects aged 40 (30.75–56) years were recruited in this study. All patients with MDD underwent DSM-IV (SCID) structured clinical interviews before inclusion. In addition, the severity of depressive symptoms was assessed by a trained medical psychologist using the 24-item Hamilton Depression Scale (HAMD) [19]. Exclusion criteria were as follows: 1) taking antipsychotic drugs in the past three months; 2) suffering from other mental diseases, such as schizophrenia, obsessive–compulsive disorder and anxiety disorder; 3) suffering from diabetes, premenstrual Syndrome (PMS), malignant tumors, autoimmune diseases, serious heart or kidney diseases, etc. This study was approved by the Medical Ethics Review Committee of Renmin Hospital of Wuhan University (WDRY2021-K041). All subjects provided written informed consent.
Collection and testing of blood samples
Blood samples were collected between the third and fifth days of the menstrual cycle [20]. All subjects were fasted at least for 8 h, the night before the examination and underwent venipathesis (about 3 mL) in the morning of the following day. The collected blood samples were centrifuged for 15 min at 3500 rpm/min at room temperature, and isolated serum samples were frozen at -80 ℃ until detection.
High-density lipoprotein cholesterol (HDL-C), uric acid (UA), creatinine (Cr), total cholesterol (TC), triglyceride (TG), urea, and low-density lipoprotein cholesterol (LDL-C) were detected by ADVIA 2400 automatic biochemical analyzer (Siemens, Germany). White blood cell counts and serum estradiol levels were measured using the Sysmex XN-20 system (Japan) and Siemens ADVIA Centaur CP (Germany), respectively. The serum levels of human serum IL-6, sgp130, and sIL-6Rα were detected by enzyme-linked immunosorbent assay (ELISA) kits (Quantikine DY206, DY227, DY008, and DY228; R&D Systems, USA). The serum levels of IL-6 were measured without diluting the sample, but the serum levels of sgp130 and serum sIL-6Rα were measured after diluting the samples 100 times.
Statistical analysis
SPSS 22.0 and GraphPad Prism 7.0 were used for analyzing data and drawing graphs. LDL-C, WBC, and TC were analyzed using an independent sample t-test (all expressed as mean ± SD). Categorical variables (e.g., smoking, etc.) were compared using the Chi-square test. Age, Urea, Cr, TG, UA, HDL-C, E2, IL-6, sgp130, TC/HDL-C, and sIL-6Rα were analyzed using the Mann–Whitney U test (all expressed as median (P25, P75)). Spearman correlation and multiple linear regression were used to analyze the correlations between IL-6, sIL6Rα, sgp130, and HAMD. Receiver operating characteristic (ROC) curve was used to evaluate the diagnostic value of relevant indicators for MDD. In addition, the prediction probability and P value of multi-indicator ROC detection were determined by binary logistic regression analysis. In the binary logistic regression model, the grouping was used as the dependent variable, IL-6,sIL-6R,sgp130 and E2 as the covariables, and the prediction probability was further calculated using SPSS 22.0 software. Two-tailed P < 0.05 showed statistically significant differences.
Results
Study population
Table 1 presents basic clinical data for all participants. There was no difference in alcohol consumption, smoking, or age between the MDD group and the healthy control group (all P > 0.05). The serum levels of TC, E2, LDL-C, and sgp130 were significantly lower in the MDD group than in the healthy control group (all P < 0.05). The serum levels of WBC, IL-6, and sIL-6Rα in the MDD group were significantly higher than that of the control group (all P < 0.05).
Correlation between serum IL-6, sIL-6Rα, sgp130 and E2
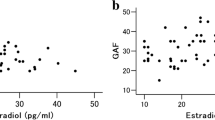
As shown in Table 2, after adjustment for smoking, TC, TG, alcohol consumption, TC/HDL-C, urea, Cr, UA, WBC, HDL-C, and LDL-C, IL-6 level was negatively correlated with the E2 level (r = -0.207, P = 0.001), but sgp130 level was positively correlated with the E2 level (r = 0.325, P < 0.001). There was no relationship between sIL-6Rα and E2 (r = -0.039, P = 0.540). Besides, spearman correlation analysis also showed that low serum levels of E2 were associated with high serum levels of IL-6 and low serum levels of sgp130 (r = -0.267, 0.370; P < 0.001) (Fig. 1).
