Abstract
Background
Studies on sleep problems among caregivers of psychiatric patients, especially during the COVID-19 pandemic, are limited. This study examined the prevalence and correlates of insomnia symptoms (insomnia hereafter) among caregivers of psychiatric inpatients during the COVID-19 pandemic as well as the association with quality of life (QoL) from a network analysis perspective.
Methods
A multi-center cross-sectional study was conducted on caregivers of inpatients across seven tertiary psychiatric hospitals and psychiatric units of general hospitals. Network analysis explored the structure of insomnia using the R program. The centrality index of “Expected influence” was used to identify central symptoms in the network, and the “flow” function was adopted to identify specific symptoms that were directly associated with QoL.
Results
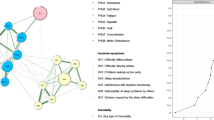
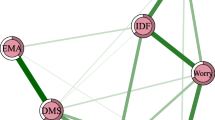
A total of 1,101 caregivers were included. The overall prevalence of insomnia was 18.9% (n = 208; 95% CI = 16.7–21.3%). Severe depressive (OR = 1.185; P < 0.001) and anxiety symptoms (OR = 1.099; P = 0.003), and severe fatigue (OR = 1.320; P < 0.001) were associated with more severe insomnia. The most central nodes included ISI2 (“Sleep maintenance”), ISI7 (“Distress caused by the sleep difficulties”) and ISI1 (“Severity of sleep onset”), while “Sleep dissatisfaction” (ISI4), “Distress caused by the sleep difficulties” (ISI7) and “Interference with daytime functioning” (ISI5) had the strongest negative associations with QoL.
Conclusion
The insomnia prevalence was high among caregivers of psychiatric inpatients during the COVID-19 pandemic, particularly in those with depression, anxiety and fatigue. Considering the negative impact of insomnia on QoL, effective interventions that address insomnia and alteration of sleep dissatisfaction should be developed.
Similar content being viewed by others
Introduction
Caregivers are defined as “primary persons who generally provide the majority of care and support to patients”, and are an extension of patients’ hospital care support system [1]. They undertake the primary task of caring for patients and play a valuable caregiving role in managing the lives of patients during their hospital stay [2]. To some extent, they are also viewed as effective legal decision-makers on medical issues for patients they are responsible for [3]. They are a hidden workforce that alleviate the burden of professional care and the costs of social care systems [2]. Caregiving burden is commonly divided into objective and subjective burdens [4]. Objective burden refers to the impact of the caregiving tasks on the caregivers’ household activities, economic resources, health and leisure activities, while subjective burden refers to the personal distress suffered as a result of giving care such as feelings of loss, shame and anger [4]. Previous studies have found a high burden in caregivers of psychiatric patients with schizophrenia (SCZ), bipolar disorder (BP), and major depressive disorder (MDD) [5], which could affect the caregivers’ quality of life (QoL) and family function [6]. Additionally, illness factors were significantly associated with a high burden in caregivers, including duration of mental illness, severity of symptoms and level of dysfunction [1, 7]. Caring for patients with severe psychiatric disorders could compromise caregivers’ mental health and result in psychological distress, anxiety, depression, insomnia and post-traumatic stress disorder [8]. Psychiatric patients often suffer from anxiety, depression, stress, insomnia, impulsivity and even suicidal ideation [9], which may contribute to their caregivers’ distress.
Insomnia is a common problem in patients with psychiatric disorders [10], which can lead to an exacerbation of a pre-existing psychiatric disorder or be a reaction to psychological distress [11]. As such, caregiving burden can directly result in sleep disturbances [12]. A previous study found that family caregivers were more likely to report insomnia than non-caregivers (46% vs. 37%) [13]. Other studies showed that caregivers had a high prevalence of sleep disturbance, for example, 50–70% of caregivers of dementia patients [14] and 40% of caregivers of cancer patients [15] experienced insomnia. Common insomnia complaints in caregivers for patients with cancers included short sleep duration, nocturnal awakenings, waking after the onset of sleep, and daytime dysfunction [15]. However, the features of insomnia among caregivers of patients with psychiatric disorders are not clear.
Insomnia in caregivers was found to be associated with various factors. Most studies found that female caregivers were more likely to experience sleep disturbance [16]. Insomnia was commonly associated with psychiatric problems among caregivers compared to non-caregivers, especially depression and anxiety disorder; for instance, 44% of caregivers of bipolar patients had a diagnosis of anxiety disorder [17]. In addition, fatigue was associated with an increased risk of sleep problems [18].
