Abstract
Purpose
The aim of present study is to measure plasma clozapine (CLZ) and N-desmethyl clozapine (DMC) as biomarkers to correlate drug concentrations with the appearance of preclinical adverse hematic effects.
Methods
A high-performance liquid chromatographic method, using a diode-array (ultraviolet) detector, was validated to obtain reliable concentrations of CLZ and DMC, its main metabolite, in plasma of 41 schizophrenic patients taking CLZ. Blood neutrophils and leucocytes counting were concurrently assessed as a proxy to subclinical adverse reactions.
Results
The analytical method employed was linear, reproducible, and stable to measure concentrations of CLZ between 30 and 1000 ng/mL, while 12.5–560 ng/mL of the metabolite. The method allowed us to correlate CLZ plasma concentrations, the time taking CLZ and CLZ dose as determinants of neutrophils’ counting with a R2 = 0.447, using a multiple regression analysis model. Likewise, the correlation of leucocyte counting vs CLZ plasma levels and CLZ time, showed a R2 = 0.461. DMC correlated significantly with both neutrophils and leucocytes counting, but was excluded from the regression when CLZ concentration was included in the model. Finally, no other hematological adverse reactions were recorded. One patient presented a cardiovascular complication. The negative correlation between clozapine and neutrophil count observed in patients, suggest that CLZ itself, but not DMC, could be related to hematologic side-effects.
Conclusion
The findings of this study, demonstrate for the first time, that plasma levels of CLZ and time taking the drug are independent determinants of blood neutrophils and leucocytes, so the monitoring of plasma CLZ may be useful in the clinic practice to determine safe dosing of the drug.
Similar content being viewed by others
Background
Schizophrenia is a chronic and debilitating disease, affecting approximately 0.5% of the world population [1]. It is a treatable condition, with many therapeutic alternatives, nevertheless, 20–30% fail to respond to treatment, and a similar fraction of treatment adherent patients relapse despite sustained maintenance therapy [2]. Treatment-resistant schizophrenia (TRS) is defined in clinical guidelines as a situation in which a significant improvement in psychopathology has not been demonstrated despite two or more different antipsychotic treatment trials, each with adequate dose and duration [3].
CLZ, a dibenzodiazepine developed in 1960’s, is considered an atypical antipsychotic, approved for the treatment of TRS [4]. It has been demonstrated that clozapine is more effective than any other first- generation agents (FGA) or second-generation antipsychotics (SGA) in the treatment of resistant schizophrenia. Even, it has been estimated that almost two thirds of those patients who do not respond adequately to treatment with FGAs or other SGAs, may respond adequately to treatment with clozapine [5].
The CLZ causes lower extrapyramidal symptoms from the side effects caused by neuroleptic drugs, mainly tardive dyskinesia [6]. However, the use of CLZ has been associated to agranulocytosis reported in 0.38% (995 cases) of treated patients, 12 deaths attributed to complications of agranulocytosis [7]. Some studies have shown that the stable active metabolites of CLZ, such as N-desmethyl clozapine (DMC), may related to the neutrophil counting in patients. The mechanism of neutrophil decline in patients treated with clozapine is still poorly understood. It has been suggested that clozapine metabolism involves an unstable reactive metabolite, nitrenium ion that is thought to bind covalently to macromolecules such as proteins or causing overproduction of reactive oxygen species. Under in vitro conditions, DMC has been reported to be more toxic than clozapine, but the concentrations of DMC used in that study were far above those obtained by clozapine metabolism under therapeutic dosing [8]. Reactive oxygen species and nitrenium ion neutralization both involve the use of reduced glutathione; noteworthy, in patients with refractory schizophrenia, the circulating glutathione levels are decreased as compared to control subjects [9].
The pharmacological treatment efficacy of CLZ in schizophrenic patients must be evaluated by the psychiatrist through clinical evaluation. However, the adherence to the clinical prescription is essential to be successful in the treatment. In this regard, 54% of the patients with schizophrenia or bipolar disorder reported intentional non-adherence [10], therefore, in those patients, the vigilance made by the pharmacist, to assess the compliance to medical indications in order to increase the probability of clinical success.
CLZ is a good option for pharmacological refractory schizophrenic patients and its popularity is increased [11]. It has been considered the gold standard treatment for both positive and negative refractory symptoms in schizophrenia [12]. However, the incidence of agranulocytosis and other hematic adverse effects, encourage the health professionals to put special attention to perform pharmacovigilance and to study the mechanism underlying such phenomenon.
The aim of the present study was to study the relationship between CLZ and the its main pharmacologically active metabolite, DMC plasma levels with neutrophils and leucocytes counting in schizophrenic patients taking CLZ, in order to explore the possibility that those variables are related, before a severe toxicological condition is displayed by patients.
Methods
Patients and study design
Forty-one patients, diagnosed with schizophrenia at the Neuropsychiatry Clinic of the National Institute of Neurology and Neurosurgery in Mexico City, were invited to participate in the study, which was conducted according to the Good Clinical Practices, the Declaration of Helsinki (October 1996 version) and with the approval of the local Institutional Bioethics Committee with registry number 92/07. Written informed consent to participate was obtained from all subjects before enrollment in the study, from a relative or guardian for each patient and two witnesses, according to Mexican Code of Health Regulations. The schizophrenia diagnosis was performed according to the Structured Clinical Interview (SCID) for DSM-V. The inclusion criteria were: either men or women, with diagnosis of refractory schizophrenia treated chronically with CLZ, older than 18. The exclusion criteria were: patients with another psychiatry illness, patients with a history of substance abuse in the last 3 months except tobacco, occasional consumption of alcohol or marijuana, and patients with severe anemia or with pregnancy.