Correlation between HAMD-24 score and serum levels of IL-6, sIL-6Rα, sgp130, and E2
We used multiple linear regression and spearman correlation to explore the relationship between the severity of MDD and serum levels of IL-6, sIL-6Rα, sgp130, and E2. In the case of multiple linear regression analysis without adjusting any variables, the score of HAMD-24 score was inversely associated with the serum level of sgp130 and E2 (r = -0.069 and -0.054, respectively, both P < 0.01). After adjustment for smoking, traumatic life events, alcohol consumption, and family history of depression in model 1, the HAMD-24 score was positively correlated with IL-6 level (r = 0.043, P < 0.05), and negatively correlated with sgp130 and E2 levels (r = -0.063 and -0.048, respectively, both P < 0.05). After additional adjustment for urea, Cr, UA, TC, TG, TC/HDL-C, WBC, HDL-C, and LDL-C in Model 2, the correlation between the above indicators remained significant (r = 0.047, -0.070, and -0.061, respectively; P < 0.05) (Table 3). In addition, the HAMD-24 score had a positive correlation with IL-6 level and a negative correlation with sgp130 and E2 levels (r = 0.251, -0.284, and -0.314, respectively; P < 0.01) (Fig. 2).
Association of IL-6, sIL-6Rα, sgp130, and E2 levels with the risk factors of MDD
Spearman correlation analysis was used to investigate the relationship between other risk factors of MDD and IL-6, sIL-6Rα, sgp130, and E2 levels. IL-6 level was positively correlated with WBC (r = 0.202, P = 0.029), and negatively correlated with TC and LDL-C levels (r = -0.239 and -0.187, respectively; both P < 0.05) (Table 4). sgp130 was negatively associated with the serum levels of urea, TC, TG, LDL-C, and TC/HDL-C (r = -0.250, -0.372, -0.392, -0.287, and -0.283, respectively; all P < 0.01). The serum level of E2 was negatively associated with the serum levels of urea, TC, TG, LDL-C and TC/HDL-C (r = -0.187, -0.458, -0.389, -0.365, and -0.326, respectively; all P < 0.05).
Prognostic values of IL-6, sIL-6Rα, sgp130 and E2 in patients with MDD
The ROC curve of the prognostic value of IL-6, sIL6Rα, sgp130, and E2 for MDD is shown in Fig. 3. AUC comparison: Combination (0.919) > IL-6 (0.885) > sgp130 (0.783) > sIL-6Rα (0.622) > E2 (0.580). Sensitivity comparison: IL-6 (85.47%) > Combination (80.34%) > sIL-6Rα (76.92%) = sgp130 (76.92%) > E2 (62.39%). Specificity comparison: Combination (89.34%) > IL-6 (79.51%) > sgp130 (69.67%) > E2 (50.82%) > sIL-6Rα (45.08%). Youden index comparison: Combination (0.6968) > IL-6 (0.6498) > sgp130 (0.4659) > sIL-6Rα (0.2200) > E2 ( 0.1312). The cut-off values of IL-6, sIL-6Rα, sgp130, and E2 were 13.57 pg/L, 5.60 ng/mL, 73.33 ng/mL, and 42.85 pg/mL, respectively (Table 5).
Discussion
This case–control study reported the evidence of associations between the IL-6/sIL-6R/sgp130 complex and E2 in patients with MDD. Firstly, we found that female patients with MDD have higher serum levels of IL-6 and sIL-6Rα, and lower serum levels of sgp130 and E2. Secondly, E2 levels were negatively correlated with IL-6 levels and positively correlated with sgp130 levels. In addition, high levels of IL-6 and low levels of sgp130 and E2 were associated with higher 24-item Hamilton scores. Thirdly, we demonstrated that the IL-6/sIL-6R/sgp130 complex has a certain diagnostic prognostic value in MDD, and the combination of several indicators can significantly improve the prognostic value.