The occurrence of mental health problems in many populations greatly increased during the COVID-19 pandemic [19]. Poor psychological status was more common among caregivers of patients with physical or intellectual disabilities [20]. Further, a comparative study found that the prevalence of insomnia among caregivers of mentally ill patients or physically disabled patients during the COVID-19 lockdown was higher than non-caregivers (69.9% vs. 44.7%) [21]. However, studies on insomnia among caregivers for psychiatric patients during the COVID-19 pandemic have been limited. Insomnia could result in adverse health outcomes, such as poor mental health status, daily functioning and caregiving quality, which in turn could worsen the QoL of caregivers [14]. Although the association between insomnia and QoL has been documented in many studies [22, 23], the relationships between individual insomnia symptoms and QoL among the caregivers of psychiatric patients are still unclear.
Traditionally, the severity of psychiatric disorders or syndromes are measured and analyzed using the sum score of the assessment tools; for example, insomnia is measured by the Insomnia Severity Index (ISI) [24]. However, traditional approaches obscure the potential differences and inter-relationships between different symptoms of a psychiatric disorder or syndrome. As an emerging novel approach to examine the complex and dynamic interactions among symptoms, network analysis can overcome this limitation, based on the premise that a particular psychiatric syndrome is viewed as an interacting cluster of symptoms, with different strength and nature of associations between the psychiatric symptoms [25]. Network analysis can identify the most influential symptoms (central nodes) and the strongest relationships (edges) between different symptoms. In a network model, the central symptoms have the strong connections with other symptoms, which play an important role in the causation and/or maintenance of the psychiatric disorder or syndrome by activating or deactivation other symptoms [25]. Thus, the identification of central symptoms is clinically important for developing effective targeted strategies or interventions to treat psychiatric disorders or syndromes.
To date, network analysis of insomnia symptoms has already been applied to general populations [26, 27] and mental health professionals [28]. However, no network analysis studies on insomnia symptoms among caregivers of psychiatric inpatients have been published. To fill this gap, this study examined the prevalence and correlates of insomnia in caregivers of psychiatric inpatients, constructed a network model of insomnia symptoms, and evaluated the relationships between insomnia symptoms and QoL.
Methods
Participants and procedures
A multi-center cross-sectional study was conducted between May 24, 2020 and January 18, 2021 among caregivers of psychiatric inpatients using a consecutive sampling method across seven tertiary psychiatric hospitals and psychiatric units of general hospitals located in Liaoning, Shandong, Beijing, Chongqing, Hunan, Guangdong, and Xinjiang in China (Fig. 1). Due to the risks of transmission of COVID-19, face-to-face assessments were not adopted. Data collection was performed using WeChat program during the period of the patients’ hospitalization. All people including caregivers needed to report their health status using WeChat when they entered hospitals during the pandemic; therefore, they were all presumably WeChat users. In China, caregivers might include family members, relatives, close friends of patients or designated hospital employees. All caregivers who visited the patients in the participating hospitals during the study period were consecutively invited by scanning a QR code linked to the invitations. The inclusion criteria were as follows: (1) aged 18 years or above; (2) caregivers of psychiatric patients hospitalized in the participating hospital during the study period such as spouses, parents, offsprings, and other kins or friends; (3) no current episodes of major psychiatric disorders and not co-hospitalized with patients; and (4) able to understand the purpose of the survey and complete the assessment. All participants who provided electronic written informed consent on a voluntary and confidential basis were included in this study. Approval of the study was granted by the Institutional Review Board of Beijing Anding hospital and respective hospitals.
Measures
Basic socio-demographic and clinical characteristics of participants were collected, including age, gender, marital status, education level, employment status, living areas, presence of any major physical diseases, perceived financial status, frequency of social media use, and difficulty in accessing mental health services during the pandemic. Additionally, the patients’ principal psychiatric diagnoses i.e., MDD, BP, SCZ, or others, and medication adherence were recorded.
The validated Chinse version of the Insomnia Severity Index (ISI) questionnaire [24] was used to assess insomnia symptoms in the participants within the past week, which included seven domains: (1) severity of sleep onset problem; (2) sleep maintenance problem; (3) early morning wakening problem; (4) sleep dissatisfaction; (5) interference with daytime function; (6) noticeability of sleep problems by others, and (7) distress caused by the sleep difficulties. Each item was scored on a scale from “0” (no problem) to “4” (very severe problem). The total score ranged from 0 to 28, with a higher score reflecting more severe insomnia symptoms. The cut-off value of 8 was considered as having insomnia symptoms, which can be further categorized into subthreshold insomnia (8–14 points), moderate clinical insomnia (15–21 points), and severe clinical insomnia (22–28 points) [29].
