Abstract
Background
Both augmented inflammatory reaction and low vitamin D status are associated with depression but the magnitude of their relationships is unclear. This study was, therefore, conducted to evaluate the effects of vitamin D supplementation on serum 25(OH)D concentration, depression severity and some pro-inflammatory biomarkers in patients with mild to moderate depression.
Methods
An 8-week double-blind randomized clinical trial (RCT) was performed on 56 (18–60 yrs) patients with mild to moderate depression, randomly assigned to intervention (50,000 IU cholecalciferol 2wks−1) and control (placebo) groups. Serum 25(OH)D, intact parathyroid hormone (iPTH), interlukin (IL)-1β, IL-6, high-sensitivity C-reactive protein (hs-CRP) and depression severity (Beck Depression Inventory-II) (BDI-II)) were initially and finally assessed.
Results
At the end point, statistically significant changes were observed only in intervention group as compared with controls including increased 25(OH)D concentration (+ 40.83 ± 28.57 vs. + 5.14 ± 23.44 nmol L−1, P < 0.001) and decreased depression severity (-11.75 ± 6.40 vs. -3.61 ± 10.40, P = 0.003). No significant within- or between group differences were observed in serum IL-1β, IL-6 and hs-CRP concentrations.
Conclusion
Increased circulating 25(OH)D concentrations following 8-week vitamin D supplementation (50,000 IU 2wks−1) resulted in a significant decrease in BDI-II scores in patients with mild to moderate depression. However, this effect was independent of the serum concentrations of the studied inflammatory biomarkers.
Trial registration
The clinical trial registration code was obtained from the Iranian Registry of Clinical Trials (date of registration: 17/09/2018, registration number: IRCT20170926036425N1) and ClinicalTrials.gov (date of registration: 04/12/2018, registration number: NCT03766074)
Similar content being viewed by others
Introduction
Background and objectives
Depression has been, and continues to be, a serious threat to the people’s mental health with an accelerated occurrence rate due to the newly emerged coronavirus disease (COVID-19) pandemic [1]. Due to the worldwide condition of home-quarantine, economic problems [1, 2] and side effects of antidepressants [3], finding simple effective practical strategies to control depression seems necessary [4]. Hence, the importance of finding the relationship between factors involved in depression is self-evident [5]. Depression is proposed the result of disorders in inter-neurons signaling that could cause difficulties in connection of different parts of brain via imbalance of stimulating and inhibitory neurons interactions [6]. Recently, an interesting theory suggests that increasing in intracellular calcium+2 (Ca+2) is one of main causes of depression at cellular level [6]. Despite progression in discovering mechanisms involved in depression such as inflammation, hypothalamic–pituitary–adrenal (HPA) axis and vitamin D [7, 8], the magnitude of their relationships is still unclear. In this context, the importance of inflammation is such that some researchers consider it as the main cause of depression [9]. It is believed that even psychosocial factors and bitter events of life, first cause an increase in pro-inflammatory biomarkers such as IL-1β, IL-6 and hs-CRP which is then followed by depression [10,11,12]. Study on people exposed to chronic inflammation has shown a two-way relationship between inflammation and depression [6]. This means that depression due to changes in function of parts of brain that are in relation with mood including hippocampus and hypothalamus could increase pro-inflammatory cytokines [6]. On the other hand, it has been suggested that increasing of pro-inflammatory cytokines including IL-1β, IL-6 and hs-CRP elevates function of mitochondria and reactive oxygen species (ROS) that by affecting function of neurons could cause depression [6].
IL-1β with T cell-stimulating ability plays a role in structure and function of neurons and their immunity responses [13]. Recently, it has been shown that IL-1β is associated with depression [6, 14] but the magnitude of this association and its relationship with other involved factors in depression needs more clarification.
IL-6, a pleiotropic cytokine, plays regulatory roles in immunity responses, acute phase reactions and in differentiation of some neurons, as well [13, 14]. As an inflammatory mediator, upregulation of IL-6 has been associated with several human diseases including obesity, inflammatory bowel disease, certain types of malignancies and ocular inflammation [15,16,17,18]. Some studies have suggested a role for IL-6 in development of depression. Even an experimental study reported resistance to stress-induced depression behavior in IL-6 knockout mice [19]. Nevertheless, the underlying mechanisms of IL-6 function and associations with other possible involved factors in depression are still unknown.
