Abstract
Background
Depression is common in older people and is associated with underlying brain change increasing the risk of dementia. Sleep disturbance is frequently reported by those with lifetime depression, however whether circadian misalignment also exists is unclear. We aimed to examine circadian rhythms and sleep associations in older patients with and without lifetime depression.
Methods
Thirty-four older people meeting DSM-IV criteria for lifetime major depression (mean age = 63.9 years), and 30 healthy controls (mean age = 65.7 years) were recruited. Participants underwent 2-weeks of actigraphy followed by a 3-night protocol including dim light melatonin onset (DLMO) assessment and overnight polysomnography (PSG) for sleep architecture. DLMO and phase angle of entrainment were computed.
Results
Compared to controls, participants with depression had a significantly longer phase angle of entrainment (6.82 h ± 1.45 vs. 5.87 h ± 1.60, p = 0.02, Cohens-d = 0.62). A small to moderate yet non-significant difference in DLMO times, with earlier DLMO (34 ± 27 min) observed in depression (20:36 ± 1:48 vs. 21:10 ± 1:48, p = 0.22, Cohens-d = 0.32). Individuals with depression had longer sleep latency and latency to rapid eye movement sleep than controls (all p < 0.05).
Conclusion
Circadian advancement and alterations to the timing of sleep and REM onset are evident in older people with lifetime major depression, despite having only mild residual symptoms. Further research examining the prognostic significance of these changes is warranted as well as chronotherapeutic treatment studies.
Similar content being viewed by others
Background
Within the increasing ageing population, depression in older people will continue to be a predominant health care problem. This syndrome is associated with significant disease burden, disability, functional decline and premature death [1]. Depression increases burden on caregivers, health services and is associated with increased health care expenditure [2]. Of significance, depression in older people is associated with cognitive impairment [3], disability [4] and progression to dementia longitudinally [5, 6]. Even sub-threshold depressive symptoms carry a significant risk for subsequent onset of major depression [7], ongoing cognitive decline [8] and dementia [9], warranting efforts focused on delineating modifiable risk factors. One potential candidate in this regard, is sleep and circadian (sleep-wake) disturbance.
The significance of sleep-wake functions for mood and cognition is underscored by a number of clinical, epidemiological and longitudinal studies showing that sleep-wake disturbance is a prodromal feature of depressive symptom onset, that it may persist in the remitted state and that it may perpetuate the illness [10,11,12,13]. Insomnia and depression are highly co-prevalent and likely to be mutually precipitating [14]. In older people with depression, insomnia is the most commonly reported sleep disturbance; with complaints of early morning wakefulness being common [15, 16]. Polysomnography (PSG) studies show alterations in slow wave sleep (SWS) and rapid eye movement (REM) sleep as well as increased latency to REM sleep, increased wake after sleep onset and reduced sleep efficiency [17], see review by [18]. Sleep disturbances in patients with depression are indicative of more severe illness and tend to be under-treated [19]. In older people with depression, sleep disturbance may be most prominent at the onset of a depressive episode [20,21,22] and is predictive of treatment responsiveness [23]. Thus, the presence of this feature has considerable clinical and prognostic significance.
To date, examination of sleep-wake patterns in older people with depression has focused largely on sleep, without concurrent examination of the circadian system. Given the increasing recognition of the integral role of circadian change in depressive disorders [24], studies examining these two systems concurrently are warranted. Whilst it is clear that circadian phase advancement occurs during healthy ageing with a concomitant reduction in circadian amplitude [14], it is not clear whether patients with lifetime major depression exhibit similar circadian patterns and/or whether the sleep and circadian changes are more pronounced. Certainly in middle-aged samples and in those with seasonal affective disorder, some [25,26,27,28,29] but not all [30] evidence suggests alterations in melatonin amplitude and rhythm and circadian changes may occur at the onset and maintenance of affective disorders [25]. In younger samples, there also appears to be a phase delay in circadian timing, and melatonin secretion may be altered even in very early stages of a depressive illness [24, 31]. However, there is some evidence to suggest that circadian alterations in affective disorders may vary according to the ageing process, with younger patients showing the greatest phase delay, and older patients showing lower circadian amplitude [32].