Depressive and anxiety symptoms were evaluated using the validated Chinese version of the Patient Health Questionnaire (PHQ-9) [30] and Generalized Anxiety Disorder scale (GAD-7) [31], respectively. Each item of the two scales was rated on a 4-point Likert scale from “0” (not at all) to “3” (nearly every day). The total scores of the PHQ-9 (0 to 27) and GAD-7 (0 to 21) were the sum of all the item scores, respectively. Subjective fatigue was assessed using a numeric rating scale (NRS), which ranged from “0” (no fatigue) to “10” (worst fatigue imaginable) [32]. The global QoL of caregivers of psychiatric inpatients was assessed using the total score of the first two items of the World Health Organization Quality of Life-brief version (WHOQOL-BREF) [33], with a higher total score indicating higher QoL.
Data analysis
Univariate and multivariate analyses
Univariate and multivariate analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, Illinois, USA). Shapiro-Wilk tests and Q-Q plots were used to test the normality of the distributions for continuous variables. Participants were divided into insomnia and non-insomnia groups. Independent sample t-tests, Mann-Whitney U tests, and Chi-square tests were conducted as appropriately to compare the differences in demographic and clinical characteristics between the two groups. Analysis of covariance (ANCOVA) was used to compare QoL between insomnia and non-insomnia groups after controlling for variables that showed significant differences in univariate analyses. Binary logistic regression analysis was performed to examine independent correlates of insomnia, with insomnia as the dependent variable, and those with significant group differences in univariate analyses were entered as independent variables. The statistical significance level was set at P < 0.05 (two-tailed) for all analyses.
Network structure
Network analysis was conducted using the R program (4.2.2 version) [34]. Symptoms were presented as nodes, and partial correlations between symptoms were presented as edges. The thickness of the edges reflected the strength of the correlations between the nodes, with green edges indicating positive correlations and red indicating negative correlations. As some of the ISI item scores did not follow normal distribution, nonparametric correlations were calculated via nonparanormal transformations. The network structure of insomnia symptoms was constructed using the Graphical Gaussian Model (GGM)following nonparanormal transformation of the data. The graphic least absolute shrinkage and selection operator (LASSO) in combination with Extended Bayesian Information Criterion (EBIC) were used to estimate a regularized GGM [35]. Considering the potential effects of age on sleep [36], the network model after controlling for age was also constructed. Network estimation was conducted using the ‘bootnet’ package [37], visualized using the ‘qgraph’ package [38] and optimized using the “ggplot2” package [39]. Additionally, the ‘flow’ function in the R package ‘qgraph’ was adopted to identify specific insomnia symptoms that were directly associated with QoL [38].
For centrality index, the expected influence (EI) index of each node was computed using “bootnet” package to determine the most central symptoms in the network model [40]. The predictability was calculated using the “mgm” package [41], reflecting the variance of a node that could be explained by its neighbor nodes in the model. To compare the original network model with the one after controlling for age, Spearman’s rank correlation coefficient was calculated to test the correlation of EI between both networks [42].
The stability and accuracy of the network model were evaluated using the “bootnet” package [35]. The correlation stability coefficient (CS-C) was used to evaluate the stability of the network model; a value of above 0.25 was regarded as acceptable stability although 0.5 was preferable [35]. Non-parametric bootstrapped 95% confidence intervals (CI) of edge weights were estimated to evaluate the network accuracy, with a narrower CI indicating a more reliable network. A nonparametric bootstrapped difference test was performed to examine whether two edge-weights significantly differed from one-another.
The Network Comparison Test (NCT) was used to compare the network structures of insomnia symptoms between genders using the “Network Comparison Test” package [43]. The indices of network structure invariance, global strength invariance, and edges or nodes centrality invariance were used to evaluate the results of comparisons [43].
Result
Participant characteristics
Of the 1,138 caregivers invited, 1,101 agreed to participate in the study and completed the assessment, giving a participation rate of 97.3%. Demographic and clinical characteristics of the participants are shown in Table 1. The mean age of participants was 43.06 (SD = 11.6) years and 40.1% (n = 442) were male. Over half of the participants had an education level of senior secondary school and above (n = 677; 61.5%). More than 80% of participants were married (n = 903; 82.0%) and employed (n = 894; 81.2%).
Prevalence and correlates of insomnia
The overall prevalence of insomnia (ISI total score ≥ 8) among caregivers of psychiatric inpatients during the COVID-19 pandemic was 18.9% (n = 208; 95% CI = 16.7–21.3%); specifically, 157 (14.3%, 95% CI = 12.3–16.5%) reported subthreshold insomnia (ISI total score: 8–14), 44 (4.0%, 95% CI = 3.0-5.3%) reported moderate clinical insomnia (ISI total score: 15–21), and 7 (0.6%, 95% CI = 0.3–1.3%) reported severe clinical insomnia (ISI total score: 22–28).