MDD is considered, in some sense, to be a chronic inflammatory disease with altered serum levels of cytokines [21, 22]. IL-6, a common inflammatory factor, is involved in the pathogenesis of MDD through various mechanisms. IL-6 can cross the blood–brain barrier (BBB)and increase synaptic contractility by directly affecting neurons or regulating microglia and other immune cells, thereby aggravating depression [23, 24]. Secondly, IL-6 can directly affect brain function and neurotransmitter production, leading to the progression and poor prognosis of MDD [25]. In addition, IL-6 can inhibit hippocampal neurogenesis in depression by acting on IL-6 receptors or a transmembrane protein, gp130, in the dentate gyrus [26, 27] or by stimulating the hypothalamic–pituitary–adrenal (HPA) axis [27, 28]. Impaired neurogenesis is an important mechanism in MDD [29]. Consistent with our results, the meta-analysis by Dahl et al. confirmed that IL-6 levels are significantly higher in patients with MDD than in the healthy control group [30]. IL-6 can induce microglial cell activation [31], oxidative stress, and neuronal apoptosis [32], and HPA axis dysfunction [33], and reduce the secretion of norepinephrine and 5-hydroxytryptamine [34], ultimately leading to MDD. In addition, Mao [35] and Roohi [36] et al. demonstrated that IL-6 is an important inflammatory factor that exacerbates MDD and is positively correlated with the severity and prognosis of MDD. Animal studies have also shown that elevated levels of central or peripheral IL-6 may be associated with depressive symptoms [24].
Spg130, a natural inhibitor of the IL-6 trans-signaling pathway, has been shown to be closely related to MDD as a potential therapeutic target [37]. Previous findings concerning sgp130 and its relationship with MDD are controversial. Sukoff et al. demonstrated that continuously increased levels of IL-6 in the central nervous system (CNS) can lead to depression-like phenotype in rodents, while sgp130 FC can significantly reduce IL-6 levels and improve depression-like behavior, suggesting the anti-inflammatory and antidepressant effects of sgp130 [38]. Besides, it has been shown that there is no significant difference in sgp130 levels in the cerebrospinal fluid between depressed and control groups [39]. The possible reason is that the sgp130 level in cerebrospinal fluid is significantly lower than that in serum, but the exact cause is unclear and more studies are needed.
In addition, numerous studies have illustrated a robust association between IL-6/sIL-6R/sgp130 complex and E2. As a steroid hormone with anti-inflammatory effects, estrogen downregulates the levels of IL-6 and gp130 levels in osteoblasts and breast cancer cells, thereby affecting the downstream IL-6/gp130 signaling pathway [39, 40]. Estradiol can also downregulate gp130 and IL-6 levels in mouse bone marrow, protecting against osteoporosis [40, 41]. However, the relationship between estrogen and the IL-6/sIL-6R/sgp130 complex has been less studied in patients with MDD. Notably, our results demonstrated that patients with MDD have low levels of E2. In particular, E2 levels were negatively correlated with IL-6 levels and positively correlated with sgp130 levels, indicating that estrogen exerts antidepressant effects by suppressing inflammation.. Therefore, balancing IL-6and sgp130 levels with estrogen may be crucial for improving depressive symptoms.