The association of C-reactive protein (CRP), another inflammatory biomarker, with depression has also been investigated. Recent studies reported elevated serum CRP concentrations in the subjects with major depressive disorder (MDD) [20, 21] and especially in those who were resistant to treatment [20]. Though the augmented inflammatory reaction in MDD might be due to smoking and dietary habits, the association between CRP and MDD remained significant even after complete adjustment for all confounders [21]. It is still unknown whether the association between inflammation and depression has a pathophysiological basis [20, 21] or it is due to some overlooked psychosocial and/or clinical confounders [21].
Regarding the repeatedly documented association of diet and inflammation [22], dietary approaches have been considered to ameliorate depression through targeting specific pathways involved in inflammation including oxidative stress, HPA and obesity, among the others [23].
One of the most studied dietary factors in relation to mental disorders is vitamin D. Exploration of vitamin D receptor (VDR) in the central nervous system (CNS) opened new insights into the so-called “non-calcemic” functions of this secosteroid. New studies indicate a wide spectrum of activities for vitamin D in CNS including energy hemostasis through central renin-angiotensin system (RAS) [24], protective effect against autoimmunity through attenuation of micoglia activation via neuron-specific signaling [25] and overall mental health in both children and adults [26, 27]. The anti-inflammatory and anti-oxidant properties of vitamin D have already been documented in other clinical settings [28,29,30,31], but assessment of these effects in relation to depression needs more studies. We have recently reported the effect of vitamin D supplementation on depression and certain neurotransmitters [32]. In this piece of work, we examined the associations among vitamin D status, depression and inflammation in an RCT.
Methods
Study design
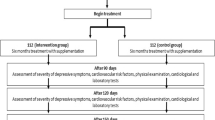
This eight-week double-blind RCT was conducted on subjects with mild to moderate depression referred to the outpatient clinics of Baharloo Hospital, between May 2018 and June 2019, Tehran, Iran. The participants had no other psychiatric disease. The detailed protocol of this study can be found elsewhere [33].
A sample size of 56 subjects was calculated through considering an effect size of 0.75 and a power of 80% based on previous study [34] according to the following formula, with 28 patients randomly assigned to each group following simple randomization procedures (according to entrance code) by head of the project [33].
The inclusion criteria were [1] 18–60 years of age and [2] having mild to moderate depression with no other psychiatric disease, as confirmed by the psychiatrist. We enrolled both incident and old cases of depression.
The non-inclusion criteria were: [1] having a history of heart infarction, angina pectoris, stroke, kidney stones, high blood pressure, liver disease, and hyperparathyroidism, [2] pregnancy and/or lactation, [3] reproductive-aged women (under 50 years old) not receiving adequate contraception, [4] consuming nutritional supplements containing vitamin D from two months prior to the intervention. Exclusion criteria were: [1] lack of willingness to continue the study and [2] failure to follow the interventional program. More details were described in protocol of the study [33].
After describing the protocol and objectives of the study to the registered volunteers, further evaluations were done on enrolled participants by psychiatrists including determination of depression severity using BDI-II questionnaire at the baseline and after the intervention. Following obtaining written informed consent, the participants were randomly allocated to either intervention or control group and received either 50,000 IU cholecalciferol 2wks−1 or placebo, respectively. Vitamin D3 supplements and placebos (made from oral paraffin) were purchased from Zahravi Pharmaceutical Company (Iran). Safety considerations to select a safe dose of vitamin D supplements were based on upper tolerable intake level of vitamin D for adults (4,000 IU day−1) [35], and the results pertaining to previous studies with higher doses of vitamin D (50,000 IU wk−1) [36]. All participants had three visits (weeks 0, 4 and 8). On the first visit, general socio-demographic data including medical history, exposure to sunlight, sunscreen use, tobacco and drug use habits and alcohol consumption were obtained during a face to face interview and completing a questionnaire. The physical activity level (PAL) was assessed using a modified questionnaire of “Intensity and Effects of Various Activities on Physical Activity level in Adults” [35]. Blood pressure and anthropometric measures were also taken. All assessments were performed at the beginning and end of the study. Subjects were instructed to stick to their usual diet, PAL, and medications during the intervention period, as prescribed by their physician.
Subjects were received their pills (vitamin D3 or placebo) in the first and second visits. Supplements and placebos were completely similar in appearance and packaging. None of the researchers or patients were aware of the treatment assignments except head of the project. Blinding in current study was done by writing the entrance code of subjects on the package of pills. All participants received a reminder call and a written "instruction of use" to consume the remaining pills. They were also asked to return the pills that were not consumed for any reason in their next visit. Therefore, the adherence assessment was carried out based on pill count and patient self-report. Summary of the study design has been shown in Fig. 1.