A greater understanding of the changes to the circadian system in older people with depression is not only of immense scientific interest, but importantly this information could inform personalised treatment approaches for both depressive symptomatology and cognitive decline [24, 33]. In this study, we aimed to examine circadian rhythms in older patients with depression, with the primary outcomes of interest being dim light melatonin onset and phase angle of entrainment. Secondary outcomes were measurements of sleep architecture and quality assessed both objectively and subjectively. We also sought to examine if there were associations between clinical correlates and circadian rhythmicity in an exploratory analysis to understand possible future clinical and therapeutic targets. We hypothesized that relative to controls, older patients with depression would show phase advancement and greater sleep disturbance.
Methods
Participants
Health-seeking older adults were recruited from specialist psychiatry clinics at the Brain & Mind Centre at the University of Sydney, Sydney, Australia. To be eligible participants had to: be aged 50 years or older; meet DSM-IV criteria for lifetime major depressive disorder; have had a depressive episode within the last 5 years; and be clinically stable on medication. Exclusion criteria were: other psychiatric illness including bipolar disorder; history of stroke; neurological disorder; head injury with loss of consciousness > 30-min; medical conditions known to affect cognition (e.g. cancer); diagnosis of dementia and /or Mini Mental State Examination Score < 24 [34]; current shift-workers; transmeridian travel within the prior 60-days; use of medication that may affect sleep and/or melatonin secretion such as beta-blockers or lithium. Individuals taking sedative hypnotics including benzodiazepines were asked to stop the medications for 2 weeks prior to the study visit. Sleep apnoea was excluded based on clinician interview and Apnoea Hypopnoea Index score when available. Age-matched control participants were from the community and had to not have any history of lifetime psychiatric disorders or any of the above mentioned exclusion criteria. Participants needed to agree to wear an actigraphy watch and complete sleep diaries for 2 weeks as well undertake overnight PSG and circadian assessments on three consecutive nights within the research facility. This research was approved by the Human Research Ethics Committee of The University of Sydney and was conducted in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Procedure
Participants were first screened over the telephone and then attended a screening visit to confirm study eligibility. Prior to attending the three-night protocol, participants were asked to wear an actigraph (Actiwatch Spectrum, Philips Respironics, OR) and complete a sleep diary for 2 weeks.
Measures
Psychiatric
As detailed elsewhere, a structured clinical assessment was conducted by an Old Age Psychiatrist, which included psychiatric history, depression onset age, risk of sleep disorders, body mass index and medication use. Medical burden was quantified using the Cumulative Illness Rating Scale, Geriatric Version [35]. Lifetime and current depression were confirmed using the affective component of the Structured Clinical Interview for DSM-IV-R [36]. The Hamilton Depression Rating Scale was used to determine depression severity [37].
Sleep and circadian measures
-
i)
Melatonin sampling: Salivary melatonin was measured to determine DLMO on the third night of the protocol, as detailed previously [31, 38]. Participants arrived 7 h prior to their Habitual Sleep Onset (HSO: determined from 14-day actigraphy and/or sleep diary) and were kept awake until 2 h after HSO time. Saliva samples (1.5 ml, using Salivette, Sarstedt, Germany) were collected 30-minutely (6-h prior to until 2-h after HSO). Participants maintained an upright seated posture and refrained from food or drink for at least 20-min prior to each saliva sample collection. Samples were immediately frozen at -20 °C. Melatonin was assayed using 200ul of saliva by double antibody radioimmunoassay (Cat# RK-DSM2; Buhlmann Laboratories AG, Schönenbuch, Switzerland). The lowest detectable level of melatonin was 1 pg/mL (4.3pM). The DLMO timing was calculated when melatonin levels passed and remained above the absolute threshold of 3 pg/mL (12.9 pM) and calculated using linear interpolation between successive 30 min saliva samples below and above the threshold for each subject [39]. The phase angle of entrainment, a measure of the relationship between the biological clock and a recurring external cue, was calculated by subtracting DLMO time from the midpoint of sleep determined from actigraphy (hh:mm) [40].
-
ii)
Self-report questionnaires: This included the Pittsburgh Sleep Quality Index to assess sleep quality over the previous month. The scale ranges from 0 to 21 with a higher value indicating greater sleep disturbance. A score > 5 is defines as impaired sleep quality [41].
-
iii)
Actigraphy monitoring: Participants were asked to wear an actigraphy watch and keep a sleep diary, under usual light and behavioural conditions, for 14 days prior to commencing the in-laboratory portion of the experimental protocol. All procedures have been previously described [42]. Habitual sleep onset, total sleep time and sleep midpoint were used to assess the participants day-to-day sleep patterns.