However, our study has certain limitations. Firstly, our study was a case–control study and could not determine causal relationships. Secondly, although this study adjusted the effects of many variables, other confounding factors were not excluded. Thirdly, the sample size of this study was small. Future studies with large sample sizes are needed to further explore the relationship between estrogen and the IL-6/sIL-6R/sgp130 complex in patients with MDD. Finally, in terms of the molecular mechanism, sevel studies have shown that the protein expression [38] and mRNA level of IL-6 are significantly increased in the peripheral blood of patients with MDD [42]. In addition, changes in IL6 DNA methylation may also be closely related to the development of MDD [43]. However, it is still unclear whether altered IL-6 protein and gene expression are related to E2, and the effect of sgp130 on MDD is currently limited to the serological studies. Therefore, more in-depth studies on the altered protein and gene expression of IL-6 and sgp130 are needed in patients with MDD to unravel the relationship between E2 and the above mechanisms.
In conclusion, our findings demonstrated that the serum levels of E2 in patients with MDD are closely related to the IL-6/sIL6R/sgp130 complex. We found that IL-6 and sgp130 have potential diagnostic value for MDD, providing a deeper insight into the pathogenesis of MDD.
Availability of data and materials
The datasets used during the current study are available from the corresponding author according to reasonable requirements.
Abbreviations
- MDD:
-
Major depressive disorder
- HAMD-24:
-
24-Item Hamilton Depression Scale
- UA:
-
Uric acid
- Cr:
-
Creatinine
- HDL-C:
-
High-density lipoprotein cholesterol
- sgp130:
-
Soluble glycoprotein 130
- sIL-6Rα:
-
Soluble interleukin-6 receptor α
- IL-6:
-
Interleukin-6
- LDL-C:
-
Low-density lipoprotein cholesterol
- TG:
-
Triglyceride
- WBC:
-
White blood cell
- TC:
-
Total cholesterol
- E2:
-
Estradiol
References
Sun T, Peng R, Sun X, Li Y. Associations between sex hormones and circulating growth differentiation factor-15 in male patients with major depressive disorder. Brain Sci. 2021;11(12):1612. https://doi.org/10.3390/brainsci11121612.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. https://doi.org/10.1371/journal.pmed.0030442.
Rossetti AC, Paladini MS, Brüning CA, Spero V, Cattaneo MG, Racagni G, et al. Involvement of the IL-6 signaling pathway in the anti-anhedonic effect of the antidepressant agomelatine in the chronic mild stress model of depression. Int J Mol Sci. 2022;23(20):12453. https://doi.org/10.3390/ijms232012453.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57. https://doi.org/10.1016/j.biopsych.2009.09.033.
Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–9. https://doi.org/10.1016/j.jad.2011.08.003.
Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82. https://doi.org/10.1016/j.coi.2015.02.008.
Kelly KM, Smith JA, Mezuk B. Depression and interleukin-6 signaling: A Mendelian Randomization study. Brain Behav Immun. 2021;95:106–14. https://doi.org/10.1016/j.bbi.2021.02.019.
Ferencova N, Visnovcova Z, Ondrejka I, Funakova D, Hrtanek I, Kelcikova S, et al. Evaluation of Inflammatory Response System (IRS) and Compensatory Immune Response System (CIRS) in Adolescent Major Depression. J Inflamm Res. 2022;15:5959–76. https://doi.org/10.2147/JIR.S387588.
Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268(1):160–7. https://doi.org/10.1046/j.1432-1327.2001.01867.x.
Zhou M, Dai W, Cui Y, Liu H, Li Y. Associations between the IL-6-neutralizing sIL-6R-sgp130 buffer system and coronary artery disease in postmenopausal women. Ann Transl Med. 2020;8(6):379. https://doi.org/10.21037/atm.2020.02.27.
Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Klöppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. https://doi.org/10.1016/j.ccr.2011.03.009.
Zhang H, Neuhöfer P, Song L, Rabe B, Lesina M, Kurkowski MU, et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J Clin Invest. 2013;123(3):1019–31. https://doi.org/10.1172/JCI64931.
Schuett H, Oestreich R, Waetzig GH, Annema W, Luchtefeld M, Hillmer A, et al. Transsignaling of interleukin-6 crucially contributes to atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2012;32(2):281–90. https://doi.org/10.1161/ATVBAHA.111.229435.
Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. https://doi.org/10.1038/nrn3856.