Assessment of depression
Assessment of depression was carried out according to the psychiatrist's assessments through the structural clinical diagnostic interview based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM–IV) criteria and BDI-II score. It is noteworthy that accuracy and reliability of BDI-II questionnaire [37], as well as, translation, cultural adaptation, and validation of its Persian version [38] have been proven before. In this study, mild to moderate depression was defined as a BDI-II score ranging from 13 to 29 [37].
Anthropometric measures
Anthropometric assessments including weight, height, waist circumference (WC) and hip circumference (HC) were done. Body mass index (BMI) and waist to hip ratio (WHR) were calculated by dividing weight (kg) by height2 (m2) and WHR by dividing WC to HC, respectively. More details were described elsewhere [38].
Blood pressure
Systolic and diastolic blood pressures (SBP and DBP) were measured in sitting position after 10 min resting using digital sphygmomanometer (BC 08; Beurer, Ulm, Germany).
Laboratory investigations
Briefly, a 10-ml venous blood sample was collected from all participants and stored on ice in cold boxes for transportation to the Laboratory of Nutrition Research, National Nutrition and Food Technology Research Institute (NNFTRI). Sera were separated from the clot samples by centrifugation at room temperature and then aliquoted in fresh microtubes and stored at -80 °C until use. In this study, enzyme immunoassay (EIA) method was employed for assessing all biochemical parameters as follows: serum 25(OH)D (Euroimmun EIA kit, Lubeck, Germany), iPTH (Euroimmun EIA kit, Lubeck, Germany), hs-CRP (Zellbio EIA kit, Ulm, Germany), IL-1β (Diaclone EIA kit, Besancon, France) and IL-6 (IBL EIA kit, Hamburg, Germany). Details of laboratory methods were described previously [33].
Classification of vitamin D status
Vitamin D status was classified based on circulating concentration of 25(OH)D as deficiency (< 50 nmol l−1), insufficiency (50–75 nmol l−1) and sufficiency (> 75 nmol l−1) [39].
Outcomes
The primary outcome was the significant elevation of serum 25(OH)D concentration from baseline until the end of intervention. Secondary outcomes included significant changes in serum pro-inflammatory biomarkers including IL-1β, IL-6 and hs-CRP. Other secondary outcomes were significant changes in serum iPTH and depression severity (BDI-II score) from baseline to the end of the eight-week intervention. More details were described elsewhere [33].
Statistical analyses
Quantitative and qualitative data were expressed as mean ± standard deviation (SD) and absolute or relative frequencies, respectively. The normality of data distribution was assessed by Shapiro–Wilk's test. To compare qualitative variables between the groups at baseline Chi-square test was used. Based on the study design, two groups were investigated within two time periods (before and after intervention); thus, paired-sample t-test or Wilcoxon test (based on the normal or non-normal distribution of data, respectively) was utilized to compare within-group changes and independent sample t-test or Mann–Whitney test (based on the normal or non-normal distribution of data) was used to compare between-group changes. The significance level was P < 0.05. Data were analyzed using Statistical Package for Social Sciences (SPSS) software v.21 (SPSS Inc., Chicago, IL, USA). Analysis was done by intention to treat. Further details were previously described [32].
Ethics
Ethical approval was obtained from the Ethics Committee of NNFTRI (IR.SBMU.NNFTRI.REC.1396.185) and all methods were performed in accordance with the relevant guidelines and regulations (NNFTRI). The clinical trial registration code was obtained from the Iranian Registry of Clinical Trials (IRCT20170926036425N1) and ClinicalTrials.gov (NCT03766074). Written informed consent was obtained from all participants or their next of kin/legally authorized representative (according to educational level) before entering the research, as well.
Results
Out of the 719 registered volunteers, 69 participants (56 incident and 13 old cases) were enrolled in the study, with almost equal number of old cases in each group (6 in intervention and 7 in control group). However, 13 subjects were excluded due to meeting exclusion criteria. Consequently, 56 subjects (intervention n = 28, control n = 28) completed the study. Participants were 50 women (89.29%) and 6 men (10.71%) aged 43.0 ± 1.15 yrs. Estimated adherence of the participants to the study protocol was approximately 100%. There was no complain about adverse drug reactions and no report of suicide attempt.
General characteristics and other parameters of the study groups had no statistically significant differences at the baseline (Tables 1 and 2). Furthermore, there were no significant within- and between-group differences in the final values of anthropometric indices, SBP, and DBP.