-
iv)
Polysomnography: Participants attended the Chronobiology and Sleep Laboratory at The Brain & Mind Centre for three consecutive nights. On the first two nights, PSG recordings were collected using an ambulatory recording system (Compumedics Siesta, Australia). Night one was considered an acclimatization night and included a clinical assessment to detect the presence of occult sleep disorders, such as obstructive sleep apnea syndrome, periodic limb movements, or restless legs syndrome. Sleep variables collected on night two, using a standardised research PSG montage (electroencephalogram, electrooculogram, electromyogram) were used for all analyses. Sleep architecture stages were visually scored in a computer using standardised criteria by an experienced sleep technician [43], with modifications for older participants [44]. Laboratory conditions were controlled with fixed lighting levels (< 30 lx during waking; < 10 lx during DLMO testing; < 1 lx during scheduled sleep periods) and ambient temperature (24 ± 1 °C). Additionally participants were physiologically and behaviourally monitored at all times. Latency to REM sleep (time from sleep onset, as defined by three contiguous epochs of stage 1 sleep, or any other stage of sleep to the first epoch of REM sleep [mins]), wake after sleep onset (mins) and the number of arousals (defined as an abrupt shift in electroencephalogram frequency of 3 seconds or longer; arousals during rapid eye movement sleep required an increase in chin electromyogram activity) were derived from the PSG. For descriptive purposes we also report the time of sleep onset (24-h clock time); latency to sleep (time from ‘lights out’ to sleep onset, as defined by three contiguous epochs of stage 1 sleep, or any other stage of sleep [mins]); the time spent in slow wave sleep (sum of stage 3 and stage 4 sleep) (mins), non-REM sleep and REM sleep (mins); sleep efficiency (total sleep time-latency to sleep onset/time in bed*100, %); and, the apnoea-hypopnoea index (number of apnoea plus hypopnoea events per hour of sleep).
Statistical analysis
We undertook purposive sampling for cases and controls from the Healthy Brain Ageing clinic database. All data are reported as means and standard deviations unless otherwise stated. All analyses were conducted using IBM® SPSS® Statistics Version 25 Chicago: IBM Inc.. Between group comparisons were performed using independent samples t-tests, Mann-Whitney U tests or Chi-squared tests where warranted. Normality of outcome variables was determined by visual inspection of histograms and then Pearson and Spearman correlation coefficients were used as appropriate. Due to the small amount of missing data, data imputation methods were not utilised. All analyses were two-tailed and employed an alpha level of 0.05.
Results
Forty-seven cases and 33 controls were screened for the study. Thirty-four participants with lifetime depression and 30 controls met eligibility criteria. Reasons for ineligibility are shown in the study flow (Fig. 1). In the cases, the mean age of depression onset was 42.2 ± 19.1 years (range 10 to 82 years), with the median number of depressive episodes being 2.0 (inter-quartile range 1.0–3.0). Nine participants met DSM-IV criteria for current major depression at the time of assessment. Twenty-three participants were taking antidepressant medications (tricyclics n = 6; Selective Serotonin Reuptake Inhibitors [SSRIs] n = 6; Serotonin Norepinephrine Reuptake Inhibitors [SNRIs] n = 8; mirtazapine n = 2; Noradrenaline Reuptake Inhibitors [NRI] n = 1).
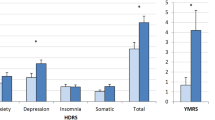
As shown in Table 1, there were no significant differences between cases and controls in terms of age, gender, years of education, global cognition (Mini Mental State Examination Score scores), body mass index or level of medical illness burden (Cumulative Illness Rating Scale). As expected, cases had significantly higher levels of depressive symptoms than control participants as evidenced by scores on the Hamilton depression rating scale and geriatric depression scale. Those with depression had poorer self-reported sleep quality as assessed by the Pittsburgh sleep quality index (Fig. 2).
Differences in dim light melatonin onset times (A) and phase angle between dim light melatonin onset time and midpoint of sleep (B) between depression cases and controls. Grey circles are controls and black diamonds are depression patients. Points are the individual values, the middle line is the mean and the error bars are the standard deviation
Differences in circadian measures between groups
DLMO could not be computed for five cases and two controls due to inability to detect melatonin within the sensitivity of the assay or not reaching the 3 pg/mL threshold. There was no statistical difference in DLMO times between groups however, cases were found to be advanced, on average, by 36 min (± 25) compared to the control groups (Table 2 and Fig. 2). The phase angle of entrainment was significantly longer in cases compared to controls (Fig. 2 and Table 2).