Albert KM, Newhouse PA. Estrogen, Stress, and Depression: Cognitive and Biological Interactions. Annu Rev Clin Psychol. 2019;15:399–423. https://doi.org/10.1146/annurev-clinpsy-050718-095557.
Rachoń D, Myśliwska J, Suchecka-Rachoń K, Wieckiewicz J, Myśliwski A. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J Endocrinol. 2002;172(2):387–95. https://doi.org/10.1677/joe.0.1720387.
Xu Y, Sheng H, Bao Q, Wang Y, Lu J, Ni X. NLRP3 inflammasome activation mediates estrogen deficiency-induced depression- and anxiety-like behavior and hippocampal inflammation in mice. Brain Behav Immun. 2016;56:175–86. https://doi.org/10.1016/j.bbi.2016.02.022.
Canellada A, Alvarez I, Berod L, Gentile T. Estrogen and progesterone regulate the IL-6 signal transduction pathway in antibody secreting cells. J Steroid Biochem Mol Biol. 2008;111(3–5):255–61. https://doi.org/10.1016/j.jsbmb.2008.06.009.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. https://doi.org/10.1136/jnnp.23.1.56.
Rajewska J, Rybakowski JK. Depression in prem.enopausal women: gonadal hormones and serotonergic system assessed by D-fenfluramine challenge test. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(4):705–9. https://doi.org/10.1016/S0278-5846(03)00085-X.
Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018;8(1):12050. https://doi.org/10.1038/s41598-018-30487-6.
Vaváková M, Ďuračková Z, Trebatická J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid Med Cell Longev. 2015;2015:898393. https://doi.org/10.1155/2015/898393.
Bai Z, Gao T, Zhang R, Lu Y, Tian J, Wang T, et al. Inhibition of IL-6 methylation by Saikosaponin C regulates neuroinflammation to alleviate depression. Int Immunopharmacol. 2023;118:110043. https://doi.org/10.1016/j.intimp.2023.110043.
Wang J, Hodes GE, Zhang H, Zhang S, Zhao W, Golden SA, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018;9(1):477. https://doi.org/10.1038/s41467-017-02794-5.
Zadka Ł, Dzięgiel P, Kulus M, Olajossy M. Clinical Phenotype of Depression Affects Interleukin-6 Synthesis. J Interferon Cytokine Res. 2017;37(6):231–45. https://doi.org/10.1089/jir.2016.0074.
McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun. 2011;25(5):850–62. https://doi.org/10.1016/j.bbi.2010.09.003.
Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86. https://doi.org/10.1016/j.pnpbp.2012.10.022.
Mastorakos G, Ilias I. Interleukin-6: a cytokine and/or a major modulator of the response to somatic stress. Ann N Y Acad Sci. 2006;1088:373–81. https://doi.org/10.1196/annals.1366.021.
Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56(3):140–5. https://doi.org/10.1016/j.biopsych.2004.02.033.
Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology. 2014;45:77–86. https://doi.org/10.1016/j.psyneuen.2014.03.019.
D’Mello C, Swain MG. Immune-to-Brain Communication Pathways in Inflammation-Associated Sickness and Depression. Curr Top Behav Neurosci. 2017;31:73–94. https://doi.org/10.1007/7854_2016_37.
Huang C, Zhang F, Li P, Song C. Low-dose IL-2 attenuated depression-like behaviors and pathological changes through restoring the balances between IL-6 and TGF-β and between Th17 and treg in a chronic stress-induced mouse model of depression. Int J Mol Sci. 2022;23(22):13856. https://doi.org/10.3390/ijms232213856.
Song C. The effect of thymectomy and IL-1 on memory: implications for the relationship between immunity and depression. Brain Behav Immun. 2002;16(5):557–68. https://doi.org/10.1016/s0889-1591(02)00012-0.
da Silva Dias IC, Carabelli B, Ishii DK, de Morais H, de Carvalho MC, Rizzo de Souza LE, et al. Indoleamine-2,3-Dioxygenase/Kynurenine Pathway as a Potential Pharmacological Target to Treat Depression Associated with Diabetes. Mol Neurobiol. 2016;53(10):6997–7009. https://doi.org/10.1007/s12035-015-9617-0.