Following the intervention, the rise in 25(OH)D concentration was significantly higher in the intervention group compared with the control group (+ 40.83 ± 28.57 vs. + 5.14 ± 23.44 nmol L−1, P < 0.001) (Table 2). Vitamin D status of both study groups had no significant difference at the beginning of the study (Table 1); however, after intervention it was significantly improved only in the intervention group compared with control one (0 (0), 1 (3.6), 27 (96.4) vs. 4 (14.3), 7 (25), 17 (60.7) as deficiency, insufficiency and sufficiency, respectively, n (%), P = 0.005)).
Final serum iPTH concentrations of both study groups had no statistical difference. However, there was a significant within-group increase just in the control group (+ 5.10 ± 6.002 pg mL−1, P < 0.001) (Table 2).
After intervention a significant reduction was observed in BDI-II scores of intervention group compared to baseline (-11.75 ± 6.40, P < 0.001) and this reduction was significant compared to the control group, as well (-11.75 ± 6.40 vs. -3.61 ± 10.40, P = 0.003); however, reduction of the BDI-II score of control group showed no significant changes compared to baseline (Table 2).
There was no significant within- or between-group difference of the initial and final serum concentrations of IL-1β, IL-6 and hs-CRP (Table 2).
Discussion
We found that expectedly increased circulating 25(OH)D concentrations following eight weeks supplementation in patients with mild to moderate depression was accompanied by a significant recuperation of depression severity as compared with the control group. However, this intervention did not significantly affect the inflammatory status, as judged by serum IL-1β, IL-6 and hs-CRP concentrations.
Similar to the present study, previous studies have reported significant improvement in depression severity following vitamin D supplementation [36, 40,41,42,43,44,45,46]. Recently some regulating roles on mood were attributed to vitamin D due to production of its active form in brain and also gene expression of VDRs especially in areas in relation with mood and social behaviors [6, 47,48,49,50,51,52]. Considering recent theory about increasing intra-neuron Ca+2 concentration as a responsible factor for disturbing balance between inhibitory and stimulating neurons [6], it has been suggested that vitamin D may have regulating roles on intra-neuron Ca+2 concentrations due to its gene-dependent function [50, 53, 54]. Vitamin D is likely to decrease Ca+2 signaling through several mechanisms including: (i) up-regulation of calbandin and parvalbumin genes expression whereby converting Ca+2 to its buffer forms [9]; (ii) up-regulation of Ca+2 pump (PMCA) and Na+/Ca+2 exchanger (NCX1) in plasma membrane and hence egression of extra Ca+2; and (iii) down-regulation of L-type Ca+2 channels (CaV1.3 & CaV1.2) of neurons of hippocampus and cortex [9]. However, some studies failed to show significant improvement in depression severity after vitamin D supplementation [34, 55,56,57]. These controversies may be attributed to design of the studies, initial serum 25(OH)D concentrations of the participants, dose and type of vitamin D supplement (D2 vs. D3), intervention duration, method of supplementation, age of the target group, existence of other comorbidities and additional simultaneous interventions. Among these, baseline circulating concentrations of 25(OH)D as well as dose and type of vitamin D supplement are of particular importance [55]. It is noteworthy that most participants of the present study had optimal levels of serum 25(OH)D at the baseline. Nevertheless, vitamin D supplementation was still efficacious in lessening depression severity. It is likely that “more than adequate” vitamin D should be targeted in patients with depression.
In the current study, eight weeks supplementation with vitamin D was not able to significantly change serum concentrations of pro-inflammatory biomarkers including IL-1β, IL-6 and hs-CRP in the subjects with mild to moderate depression. The results of some previous observational studies showed an inverse association between circulating concentrations of inflammatory mediators (IL-6 and hs-CRP) and 25(OH)D in subjects with depression [8, 58]. However, the results of RCTs aimed to investigate the effects of vitamin D on depression severity and pro-inflammatory biomarkers have been controversial. In agreement with our results, an interventional study showed that supplementation with vitamin D (50,000 IU 2wks−1) for eight weeks did not decrease serum IL-6 [59] and hs-CRP concentrations in adult subjects with depression [60]. In contrast, some investigations reported a significant suppressive effect of 12 weeks vitamin D supplementation (50,000 IU 2wks−1) on serum hs-CRP in depressed patients as compared with control group [43, 61].