Correlates of phase angle in people with depression
In cases, phase angle was not significantly correlated with clinical measures including age (r = − 0.06, p = 0.75), age of onset of depression (r = 0.14, p = 0.41), depression severity as measured by the Hamilton depression rating scale (rho = − 0.07, p = 0.72), subjective sleepiness (Pittsburgh sleep quality index, r = 0.29 p = 0.13), body mass index (r = − 0.29, p = 0.14) or global cognition (rho = 0.26, p = 0.21). There was no difference in phase angle (mean difference 0.14 h; 95%CI − 0.86 to 1.13), DLMO (0:59 h; − 1:33 to 0:54) between those with depression who did and did not take antidepressant medication nor were there any differences between those who had current major depression (n = 9) and those with a history of depression (Phase angle: mean difference − 0.08; 95%CI − 1.59 to 1.42; DLMO 0:14 h, 95%CI − 1:05 to 1:34).
Table 2 shows that there were no differences in actigraphic measured pre-study sleep durations or sleep onset times between the depression and control groups. Furthermore, there were no significant differences between patients and controls in sleep onset time, total sleep time and wake after sleep onset using PSG. Habitual sleep offset was later in individuals with depression compared to controls which was driven by three people who were late sleepers. After removal of these three late sleepers the difference in the offset had gone but the effects observed in the circadian measurements remained.
In comparison to control subjects, those with depression demonstrated longer latency to sleep onset and latency to REM sleep times (Table 2 and Fig. 3). There was no difference in the apnea hypopnea index, arousal index or number of arousals between the two groups. There was no difference between REM latency (z statistic, − 1.40, p = 0.18) between those with depression who did (median [IQR] = 136 min [91.5, 269.25]) and did not (99.5 min [65.625, 137.625]) take antidepressant medication.
Differences in subjective sleep quality assessed using Pittsburgh Sleep Quality Index (a), and latency to REM sleep (b) measured using polysomnography between depression cases and controls. Grey circles are depression patients and black diamonds are controls. Points show individual values, middle line is the mean and error bars are the standard deviation
Discussion
This study shows for the first time that older people with largely remitted lifetime depression have a longer phase angle of entrainment relative to healthy controls, suggesting that the relationship between melatonin onset and sleep is misaligned. Indeed, DLMO was 34 min earlier on average in the depression cases, which corresponds to a small to medium effect size difference. This may be clinically relevant in this population; however, future studies are required to confirm. In addition people with depression had worse subjective sleep quality and longer latency to REM sleep onset however no differences in other objective sleep quality measures were observed.
Our finding that older adults with lifetime depression have advanced patterns of Melatonin secretion is aligned with very early theories that people with major depressive disorder had advanced biological rhythms [45]. A small number of studies have shown individuals with affective disorders to have advanced circadian rhythmicity [46,47,48] with one reporting disruptions in melatonin secretion as well [46]. However, these studies had small sample sizes and to date findings from the current study represent the largest sample size of sleep and circadian assessment in older people with lifetime depression. Importantly, while it has been long documented that there are changes to the circadian clock during normal ageing including a reduction in amplitude of melatonin secretion and an advancement of phase [49, 50], our findings demonstrate that these are not due to normal ageing alone and are more pronounced in those with a history of affective disorder.
Conversely, a number of studies have also shown delayed melatonin secretion [31, 51, 52] but most of these latter studies have been in adolescent and young adult populations. One study in 72 older adults aged over 60 reported no significant associations between affective symptoms and melatonin secretion however only 55% had a lifetime history of affective disorder [53]. While in this study, we did not find that depression severity was associated with circadian misalignment, this may be due to a restricted range since our sample had largely sub-threshold symptoms. Certainly in symptomatic and samples under 60 years, prior studies have shown that an increased phase angle between melatonin onset and mid-sleep (in the delayed direction) was associated with severity of mood symptoms [54]. Overall, there is therefore emerging evidence for circadian disruption within affective disorders across the lifespan, which may differ somewhat according to age, depression subtype and symptom severity.