Compared with the non-insomnia group, caregivers with insomnia were more likely to be male (P = 0.034) and had major physical diseases (P = 0.009), difficulty in visiting mental health services during the pandemic (P = 0.001), and perceived poor financial status (P < 0.001). Moreover, participants with insomnia were more likely to report a higher score on PHQ-9 (P < 0.001), GAD-7 (P < 0.001), and fatigue (P < 0.001), but a lower score on QoL (P < 0.001) (Table 1). ANCOVA results indicated that caregivers with insomnia had a poorer global QoL (F (1,101) = 82.276, P < 0.001) compared to those without insomnia. Binary logistic regression analysis revealed that more severe depressive (OR = 1.185; P < 0.001) and anxiety symptoms (OR = 1.099; P = 0.003) and fatigue (OR = 1.320; P < 0.001) were significantly associated with higher risk of insomnia (Table 2).
Network structure of insomnia symptoms
Figure 2 presents the original network structure of insomnia symptoms and the adjusted network model after controlling for age among caregivers of psychiatric inpatients during the COVID-19 pandemic. Figure 3 shows that the top three nodes with the highest centrality were ISI2 (“Sleep maintenance”), ISI7 (“Distress caused by the sleep difficulties”) and ISI1 (“Severity of sleep onset”) in both networks. The mean predictability in the original insomnia network was 0.654, indicating that there was an average of 65.4% of the variance in each node that could be accounted for by its neighboring nodes in the model. Table S1 shows the descriptive information of insomnia symptoms in the original and adjusted network models. The similarity tests showed that the EI generated from the original and adjusted insomnia network models were highly correlated (r = 0.99, P < 0.01). Furthermore, the NCT results did not show any significant differences in the network structure (M = 0.227, P = 0.08), global strength (S = 0.052, P = 0.677) and nodes centrality test (C=-0.032, P = 0.09) between the male and female network models of insomnia symptoms. The flow network (Fig. 4) shows that the edge between ISI4 (“Sleep dissatisfaction”; average edge weight=-0.18) and QoL is the thickest one marked in red, indicating that ISI4 (“Sleep dissatisfaction”) had the strongest negative association with QoL, followed by the ISI7 (“Distress caused by the sleep difficulties”; average edge weight=-0.12) and ISI5 (“Interference with daytime functioning”; average edge weight=-0.07). The details of the edge weights of these symptoms are shown in Table S2. Figure S1 presents the network stability based on the case-dropping bootstrap procedure. The CS-C for EI was 0.75, suggesting sufficient stability. For network accuracy, a narrow range was shown in the bootstrap 95% CIs for the estimated edge weights (Figure S2), and most of them were non-zero, indicating that most edges were stable and accurate. The bootstrapped difference tests for edge weights in Figure S3 show that most comparisons among edge weights were statistically significant, indicating a reliable network model.
Discussion
To the best of our knowledge, this was the first study to explore the prevalence and network structure of insomnia among caregivers of psychiatric inpatients during the COVID-19 pandemic. Of the participants, 18.9% suffered from insomnia (ISI total score ≥ 8; 95% CI: 16.7–21.3%), which is higher than the corresponding figure in the general population reported in previous studies in China both before (15.0%; 95%CI: 12.1–18.5%) [44] and during the COVID-19 pandemic (16.5%; 95% CI: 8.4–29.7%) [45]. However, another population survey conducted in 125 cities in mainland China during COVID-19 pandemic found a higher rate (24.7%) than our study [46]. There might be common COVID-19 related factors associated with insomnia among the caregivers, such as the fear of contracting the infection, being quarantined, and financial burden [45]. Psychiatric inpatients were at higher risk of infection compared to the general population due to several reasons: psychiatric hospitals have closed wards with restricted space and poor quality of air; the use of antipsychotics is associated with reduced ability to eliminate pathogenic bacteria in the respiratory tract; and psychiatric inpatients who are mentally unwell often lack the capacity to practice self-protection measures [47]. Additionally, the strict infection prevention policies of hospitals to minimize hospital transmission substantially restricted the caregivers’ access to patients or ability to provide caregiving, which might aggravate their anxiety and uncertainty about the patients [47]. Interestingly, the insomnia rate in our study was much lower than the findings using the same assessment scale and cutoff value in caregivers living with family members with disabilities in the same household (69.9%) [21], hospice family caregivers (49.1%) [48] and caregivers of children with kidney diseases (38.5%) [49]. It is likely that the different types and severity of diseases were associated with different levels of the distress among the caregivers [50].