Since inflammation can affect the neuronal functions responsible for depression in several ways, anti-inflammatory factors such as vitamin D may play a role in controlling inflammation and hence depression severity through various mechanisms [9]. It should be noted that inflammation elevates the function of mitochondria and production of ROS that deeply affect the function of neurons [9]. It seems ROS by inhibiting the synthesis of key neurotransmitters involved in depression such as serotonin and also increasing the Ca+2 signaling through increasing the sensitivity of inositol triphosphate (IP3) receptors and depleting of glutathione resources of neurons can contribute in development of depression [9]. Following finding of VDRs in brain, it was suggested that gene-dependent function of vitamin D probably could decrease the production of ROS by affecting gene expression of DNAFootnote 1-demethylase through inhibition of hyper-methylation of promoters responsible for gene transcription [9]. On the other hand, it was proposed that vitamin D may also control the function of ROS probably by maintenance of the homeostasis of serotonin through modulation of gene expression of tryptophan hydroxylase 1 & 2 (TPH1 & TPH2), the key enzymes of tryptophan synthesis pathways. Besides, vitamin D may also affect the function of ROS by up-regulation of glutathione to compensate for lost resources [9].
Interestingly, most commonly used anti-depressant medications, including Fluxetine, have anti-inflammatory properties [62, 63] and even recently have been proposed as an anti-inflammatory therapy against newly emerged coronavirus infection [64].
Limitations
This study had some limitations. Firstly, short-term effects of vitamin D supplementation on severity of depression may not necessarily reflect the long-term outcomes. Secondly, most of our participants had adequate baseline circulating 25(OH)D concentrations, which may have concealed any possible association among depression, inflammation and vitamin D. There is a great need for well-designed RCTs with longer durations to elucidate the possible effects of vitamin D on depressive disorders. Besides, simultaneous evaluation of vitamin D supplementation effects on glutathione and cytokines in depressed patients could be very helpful to explore the novel pathways of depression pathogenesis. Additionally, evaluation of the effects of vitamin D on gene expression of PMCA, NCX1 and L-type CaV1.2 and CaV1.3 necessitates further research. Current study had some advantages including, double-blind RCT design, safe dose of vitamin D supplements and control of confounding factors (e.g., anthropometric indices, SBP and DBP).
Conclusion
Eight-week supplementation with vitamin D (50,000 IU 2wks−1) resulted in a significant increase in serum 25(OH)D concentrations of adult subjects with mild to moderate depression which was accompanied with amelioration for their depression severity. This effect was independent of circulating concentrations of IL-1β, IL-6 and hs-CRP.
Our findings shed some light on the mechanisms underlying the pathophysiology of depression and may also be helpful for future preventive and therapeutic approaches.
Availability of data and materials
The data used and analyzed during the current study are available from the corresponding author.
Notes
DNA: deoxyribonucleic acid.
Abbreviations
- BDI-II:
-
Beck Depression Inventory-II
- BMI:
-
Body mass index
- COVID-19:
-
Coronavirus disease
- CRP:
-
C-reactive protein
- Ca+2 :
-
Calcium+2
- CNS:
-
Central nervous system
- DBP:
-
Diastolic blood pressure
- DSM–IV:
-
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
- EIA:
-
Enzyme immunoassay
- HC:
-
Hip circumference
- HPA:
-
Hypothalamus–pituitary–adrenal
- hs-CRP:
-
High-sensitivity C-reactive protein
- IL:
-
Interlukin
- iPTH:
-
Intact parathyroid hormone
- IP3:
-
Inositol triphosphate
- CaV1.3 & CaV1.2:
-
L-type Ca+2 channels
- MDD:
-
Major depressive disorder
- NCX1:
-
Na+/Ca2+ exchanger
- NNFTRI:
-
National Nutrition and Food Technology Research Institute
- PAL:
-
Physical activity level
- PMCA:
-
Calcium pump
- RCT:
-
Randomized clinical trial
- RAS:
-
Renin-angiotensin system
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SPSS:
-
Statistical Package for Social Sciences
- SBP:
-
Systolic blood pressures
- TPH:
-
Tryptophan hydroxylase
- WC:
-
Waist circumference
- WHR:
-
Waist to hip ratio
- VDR:
-
Vitamin D receptor
References
Salari N, Hosseinian-Far A, Jalali R, Vaisi-Raygani A, Rasoulpoor S, Mohammadi M, et al. Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Globalization Health. 2020;16(1):57.
Gururajan A, Clarke G, Dinan TG, Cryan JF. Molecular biomarkers of depression. Neurosci Biobehav Rev. 2016;64:101–33.
Li G, Mbuagbaw L, Samaan Z, Falavigna M, Zhang S, Adachi JD, et al. Efficacy of vitamin D supplementation in depression in adults: a systematic review. J Clin Endocrinol and Metabol. 2014;99(3):757–67.