Importantly, despite 67% of our population using antidepressants, individuals with lifetime depression had a significantly longer phase angles than controls. There has been some evidence that treatment with antidepressants can phase realign the circadian clock [55, 56] particularly SSRIs. Of those using antidepressants only 26% were using SSRIs and therefore there was not an overall dampening effect. However, it is important to note, that there is an emerging literature suggesting that antidepressants increase sensitivity to light, which in turn, affects melatonin secretion [57] and circadian rhythmicity. In combination with external zeitgebers, activity, feeding patterns and other medical comorbidities, the effect of increased light sensitivity due to antidepressants could be pronounced, and could serve to either perpetuate circadian misalignment, sleep quality and timing, and depressive symptoms, or could play a vital role in symptom remission. Further research is required to ascertain the degree of light sensitivity in older people and antidepressant medications.
Consistent with prior research, we additionally found that subjective and aspects of objectively assessed sleep differed in those with lifetime depression. Poor sleep quality is in turn associated with poor cognitive functioning in both symptomatic and asymptomatic samples [58, 59] and brain connectivity in those with lifetime depression [60]. We found that those with depression had longer REM latency than healthy control participants. This could be attributed to a number of underlying mechanisms including alterations in the homeostatic drive for REM sleep as well as disturbances of the circadian system [61]. Alternatively, some antidepressant medications can suppress REM sleep and/or impact on latency [62]. In this study, 67% of participants with depression were taking antidepressants, however REM latency was not different between those who were and were not taking anti-depressants suggesting this was not the driving factor for this difference. The observed greater latency to REM in individuals with depression is in contrast to previous research, often focussing on middle-aged populations in which latency to REM sleep if often shortened. This could be due to an effect of ageing and emerging neurodegenerative pathology that may well be present in this population. We did not see any other significant differences in more traditional objective measures of sleep disturbance including wake after sleep onset and arousal index which is in line with other studies [63]. This suggests that circadian changes in older people with lifetime depression can occur without the concurrence of overt sleep disturbance.
An improved scientific understanding of the nature and mechanisms mediating the relationships between sleep and mood will ultimately assist in the delivery of more targeted and personalized treatments for this patient group. The need for this research is underscored by the fact that most antidepressant therapies actually alter sleep architecture, and can even exacerbate sleep disturbance, warranting interventions that are tailored to the individual sleep-wake profile [24]. Even though such research in its infancy, emerging data suggests that younger people with circadian phase delay may preferentially benefit from melatoninergic agents, aiming to advance the circadian rhythm [64]. Conversely, in older people, interventions aimed at specifically targeting sleep and the circadian system are required. Light therapy, physical exercise, sleep psychoeducation, cognitive activity, and transcranial magnetic stimulation have all been used as interventions aimed at realigning circadian rhythmicity, reducing nocturnal sleep disruption or enhancing sleep consolidation [65, 66]. Finally, the effects of antidepressants on sleep-wake systems cannot be under-stated. Further research exploring optimal maintenance treatments for those with remitted symptoms and persistent sleep-wake disruption is now needed. Older people with remitted symptoms and sleep-wake disturbance may be best suited to use of non-pharmacological treatments only such as psychological therapy [58]; non-REM suppressing medications, or those that are least disruptive to sleep architecture (e.g. Agomelatine).
We do need to acknowledge that this is a cross-sectional study and therefore we cannot draw causal inferences. Furthermore we studied a specific population with generally remitted depression symptoms and additionally had to exclude many other populations due to the nature of the outcomes and therefore our findings may not be generalizable to all older people with lifetime depression.
Conclusion
Overall this study shows that in older people with lifetime depression and largely subthreshold symptoms, changes circadian functions are evident, compared to healthy controls, even when only small changes to sleep quality are apparent. Circadian disturbances may be possible targets of interventions to reduce depressive symptoms in older adults.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author at the discretion of the investigators.
Abbreviations
- DLMO:
-
Dim light melatonin onset
- HSO:
-
Habitual sleep onset
- PSG:
-
Polysomnography
- REM:
-
Rapid-eye movement
- SWS:
-
Slow wave sleep
References
Australian Institute of Health and Welfare. Australia’s health 2008. Canberra: Australian Institute of Health and Welfare; 2008.
Katon WJ, Lin E, Russo J, Unutzer J. Increased medical costs of a population-based sample of depressed elderly patients. Arch Gen Psychiatry. 2003;60(9):897–903.