In both original and adjusted network models of insomnia symptoms, “Sleep maintenance” (ISI2), “Distress caused by the sleep difficulties” (ISI7), and “Severity of sleep onset” (ISI1) were the most influential symptoms. As both network models were highly similar and no gender differences in the network model of insomnia symptoms were found, this suggests that the insomnia symptoms network was stable and not affected by age or gender. Nocturnal symptoms (e.g., ISI1, ISI2, ISI3) appeared to be dominant in this network model. Inpatients with psychiatric disorders often have sleep problems and even an increased risk of suicide at night time [51], and as such, due to increased vigilance, this would more likely cause difficulty for their caregivers in getting to sleep or maintaining sleep at night [52]. Previous studies found that caring for patients at night was a major cause of sleep deprivation and poor sleep quality for caregivers [52]. This finding however was inconsistent with the insomnia network model of mental health professionals [28] that found “Interference with daytime functioning” (ISI5), “Sleep maintenance” (ISI2), “Noticeability of sleep problems by others” (ISI6), and daytime insomnia symptoms (ISI5, ISI6, ISI7) as influential symptoms, probably due to the working shifts of mental health professionals. The discrepancy between different insomnia network models indicates that the interventions for insomnia symptoms should be individualized for different populations in need. However, this study did not find any clear association between caregivers’ insomnia and the psychiatric diagnoses of patients they cared for in terms of insomnia prevalence and the network structures of insomnia symptoms.
Comorbid psychiatric problems were found to be common in persons with insomnia [53, 54]. Similarly, in this study, more severe depression and anxiety were significantly associated with a higher risk of insomnia. A previous study found that worry about the night-time activities of inpatients could lead to anxiety among the caregivers [52], and if the anxiety and stress continued, the risk of sleep problems might significantly increase. In addition, the bidirectional relationships between insomnia and depression and anxiety have been well documented [53]. On one hand, depression and anxiety could act as risk factors for insomnia; on the other hand, insomnia might manifest as a symptom of depression and anxiety [54]. Further, stressful life events could trigger the development of these problems with insomnia often manifesting first and continue to be persistent [54].
Fatigue was also a significant factor of insomnia among caregivers of psychiatric inpatients. Previous studies found that people suffering from insomnia were more likely to suffer from fatigue than those with any other sleep problems [55, 56]. Fatigue is defined as subjective feelings ranging from tiredness to exhaustion and accompanied by interference with daily functioning [57]. From a psychological perspective, it is associated with stress and other strong emotional experiences that may coexist with depression and anxiety disorders [57]. Caring for patients with severe psychiatric symptoms may lead to high levels of physical, mental and work fatigue among their caregivers [58]. A previous study conducted among insomnia patients found that the relationship between insomnia and fatigue was moderated by comorbid depression [55]. Therefore, it may be that preventing or treating depression can improve fatigue in patients with insomnia.
Consistently, in this study we found that caregivers with insomnia had lower QoL compared to those without insomnia. Insomnia could result in both physical and psychiatric distress [59], which are risk factors of low QoL [60]. In addition, “Sleep dissatisfaction” (ISI4), “Distress caused by the sleep difficulties” (ISI7) and “Interference with daytime functioning” (ISI5) had the strongest negative associations with QoL among caregivers of psychiatric inpatients, which is partly consistent with previous findings in bipolar patients [59]. Disturbed sleep patterns (e.g., insufficient or excessive sleep) were associated with physical and mental health problems such as cardiovascular diseases, diabetes, obesity, depression, suicidal behavior and mortality [61]. Previous studies found that the distress caused by insomnia such as anxiety, depression, irritable feelings and tiredness could lower QoL [62]. Furthermore, persons with insomnia might experience daytime sleepiness and decreased productivity, which could impair social and occupational domains and lower QoL [63]. Targeting these individual symptoms may be beneficial to improve the QoL among caregivers of psychiatric inpatients.
The strengths of this study included the multicenter study design, a large sample size, and use of a novel, sophisticated statistical approach at the symptom level from the perspective of network analysis. However, several limitations should be noted. First, the cross-sectional study design could not ascertain the causal relationships between variables. Second, for logistical reasons, consecutive sampling methods were used, which may limit the representativeness of the study sample. Third, the assessment was based on self-report, therefore, recall bias might occur. Finally, for logistical reasons, the history of psychiatric disorders of caregivers could not be verified as this study based on self-report.
In conclusion, insomnia was common among caregivers of psychiatric inpatients during the COVID-19 pandemic, particularly in those with depression, anxiety and fatigue. Considering the negative impact of insomnia on QoL, regular screening and effective interventions for insomnia should be developed for caregivers of psychiatric inpatients, particularly targeting the central symptoms identified in this study.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Awad AG, Voruganti LNP. The Burden of Schizophrenia on Caregivers. PharmacoEconomics. 2008;26(2):149–62.