Wang YP, Gorenstein C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics (Sao Paulo, Brazil). 2013;68(9):1274–87.
Adibfar A, Saleem M, Lanctot KL, Herrmann N. Potential biomarkers for depression associated with coronary artery disease: a critical review. Curr Mol Med. 2016;16(2):137–64.
Wong SK, Chin KY, Ima-Nirwana S. Vitamin D and depression: the evidence from an indirect clue to treatment strategy. Curr Drug Targets. 2018;19(8):888–97.
Aghajafari F, Letourneau N, Mahinpey N, Cosic N, Giesbrecht G. Vitamin D Deficiency and Antenatal and Postpartum Depression: A Systematic Review. Nutrients. 2018;10(4):478.
Verduijn J, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman AT, Penninx BW. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Transl Psychiatry. 2015;5:e649.
Berridge MJ. Vitamin D and depression: cellular and regulatory mechanisms. Pharmacol Rev. 2017;69(2):80–92.
Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200.
Singhal G, Baune BT. Do chemokines have a role in the pathophysiology of depression? In: Baune Bernhard, editor. Inflammation and immunity in depression. Cambridge: Academic Press; 2018. p. 135–59.
Singhal G, Baune BT. Chapter 9 - Inflammasomes Action as an Important Mechanism in Experimental and Clinical Depression. In: Baune BT, editor. Inflammation and Immunity in Depression: Academic Press; 2018. p. 161–71.
Toben C, Baune BT. The roles of T cells in clinical depression. In: Baune Bernhard, editor. Inflammation and immunity in depression. Cambridge: Academic Press; 2018. p. 115–33.
Musker M, Licinio J, Wong M. Inflammation genetics of depression. In: Baune Bernhard, editor. Inflammation and immunity in depression. Cambridge: Academic Press; 2018. p. 411–25.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295-a.
Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–7.
Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE. 2015;10(7):e0133494.
Ghasemi H. Roles of IL-6 in Ocular Inflammation: A Review. Ocular Immunol Inflamm. 2018;26(1):37–50.
Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, et al. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23(3):587–94.
Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry. 2019;214(1):11–9.
Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JRI, Hotopf M, Lewis CM, et al. Elevated C-Reactive Protein in Patients With Depression, Independent of Genetic, Health, and Psychosocial Factors: Results From the UK Biobank. Am J psychiatry. 2021;178(6):522–9.
Demetrowitsch TJ, Schlicht K, Knappe C, Zimmermann J, Jensen-Kroll J, Pisarevskaja A, et al. Precision Nutrition in Chronic Inflammation. Front Immunol. 2020;11:587895.
Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder K, et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2021;26(1):134–50.
Su H, Liu N, Zhang Y, Kong J. Vitamin D/VDR regulates peripheral energy homeostasis via central renin-angiotensin system. J Adv Res. 2021;33:69–80.
Lee PW, Selhorst A, Lampe SG, Liu Y, Yang Y, Lovett-Racke AE. Neuron-Specific Vitamin D Signaling Attenuates Microglia Activation and CNS Autoimmunity. Front Neurol. 2020;11:19.
Głąbska D, Kołota A. The Influence of Vitamin D Intake and Status on Mental Health in Children: A Systematic Review. 2021;13(3).
Cuomo A, Maina G, Bolognesi S, Rosso G, Beccarini Crescenzi B, Zanobini F, et al. Prevalence and correlates of vitamin D deficiency in a sample of 290 inpatients with mental illness. Front Psychiatry. 2019;10:167.
Nikooyeh B, Neyestani TR. Oxidative stress, type 2 diabetes and vitamin D: past, present and future. Diabetes/Metabolism Res Rev. 2016;32(3):260–7.
Motamed S, Nikooyeh B. Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes. 2019;15(4):e12867
Neyestani TR, Nikooyeh B, Alavi-Majd H, Shariatzadeh N, Kalayi A, Tayebinejad N, et al. Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in the subjects with type 2 diabetes. J Clin Endocrinol Metab. 2012;97(6):2005–11.
Nikooyeh B, Anari R, Neyestani TR. Chapter 38 - Vitamin D, oxidative stress, and diabetes: crossroads for new therapeutic approaches. In: Preedy VR, editor. Diabetes (Second Edition): Academic Press; 2020. p. 385–95.
Kaviani M, Nikooyeh B, Zand H, Yaghmaei P, Neyestani TR. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J Affect Disord. 2020;269:28–35.