Naismith SL, Hickie IB, Turner K, Little CL, Winter V, Ward PB, Wilhelm K, Mitchell P, Parker G. Neuropsychological performance in patients with depression is associated with clinical, etiological and genetic risk factors. J Clin Exp Neuropsychol. 2003;25(6):866–77.
Naismith S, Hickie I, Longley W, Scott E. How well do self-reported and objectively measured cognitive deficits predict disability in major depression? BMC Psychiatry. 2007;7:32.
Steffens DC, Potter GG, McQuoid DR, MacFall JR, Payne ME, Burke JR, Plassman BL, Welsh-Bohmer KA. Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry. 2007;15(10):839–49.
Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression--a longitudinal evaluation. Biol Psychiatry. 1997;42(5):367–74.
Lyness JM, Yu Q, Tang W, Tu X, Conwell Y. Risks for depression onset in primary care elderly patients: potential targets for preventive interventions. Am J Psychiatry. 2009;166(12):1375–83.
Yaffe K, Blackwell T, Gore R, Sands L, Reus V, Browner WS. Depressive symptoms and cognitive decline in nondemented elderly women: a prospective study. Arch Gen Psychiatry. 1999;56(5):425–30.
Ritchie K, Carriere I, Ritchie CW, Berr C, Artero S, Ancelin ML. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. Br Med J. 2010;341.
Pigeon WR, Hegel M, Unutzer J, Fan MY, Sateia MJ, Lyness JM, Phillips C, Perlis ML. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. 2008;31(4):481–8.
Mallon L, Broman J-E, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12(03):295–306.
Perlis ML, Smith LJ, Lyness JM, Matteson SR, Pigeon WR, Jungquist CR, Tu X. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13.
Cho H, Lavretsky H, Olmstead R, Levin M, Oxman M, Irwin M. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatr. 2008;165(12):1543–50.
Costa IC, Carvalho HN, Fernandes L. Aging, circadian rhythms and depressive disorders: a review. Am J Neurodegener Dis. 2013;2(4):228–46.
Benca RM, Peterson MJ. Insomnia and depression. Sleep Med. 2008;9(Suppl 1):S3–9.
Buysse DJ. Insomnia, depression and aging. Assessing sleep and mood interactions in older adults. Geriatrics. 2004;59(2):47–51.
Lauer CJ, Riemann D, Wiegand M, Berger M. From early to late adulthood. Changes in EEG sleep of depressed patients and healthy volunteers. Biol Psychiatry. 1991;29(10):979–93.
Naismith SL, Lewis SJ, Rogers NL. Sleep-wake changes and cognition in neurodegenerative disease. Prog Brain Res. 2011;190:21–52.
Sunderajan P, Gaynes BN, Wisniewski SR, Miyahara S, Fava M, Akingbala F, DeVeaugh-Geiss J, Rush AJ, Trivedi MH. Insomnia in patients with depression: a STAR*D report. CNS Spectr. 2010;15(6):394–404.
Dew MA, Reynolds CF 3rd, Buysse DJ, Houck PR, Hoch CC, Monk TH, Kupfer DJ. Electroencephalographic sleep profiles during depression. Effects of episode duration and other clinical and psychosocial factors in older adults. Arch Gen Psychiatry. 1996;53(2):148–56.
Dridi D, Zouiten A, Ben Mansour H. Depression: chronophysiology and chronotherapy. Biol Rhythm Res. 2014;45(1):77–91.
Palagini L, Baglioni C, Ciapparelli A, Gemignani A, Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17(5):377–90.
Dew MA, Reynolds CF 3rd, Houck PR, Hall M, Buysse DJ, Frank E, Kupfer DJ. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54(11):1016–24.
Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378(9791):621–31.
Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Parry B, Cardinali DP. Melatonin in mood disorders. World J Biol Psychiatry. 2006;7(3):138–51.
Branchey L, Weinberg U, Branchey M, Linkowski P, Mendlewicz J. Simultaneous study of 24-hour patterns of melatonin and cortisol secretion in depressed patients. Neuropsychobiology. 1982;8(5):225–32.
Claustrat B, Chazot G, Brun J, Jordan D, Sassolas G. A chronobiological study of melatonin and cortisol secretion in depressed subjects: plasma melatonin, a biochemical marker in major depression. Biol Psychiatry. 1984;19(8):1215–28.
Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(5 Suppl):S102–8.
Wehr TA, Rosenthal NE, Sack DA, Gillin JC. Antidepressant effects of sleep deprivation in bright and dim light. Acta Psychiatr Scand. 1985;72(2):161–5.
Lewy A. Circadian phase sleep and mood disorders. Philadelphia: Lipincott Williams and Wilkins 1880-1893; 2002.
Naismith SL, Hermens DF, Ip TK, Bolitho S, Scott E, Rogers NL, Hickie IB. Circadian profiles in young people during the early stages of affective disorder. Transl Psychiatry. 2012;2:e123.
Robillard R, Naismith SL, Smith KL, Rogers NL, White D, Terpening Z, Ip TK, Hermens DF, Whitwell B, Scott EM, et al. Sleep-wake cycle in young and older persons with a lifetime history of mood disorders. PLoS One. 2014;9(2):e87763.
Hickie IB, Naismith SL, Norrie LM, Scott EM. Managing depression across the life cycle: new strategies for clinicians and their patients. Intern Med J. 2009;39(11):720–7.
Folstein MF, Folstein SE, PR MH. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98.
Miller MD, Towers A. Manual of guidelines for scoring the cumulative illness rating scale for geriatrics (CIRS-G). Pittsburgh: University of Pittsburgh; 1991.
First MB, Spitzer RL, Gibson M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders, patient Ed, SCID I/P. Washington, DC: American Psychiatric Association; 1996.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Naismith SL, Hickie IB, Terpening Z, Rajaratnam SW, Hodges JR, Bolitho S, Rogers NL, Lewis SJG. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis. 2014;38(4):857–66.
Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythm. 2002;17(2):181–93.
Burgess HJ, Eastman CI. The dim light melatonin onset following fixed and free sleep schedules. J Sleep Res. 2005;14(3):229–37.
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
Naismith SL, Rogers NL, Lewis SJ, Terpening Z, Ip T, Diamond K, Norrie L, Hickie IB. Sleep disturbance relates to neuropsychological functioning in late-life depression. J Affect Disord. 2011;132(1–2):139–45.
Rechtschaffen K. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. In: Rechtschaffen A, Kales A, editors. . Bethesda: U. S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968.
Webb WB, Dreblow LM. A modified method for scoring slow wave sleep of older subjects. Sleep. 1982;5(2):195–9.
Wehr TA, Wirz-Justice A, Goodwin FK, Duncan W, Gillin JC. Phase advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206(4419):710–3.
Nair NP, Hariharasubramanian N, Pilapil C. Circadian rhythm of plasma melatonin in endogenous depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 1984;8(4–6):715–8.
Millet B, Touitou Y, Poirier MF, Bourdel MC, Hantouche E, Bogdan A, Olie JP. Plasma melatonin and cortisol in patients with obsessive-compulsive disorder: relationship with axillary temperature, physical activity, and clinical symptoms. Biol Psychiatry. 1998;44(9):874–81.
Novakova M, Prasko J, Latalova K, Sladek M, Sumova A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. 2015;17(3):303–14.
Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Phys. 1998;275(5 Pt 2):R1478–87.
Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3(4):299–309.
Robillard R, Carpenter JS, Rogers NL, Fares S, Grierson AB, Hermens DF, Naismith SL, Mullin SJ, Feilds KL, Glozier N, et al. Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl Psychiatry. 2018;8(1):213.
Crasson M, Kjiri S, Colin A, Kjiri K, L'Hermite-Baleriaux M, Ansseau M, Legros JJ. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology. 2004;29(1):1–12.
Kripke DF, Youngstedt SD, Rex KM, Klauber MR, Elliott JA. Melatonin excretion with affect disorders over age 60. Psychiatry Res. 2003;118(1):47–54.
Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168(3):259–61.
Morin LP. Serotonin and the regulation of mammalian circadian rhythmicity. Ann Med. 1999;31(1):12–33.
Carvalho LA, Gorenstein C, Moreno R, Pariante C, Markus RP. Effect of antidepressants on melatonin metabolite in depressed patients. J Psychopharmacol. 2009;23(3):315–21.
McGlashan EM, Nandam LS, Vidafar P, Mansfield DR, Rajaratnam SMW, Cain SW. The SSRI citalopram increases the sensitivity of the human circadian system to light in an acute dose. Psychopharmacology. 2018;235(11):3201–9.