Onwumere J, Kuipers E, Wildman E, Mason A, Stahl D. Caregiver wellbeing during Covid-19: does being hopeful play a role? J Affect Disord Rep. 2021;6:100239.
Ricotta DN, Parris JJ, Parris RS, Sontag DN, Bioethics M, Mukamal KJ. The Burden of Guardianship: a matched cohort study. J Hosp Med. 2018;13(9):595–601.
Fadden G, Bebbington P, Kuipers L. The burden of care: the impact of functional psychiatric illness on the patient’s family. Br J Psychiatry. 1987;150(3):285–92.
Gianfrancesco FD, Wang R-h, Yu E. Effects of patients with bipolar, schizophrenic, and major depressive disorders on the mental and other healthcare expenses of family members. Soc Sci Med. 2005;61(2):305–11.
Khatimah CH, Adami A, Abdullah A, Marthoenis. Quality of life, mental health, and family functioning of schizophrenia caregivers: a community-based cross-sectional study. Asia-Pacific Psychiatry. 2022;14(1):e12467.
Peng MM, Xing J, Tang X, Wu Q, Wei D, Ran MS. Disease-related risk factors for caregiver burden among family caregivers of persons with schizophrenia: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19(3):1862.
Eckardt JP. Caregivers of people with severe mental illness in the COVID-19 pandemic. The Lancet Psychiatry. 2020;7:e53–3.
Hao F, Tan W, Jiang L, Zhang L, Zhao X, Zou Y, Hu Y, Luo X, Jiang X, McIntyre RS, et al. Do psychiatric patients experience more psychiatric symptoms during COVID-19 pandemic and lockdown? A case-control study with service and research implications for immunopsychiatry. Brain Behav Immun. 2020;87:100–6.
Collier E, Skitt G, Cutts H. A study on the experience of insomnia in a psychiatric inpatient population. J Psychiatr Ment Health Nurs. 2003;10(6):697–704.
Weissman MM, Greenwald S, Niño-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19(4):245–50.
McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11(2):143–53.
Hopps M, Iadeluca L, McDonald M, Makinson GT. The burden of family caregiving in the United States: work productivity, health care resource utilization, and mental health among employed adults. J Multidiscip Healthc. 2017;10:437–44.
Peng HL, Chang YP. Sleep disturbance in family caregivers of individuals with dementia: a review of the literature. Perspect Psychiatr Care. 2013;49(2):135–146.
Kotronoulas G, Wengstrom Y, Kearney N. Sleep patterns and sleep-impairing factors of persons providing informal care for people with cancer: a critical review of the literature. Cancer Nurs 2013;36(1):E1–E15.
Byun E, Lerdal A, Gay CL, Lee KA. How adult caregiving impacts sleep: a systematic review. Curr Sleep Med Rep. 2016;2(4):191–205.
Goldstein TR, Miklowitz DJ, Richards JA. Expressed emotion attitudes and individual psychopathology among the relatives of bipolar patients. Fam Process. 2002;41(4):645–57.
Leysen L, Lahousse A, Nijs J, Adriaenssens N, Mairesse O, Ivakhnov S, Bilterys T, Van Looveren E, Pas R, Beckwée D. Prevalence and risk factors of sleep disturbances in breast cancersurvivors: systematic review and meta-analyses. Support Care Cancer. 2019;27(12):4401–33.
Lazzari C, Shoka A, Nusair A, Rabottini M. Psychiatry in time of COVID-19 pandemic. Psychiatria Danubina. 2020;32(2):229–35.
Willner P, Rose J, Stenfert Kroese B, Murphy GH, Langdon PE, Clifford C, Hutchings H, Watkins A, Hiles S, Cooper V. Effect of the COVID-19 pandemic on the mental health of carers of people with intellectual disabilities. J Appl Res Intellect Disabil. 2020;33(6):1523–33.
Fusar-Poli L, Surace T, Meo V, Patania F, Avanzato C, Pulvirenti A, Aguglia E, Signorelli MS. Psychological well-being and family distress of italian caregivers during the COVID-19 outbreak. J Community Psychol. 2022;50(5):2243–59.
Ishak WW, Bagot K, Thomas S, Magakian N, Bedwani D, Larson D, Brownstein A, Zaky C. Quality of life in patients suffering from insomnia. Innovations in Clinical Neuroscience. 2012;9(10):13.
Jermann F, Perroud N, Favre S, Aubry J-M, Richard-Lepouriel H. Quality of life and subjective sleep-related measures in bipolar disorder and major depressive disorder. Qual Life Res 2021:1–8.
Morin CM. Insomnia: psychological assessment and management. Guilford press; 1993.
Borsboom D, Cramer AO. Network analysis: an integrative approach to the structure of psychopathology. Ann Rev Clin Psychol. 2013;9(1):91–121.