Kaviani M, Nikooyeh B, Zand H, Yaghmaei P, Neyestani TR. Effects of Vitamin D Supplementation on Depression Status, Selected Pro-inflammatory Biomarkers and Neurotransmitters in Depressive Patients: A Study Protocol. Nutr Food Sci Res. 2019;6(4):1–7.
Wang Y, Liu XJ, Robitaille L, Eintracht S, MacNamara E, Hoffer LJ. Effects of vitamin C and vitamin D administration on mood and distress in acutely hospitalized patients. Am J Clin Nutr. 2013;98(3):705–11.
Demarest Litchford M. Clinical, biochemical, physical and functional assessment. In: Kathleen Mahan L, Raymond Janice L, editors. Food and the nutrition care process. 14th ed. St. Louis: Elsevier; 2017. p. 118.
Alavi NM, Khademalhoseini S, Vakili Z, Assarian F. Effect of vitamin D supplementation on depression in elderly patients: A randomized clinical trial. Clin Nutr. 2019;38(5):2065–70.
Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35(4):416–31.
Kaviani M, Nikooyeh B, Zand H, Yaghmaei P, Neyestani TR. Assessment of Overweight and Obesity Status in Patients with Depression Referred to Baharloo Hospital in Tehran: Possible Roles For Vitamin D? Nutr Food Sci Res. 2021;8(4):19–27.
Rosen CJ, Gallagher JC. The 2011 IOM report on vitamin D and calcium requirements for north america: clinical implications for providers treating patients with low bone mineral density. J Clin Densitom. 2011;14(2):79–84.
Jalali-Chimeh F, Gholamrezaei A, Vafa M, Nasiri M, Abiri B, Darooneh T, et al. Effect of Vitamin D Therapy on Sexual Function in Women with Sexual Dysfunction and Vitamin D Deficiency: A Randomized, Double-Blind, Placebo Controlled Clinical Trial. J Urol. 2019;201(5):987–93.
Mousa A, Naderpoor N, de Courten MPJ, de Courten B. Vitamin D and symptoms of depression in overweight or obese adults: A cross-sectional study and randomized placebo-controlled trial. J Steroid Biochem Mol Biol. 2018;177:200–8.
Mozaffari-Khosravi H, Nabizade L, Yassini-Ardakani SM, Hadinedoushan H, Barzegar K. The effect of 2 different single injections of high dose of vitamin D on improving the depression in depressed patients with vitamin D deficiency: a randomized clinical trial. J Clin Psychopharmacol. 2013;33(3):378–85.
Ghaderi A, Banafshe HR, Motmaen M, Rasouli-Azad M, Bahmani F, Asemi Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):84–9.
Penckofer S, Byrn M, Adams W, Emanuele MA, Mumby P, Kouba J, et al. Vitamin D Supplementation Improves Mood in Women with Type 2 Diabetes. J Diabetes Res. 2017;2017:8232863.
Jamilian H, Amirani E, Milajerdi A, Kolahdooz F, Mirzaei H, Zaroudi M, et al. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94:109651.
Bagheri S, Saghazade AR, Abbaszadeh-Mashkani S, Banafshe HR, Ghoreishi FS, Mesdaghinia A, et al. The effect of vitamin D supplementation on tobacco-related disorders in individuals with a tobacco use disorder: a randomized clinical trial. J Add Dis. 2022;40(3):382–93.
Libuda L, Antel J, Hebebrand J, Focker M. Nutrition and mental diseases : Focus depressive disorders. Nervenarzt. 2017;88(1):87–101.
Kesby JP, Turner KM, Alexander S, Eyles DW, McGrath JJ, Burne THJ. Developmental vitamin D deficiency alters multiple neurotransmitter systems in the neonatal rat brain. Intl J Dev Neurosci. 2017;62:1–7.
Caldwell JD, Londe K, Ochs SD, Hajdu Z, Rodewald A, Gebhart VM, et al. Three steroid-binding globulins, their localization in the brain and nose, and what they might be doing there. Steroids. 2019;142:48–54.
Briggs R, McCarroll K, O’Halloran A, Healy M, Kenny RA, Laird E. Vitamin D Deficiency Is Associated With an Increased Likelihood of Incident Depression in Community-Dwelling Older Adults. J Am Med Dir Assoc. 2019;20(5):517–23.
Casseb GAS, Kaster MP, Rodrigues ALS. Potential Role of Vitamin D for the Management of Depression and Anxiety. CNS Drugs. 2019;33(7):619–37.