Naismith SL, Rogers NL, Lewis SJ, Diamond K, Terpening Z, Norrie L, Hickie IB. Sleep disturbance in mild cognitive impairment: differential effects of current and remitted depression. Acta Neuropsychiatr. 2011;23(4):167–72.
Naismith SL, Norrie L, Lewis SJ, Rogers NL, Scott EM, Hickie IB. Does sleep disturbance mediate neuropsychological functioning in older people with depression? J Affect Disord. 2009;116(1–2):139–43.
McKinnon AC, Hickie IB, Scott J, Duffy SL, Norrie L, Terpening Z, Grunstein RR, Lagopoulos J, Batchelor J, Lewis SJG, et al. Current sleep disturbance in older people with a lifetime history of depression is associated with increased connectivity in the default mode network. J Affect Disord. 2018;229:85–94.
Schulz H, Lund R. Sleep onset REM episodes are associated with circadian parameters of body temperature. A study in depressed patients and normal controls. Biol Psychiatry. 1983;18(12):1411–26.
Wichniak A, Wierzbicka A, Walecka M, Jernajczyk W. Effects of antidepressants on sleep. Curr Psychiatry Rep. 2017;19(9):63.
Difrancesco S, Lamers F, Riese H, Merikangas KR, Beekman ATF, van Hemert AM, Schoevers RA, Penninx B. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: a 2-week ambulatory assessment study. Depress Anxiety. 2019;36(10):975–86.
Robillard R, Carpenter JS, Feilds KL, Hermens DF, White D, Naismith SL, Bartlett D, Whitwell B, Southan J, Scott EM, et al. Parallel changes in mood and melatonin rhythm following an adjunctive multimodal Chronobiological intervention with Agomelatine in people with depression: a proof of concept open label study. Front Psychiatry. 2018;9:624.
Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23(7):571–85.
Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–89.
Acknowledgements
The authors wish to thank the participants for their involvement in the study and A/Prof David Kennaway for his expertise with melatonin assays.
Funding
This work was supported by a National Health and Medical Research Council Project Grant (NHMRC, No. 632689) and a NHMRC Australia Fellowship to Professor Ian Hickie. Dr. Camilla Hoyos and Professor Simon Lewis are funded by NHMRC-ARC Dementia Research Development Fellowships (APP1104003 and APP1110414 respectively) and Professor Sharon Naismith is funded by a NHMRC Dementia Leadership Fellowship. The funding body did not have any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
SN, SL and IH conceived the idea for the original study and obtained funding. SN, SL and ZT were involved in data collection. SN oversaw all aspects of the study. CH was responsible for data cleaning, data processing, data analysis. CG calculated the melatonin times. CH prepared the manuscript and all authors contributed to the interpretation of results and the planning, editing and revising of the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the Human Research Ethics Committee of The University of Sydney and was conducted in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Consent for publication
No individual participant data or any identifiable data is being published so no specific consent has been given. Participants were informed in the information statement that the de-identifiable data would be published.
Competing interests
Dr. Hoyos, A/Prof Gordon, Prof Lewis, Dr. Norrie, Dr. Terpening and Prof Naismith have no conflicts to declare. Professor Ian Hickie was an inaugural Commissioner on Australia’s National Mental Health Commission (2012–18). He is the Co-Director, Health and Policy at the Brain and Mind Centre (BMC) University of Sydney. The BMC operates an early-intervention youth services at Camperdown under contract to headspace. Professor Hickie has previously led community-based and pharmaceutical industry-supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) projects focused on the identification and better management of anxiety and depression. He was a member of the Medical Advisory Panel for Medibank Private until October 2017, a Board Member of Psychosis Australia Trust and a member of Veterans Mental Health Clinical Reference group. He is the Chief Scientific Advisor to, and an equity shareholder in, Innowell. Innowell has been formed by the University of Sydney and PwC to deliver the $30 m Australian Government-funded ‘Project Synergy’. Project Synergy is a 3 year program for the transformation of mental health services through the use of innovative technologies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hoyos, C.M., Gordon, C., Terpening, Z. et al. Circadian rhythm and sleep alterations in older people with lifetime depression: a case-control study. BMC Psychiatry 20, 192 (2020). https://doi.org/10.1186/s12888-020-02606-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-020-02606-z