Bai W, Zhao Y-J, Cai H, Sha S, Zhang Q, Lei S-M, et al. Network analysis of depression, anxiety, insomnia and quality of life among Macau residents during the COVID-19 pandemic. J Affect Disord. 2022;311:181–188.
Cha EJ, Jeon HJ, Chung S. Central symptoms of Insomnia in Relation to Depression and COVID-19 anxiety in General Population: A Network Analysis. J Clin Med. 2022;11(12):3416.
Bai W, Zhao Y, An F, Zhang Q, Sha S, Cheung T, Cheng CP-W, Ng CH, Xiang Y-T. Network analysis of insomnia in chinese mental health professionals during the covid-19 pandemic: a cross-sectional study. Nat Sci Sleep. 2021;13:1921.
Schulte T, Hofmeister D, Mehnert-Theuerkauf A, Hartung T, Hinz A. Assessment of sleep problems with the Insomnia Severity Index (ISI) and the sleep item of the Patient Health Questionnaire (PHQ-9) in cancer patients. Support Care Cancer. 2021;29(12):7377–84.
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13.
Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7.
Butt Z, Wagner LI, Beaumont JL, Paice JA, Peterman AH, Shevrin D, Von Roenn JH, Carro G, Straus JL, Muir JC, et al. Use of a single-item Screening Tool to detect clinically significant fatigue, Pain, Distress, and Anorexia in Ambulatory Cancer Practice. J Pain Symptom Manag. 2008;35(1):20–30.
Cheung YB, Yeo KK, Chong KJ, Khoo EYH, Wee HL. Measurement equivalence of the English, Chinese and malay versions of the World Health Organization quality of life (WHOQOL-BREF) questionnaires. Health Qual Life Outcomes. 2019;17(1):1–6.
R. : a language and environment for statistical computing. R Foundation for Statistical Computing. [https://www.r-project.org/].
Epskamp S, Borsboom D, Fried EI. Estimating psychological networks and their accuracy: a tutorial paper. Behav Res Methods. 2018;50(1):195–212.
Paine S-J, Gander PH, Harris R, Reid P. Who reports insomnia? Relationships with age, sex, ethnicity, and socioeconomic deprivation. Sleep. 2004;27(6):1163–9.
Epskamp S, Fried EI. Package ‘bootnet’. R package version 1.5.5. 2023. https://CRAN.R-project.org/package=bootnet
Epskamp S, Cramer AO, Waldorp LJ, Schmittmann VD, Borsboom D. qgraph: Network visualizations of relationships in psychometric data. J Stat Softw. 2012;48:1–18.
Gómez-Rubio V. ggplot2-elegant graphics for data analysis. J Stat Softw. 2017;77:1–3.
Robinaugh DJ, Millner AJ, McNally RJ. Identifying highly influential nodes in the complicated grief network. J Abnorm Psychol. 2016;125(6):747–57.
Haslbeck J, Waldorp LJ. How well do network models predict observations? On the importance of predictability in network models. Behav Res Methods. 2018;50(2):853–61.
Li W, Zhao Y-J, Zhang S-F, Yang B, Cheung T, Jackson T, Sha S, Xiang Y-T. Mapping post-traumatic stress disorder symptoms and quality of life among residents of Wuhan, China after the COVID-19 outbreak: a network perspective. J Affect Disord. 2022;318:80–7.
Van Borkulo CD, van Bork R, Boschloo L, Kossakowski JJ, Tio P, Schoevers RA, et al. Comparing network structures on three aspects: a permutation test. Psychol Methods. 2022:1–42.
Cao X-L, Wang S-B, Zhong B-L, Zhang L, Ungvari GS, Ng CH, Li L, Chiu HFK, Lok GKI, Lu J-P, et al. The prevalence of insomnia in the general population in China: a meta-analysis. PLoS ONE. 2017;12(2):e0170772.
Cénat JM, Blais-Rochette C, Kokou-Kpolou CK, Noorishad P-G, Mukunzi JN, McIntee S-E, Dalexis RD, Goulet M-A, Labelle PR. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. 2021;295:113599.
Huang Y, Wang Y, Zeng L, Yang J, Song X, Rao W, et al. Prevalence and correlation of anxiety, insomnia and somatic symptoms in a Chinese population during the COVID-19 epidemic. Front Psychiatry. 2020;11:568329.
Yang M, Wang H, Li Z, Zhang Q, Liu X, He M, Gao S. Prevention and control of COVID-19 infection in a Chinese Mental Health Center. Front Med. 2020;7:356.