Ikonen H, Palaniswamy S, Nordstrom T, Jarvelin MR, Herzig KH, Jaaskelainen E, et al. Vitamin D status and correlates of low vitamin D in schizophrenia, other psychoses and non-psychotic depression - The Northern Finland Birth Cohort 1966 study. Psychiatry Res. 2019;279:186–94.
Khundmiri SJ, Murray RD, Lederer E. PTH and Vitamin D. Compr Physiol. 2016;6(2):561–601.
Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–29.
Kjaergaard M, Waterloo K, Wang CE, Almas B, Figenschau Y, Hutchinson MS, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360–8.
Dean AJ, Bellgrove MA, Hall T, Phan WM, Eyles DW, Kvaskoff D, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults–a randomised controlled trial. PLoS ONE. 2011;6(11):e25966.
Yalamanchili V, Gallagher JC. Dose ranging effects of vitamin D3 on the geriatric depression score: A clinical trial. J Steroid Biochem Mol Biol. 2018;178:60–4.
Grudet C, Malm J, Westrin A, Brundin L. Suicidal patients are deficient in vitamin D, associated with a pro-inflammatory status in the blood. Psychoneuroendocrinology. 2014;50:210–9.
Amini S, Amani R, Jafarirad S, Cheraghian B, Sayyah M, Hemmati AA. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutr Neurosci. 2022;25(1):22–32.
Abiri B, Sarbakhsh P, Vafa M. Randomized study of the effects of vitamin D and/or magnesium supplementation on mood, serum levels of BDNF, inflammation, and SIRT1 in obese women with mild to moderate depressive symptoms. Nutr Neurosci. 2022;25(10):2123–35.
Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84(Pt A):50–5.
Alboni S, Poggini S, Garofalo S, Milior G, El Hajj H, Lecours C, et al. Fluoxetine treatment affects the inflammatory response and microglial function according to the quality of the living environment. Brain Behav Immun. 2016;58:261–71.
Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain, Behav Immun. 2012;26(3):469–79.
Creeden JF, Imami AS, Eby HM, Gillman C, Becker KN, Reigle J, et al. Fluoxetine as an anti-inflammatory therapy in SARS-CoV-2 infection. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2021;138:111437.
Acknowledgments
All laboratory bench works were performed at the Laboratory of Nutrition Research, NNFTRI. The assistance of Mrs. Marjan Rismanchi and Mr. Ali Kalayi in laboratory analyses is appreciated. We acknowledge the Baharloo Hospital and its outpatient clinics managers with special thanks to Ms. Arab and Mrs. Nami, head nurses of Baharloo Hospital internal medicine sections for blood sampling. We also appreciate all participants of this study.
Submission declaration
It is declared that the work described has not been published previously and it is not under consideration for publication elsewhere. Also, publication of manuscript is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Funding
This work was funded by National Nutrition and Food Technology Research Institute (NNFTRI) [grant numbers 450/1701].
Author information
Authors and Affiliations
Contributions
Author Mina Kaviani: She contributed in study design, wrote the protocol and performed all field works (sampling, intervention, laboratory tests, data analysis), as well as literature searches. Also, the initial manuscript was written by her. Author Baharehe Nikooyeh: Statistical analyses were performed under her supervision and directions. Author Farnaz Etesam: She contributed in study design (psychiatry part), patients’ depression diagnosis and selecting participants. Author Siroos Jahangiri Behnagh: He contributed in patients’ depression diagnosis and selecting participants. Author Hamed Mohammadi Kangarani: He contributed in patients’ depression diagnosis and selecting participants. Author Mohammad Arefi: He contributed in accepting the proposal and coordinating the implementation of the project in the clinics of Baharloo Hospital. Author Parichehreh Yaghmaei: She was the advisor of dissertation. Author Tirang R. Neyestani (corresponding author): The initial idea of the study was from him. He contributed in study design, as well as managed the laboratory tests and literature searches, and finalized the manuscript. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of NNFTRI (IR.SBMU.NNFTRI.REC.1396.185) and all methods were performed in accordance with the relevant guidelines and regulations (NNFTRI). The clinical trial registration code was obtained from the Iranian Registry of Clinical Trials (IRCT20170926036425N1) and ClinicalTrials.gov (NCT03766074). Written informed consent was obtained from all participants or their next of kin/legally authorized representative (according to educational level) before entering the research, as well.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kaviani, M., Nikooyeh, B., Etesam, F. et al. Effects of vitamin D supplementation on depression and some selected pro-inflammatory biomarkers: a double-blind randomized clinical trial. BMC Psychiatry 22, 694 (2022). https://doi.org/10.1186/s12888-022-04305-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-022-04305-3