Mao L, Ren X, Wang X, Tian F. Associations between autoimmunity and depression: serum IL-6 and IL-17 have directly impact on the HAMD scores in patients with first-episode depressive disorder. J Immunol Res. 2022;2022:6724881. https://doi.org/10.1155/2022/6724881.
Roohi E, Jaafari N, Hashemian F. On inflammatory hypothesis of depression: what is the role of IL-6 in the middle of the chaos? J Neuroinflammation. 2021;18(1):45. https://doi.org/10.1186/s12974-021-02100-7.
Li Y, Mei T, Sun T, Xiao X, Peng R. Altered circulating GDF-15 level predicts sex hormone imbalance in males with major depressive disorder. BMC Psychiatry. 2023;23(1):28. https://doi.org/10.1186/s12888-023-04527-z.
Sukoff Rizzo SJ, Neal SJ, Hughes ZA, Beyna M, Rosenzweig-Lipson S, Moss SJ, et al. Evidence for sustained elevation of IL-6 in the CNS as a key contributor of depressive-like phenotypes. Transl Psychiatry. 2012;2(12):e199. https://doi.org/10.1038/tp.2012.120.
Stübner S, Schön T, Padberg F, Teipel SJ, Schwarz MJ, Haslinger A, et al. Interleukin-6 and the soluble IL-6 receptor are decreased in cerebrospinal fluid of geriatric patients with major depression: no alteration of soluble gp130. Neurosci Lett. 1999;259(3):145–8. https://doi.org/10.1016/s0304-3940(98)00916-1.
Lin SC, Yamate T, Taguchi Y, Borba VZ, Girasole G, O’Brien CA, et al. Regulation of the gp80 and gp130 subunits of the IL-6 receptor by sex steroids in the murine bone marrow. J Clin Invest. 1997;100(8):1980–90. https://doi.org/10.1172/JCI119729.
Cui Y, Dai W, Li Y. Circulating levels of sgp130 and sex hormones in male patients with coronary atherosclerotic disease. Atherosclerosis. 2017;266:151–7. https://doi.org/10.1016/j.atherosclerosis.2017.09.002.
Rizavi HS, Ren X, Zhang H, Bhaumik R, Pandey GN. Abnormal gene expression of proinflammatory cytokines and their membrane-bound receptors in the lymphocytes of depressed patients. Psychiatry Res. 2016;240:314–20. https://doi.org/10.1016/j.psychres.2016.04.049.
Ryan J, Pilkington L, Neuhaus K, Ritchie K, Ancelin ML, Saffery R. Investigating the epigenetic profile of the inflammatory gene IL-6 in late-life depression. BMC Psychiatry. 2017;17(1):354. https://doi.org/10.1186/s12888-017-1515-8.
Acknowledgements
All authors agree with the content of the manuscript. We are grateful to all those who voluntarily participated in our research.
Funding
The National Natural Science Foundation of China (81772265) have contributed to analysis and interpretation of data.
Author information
Authors and Affiliations
Contributions
Ting Sun: Methodology, Formal analysis, Data analysis, Writing - original draft. Qian Chen: Formal analysis, Data analysis, Methodology. Junchi Mei: Investigation. Yan Li: Validation, Data curation, Funding acquisition, Supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All subjects provided written informed consent. The present study was approved by the Medical Ethics Review Committee of Renmin Hospital at Wuhan University (WDRY2021-K041) and conducted in accordance with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, T., Chen, Q., Mei, J. et al. Associations between serum estradiol and IL-6/sIL-6R/sgp130 complex in female patients with major depressive disorder. BMC Psychiatry 23, 742 (2023). https://doi.org/10.1186/s12888-023-05248-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05248-z