Starr LT, Washington K, McPhillips MV, Pitzer K, Demiris G, Oliver DP. Insomnia symptoms among Hospice Family Caregivers: Prevalence and Association with Caregiver Mental and Physical Health, Quality of Life, and Caregiver Burden. Am J Hosp Palliat Care 2022:10499091221105882.
Sharma R, Jafra BS, Tiewsoh K, Kumar K, Kaur N, Sharawat IK, Dawman L. Distress, anxiety, and its correlates among caregivers of children with kidney diseases during COVID-19 pandemic lockdown. Archives de Pédiatrie. 2022;29(3):243–8.
Guan Z, Wang Y, Lam L, Cross W, Wiley JA, Huang C, Bai X, Sun M, Tang S. Severity of illness and distress in caregivers of patients with schizophrenia: do internalized stigma and caregiving burden mediate the relationship? J Adv Nurs. 2021;77(3):1258–70.
Perlis ML, Grandner MA, Brown GK, Basner M, Chakravorty S, Morales KH, Gehrman PR, Chaudhary NS, Thase ME, Dinges DF. Nocturnal wakefulness as a previously unrecognized risk factor for suicide. J Clin Psychiatry. 2016;77(6):12828.
Liu S, Li C, Shi Z, Wang X, Zhou Y, Liu S, Liu J, Yu T, Ji Y. Caregiver burden and prevalence of depression, anxiety and sleep disturbances in Alzheimer’s disease caregivers in China. J Clin Nurs. 2017;26(9–10):1291–300.
Jansson-Fröjmark M, Lindblom K. A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res. 2008;64(4):443–9.
Vargas I, Perlis ML. Insomnia and depression: clinical associations and possible mechanistic links. Curr Opin Psychol. 2020;34:95–9.
Kim SJ, Kim S, Jeon S, Leary EB, Barwick F, Mignot E. Factors associated with fatigue in patients with insomnia. J Psychiatr Res. 2019;117:24–30.
Lichstein KL, Means MK, Noe SL, Aguillard R. Fatigue and sleep disorders. Behav Res Ther. 1997;35(8):733–40.
Aaronson LS, Teel CS, Cassmeyer V, Neuberger GB, Pallikkathayil L, Pierce J, Press AN, Williams PD, Wingate A. Defining and measuring fatigue. Image: The Journal of Nursing Scholarship. 1999;31(1):45–50.
Sfeir M, Zeitoun A, Hallit S, Obeid S. Presence of a psychiatric patient at home and work fatigue in family caregivers: the moderating effect of spirituality. Perspect Psychiatr Care. 2022;58(4):2664–75.
De la Fuente-Tomás L, Sierra P, Sanchez-Autet M, García-Blanco A, Safont G, Arranz B, García-Portilla MP. Sleep disturbances, functioning, and quality of life in euthymic patients with bipolar disorder. Psychiatry Res. 2018;269:501–7.
Middleton JW, Simpson GK, De Wolf A, Quirk R, Descallar J, Cameron ID. Psychological distress, quality of life, and burden in caregivers during community reintegration after spinal cord injury. Arch Phys Med Rehabil. 2014;95(7):1312–9.
Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthc (Basel). 2018;7(1):1.
Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM. Relations between sleep, fatigue, and health-related quality of life in individuals with insomnia. J Psychosom Res. 2010;69(5):475–83.
Bolge SC, Doan JF, Kannan H, Baran RW. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18(4):415–22.
Acknowledgements
The authors are grateful to all participants and clinicians involved in this study.
Funding
The study was supported by the National Science and Technology Major Project for investigational new drug (2018ZX09201-014), the Beijing Hospitals Authority Clinical Medicine Development of special funding support (XMLX202128), and the University of Macau (MYRG2019-00066-FHS; MYRG2022-00187-FHS).
Author information
Authors and Affiliations
Contributions
Study design: Feng-Rong An, Yan-Jie Zhao, Qinge Zhang, Yu-Tao Xiang. Data collection, analysis and interpretation: Pan Chen, Yan-Jie Zhao, Feng-Rong An, Mei Ieng Lam, Ka-In Lok, Yue-Ying Wang, Jia-Xin Li, Zhaohui Su, Teris Cheung, Gabor S. Ungvari. Drafting of the manuscript: Pan Chen, Yu-Tao Xiang. Critical revision of the manuscript: Chee H. Ng. Approval of the final version for publication: all co-authors. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations. Approval of the study was granted by the Institutional Review Board of Beijing Anding hospital and respective hospitals. Informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, P., Zhao, YJ., An, FR. et al. Prevalence of insomnia and its association with quality of life in caregivers of psychiatric inpatients during the COVID-19 pandemic: a network analysis. BMC Psychiatry 23, 837 (2023). https://doi.org/10.1186/s12888-023-05194-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-023-05194-w