The psychiatrist evaluated the efficacy of the treatment by clinical parameters (PANSS scale). The pharmacist offered treatment and illness education to patients and their families and looked for adverse reactions, evaluated the psychiatrist’s prescription, counted the patient’s medication and evaluated the safety of pharmacological treatment. CLZ treatment started with 25 mg/day dose and escalated up to 900 mg/day, according to the specialist directions. Blood samples were collected by venous puncture and centrifuged to obtain plasma samples that were stored at − 20 °C until analyzed. Clinical variables were also recorded for each patient, such as: concomitant drug treatment, and the time patients were under CLZ medication (Time taking CLZ). Even when the study is cross-sectional, the inclusion of a time variable allowed us to perform an evaluation of the impact of time on blood cells ‘counting. The time taking CLZ is also an indirect assessment of the cumulative dose taken by each patient.
Chromatographic analysis
We used a chromatographic system (Agilent technologies, model 1100), equipped with a diode array detector. The wavelength was set at 245 nm and 100 μl volume injection. An analytical column Brava Cyano, 5 μm, 250 mm × 4.6 mm (Grace, Alltech) was used. The mobile phase consisted of acetonitrile/ammonium acetate buffer 1 M (36:64 v/v), pH 5.7 and flow adjusted to 1 ml/min.
We use the protriptyline as internal standard, 250 ng/μl, clozapine and N-desmethylclozapine. Sample preparation was performed as reported by Volpicelli et al. [13] with minor modifications. The analytical method was previously validated for linearity, precision, and accuracy. We analyzed recovery, selectivity and the stability for the samples after 90 days [14].
Blood neutrophils and leukocytes counting
The neutrophils and leukocytes counting were performed at the clinical laboratory of the National Institute of Neurology and Neurosurgery. All blood samples were assessed using a XN-1000 Sysmex hematology analyzer, according to the manufacturer’s protocol.
Statistical analyses
Data were analyzed by using statistical software SPSS (IBM. Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY. USA: IBM Corp). Exploratory analysis was applied to data in order to check for normality distribution. Bivariate analyses were then applied to select variables association, using bivariate Spearman’s and Pearson’s correlations.
For the construction of multiple regression models, bivariate analyses in the form of simple lineal regression were first carried out to select variables. For those analyses, variables were selected when p < 0.05 in one-tailed tests. Multivariate modeling was done through least-squared multiple regression, considering neutrophils or leukocyte counts as dependent variables, and plasma concentrations of CLZ, DMC, age, time taking CLZ, and CLZ dose, as independent ones.
The assumption of normality of the data, after applying multiple regression models, was verified by residual analysis plots.
Results
Chromatographic analysis
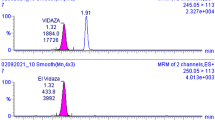
The HPLC-UV method was linear, exact, intra inter-day precision and stable to measure concentrations of CLZ between 30 and 1000 ng/mL, while 12.5–560 ng/mL of the metabolite. It was a selective method and also allowed the adequate resolution of internal standard, from CLZ and DMC, see Fig. 1. The intermediate mean recovery points ranged − 3.36 to 0.06% for CLZ and − 0.39 to − 1.02% for DMC.
The absolute deviation was within a range of − 5.61 to − 0.39% of absolute deviation for CLZ and − 5.66 to 4.59% of absolute deviation for DMC.
From the stability study, plasma samples and stock solutions of CLZ and DMC were stable for 90 days at − 70 °C, the absolute deviation has a range of − 4.18 to − 0.33% and − 5.66 to 4.59%, respectively. The stability study demonstrated that plasma samples can be maintained frozen, for up to 1 month without change in their CLZ or DMC concentrations.
Clinical features of selected patients
Forty-one patients were included in this study and the descriptive statistics are shown in the Table 1. Fifty-percent of the subjects showed concomitant illnesses, the more frequent of those was depression. Twelve percent of patients underwent a non-pharmacological treatment, a regional tea (tila tea), and massages in the head with relaxing oils. The concomitant medications taken by the patients are described in Table 2. None of them modified the effect of CLZ on cells’ counting.
Three adverse reactions were observed: only one was severe, detected as an increased QT interval for which the psychiatrist decreased the dose of CLZ employed, returning the QT interval to normality after 1 week. The pharmaceutical accessories helped the patients to comply adherence to treatment, in this study the partial adherence was 9.5%, lower than that reported by studies in the literature [10, 15]. Fifty-four percent of patients were back to work after 10 weeks on average clozapine treatment.
Bivariate determinants of neutrophils and leucocytes counting
After applying bivariate analyses, the plasma concentrations of CLZ and DMC were negatively correlated with both neutrophils and leucocytes counting (see Figs. 2 and 3 and Table 3 for bivariate correlation coefficients estimates). Also, the time taking CLZ and the CLZ dose were significantly associated to neutrophils and leucocytes counting, after bivariate analysis.
Multiple linear regression models were applied to data, in order to obtain parameter estimations for the independent determinants of the countings, taking as dependent variable either neutrophils or leucocytes counting (Table 4).
CLZ plasma concentrations, the time taking CLZ and CLZ dose remained significant determinants of neutrophils’ counting with a R2 = 0.447, using the multiple regression analysis model of Table 4. Likewise, the correlation of leucocyte counting vs CLZ plasma levels and CLZ time, showed a R2 = 0.461. As can be observed, DMC concentration was no longer an independent determinant of the countings (Table 4). In the case of leucocytes counting, time taking CLZ time is still significant after applying regression analysis, but CLZ dose was no longer a determinant.
A reduced number of patients in the study population were smokers, compared to other studies that support that more than 50% of patients with schizophrenia are smokers [16]. When tobacco smoking was included in the multiple linear model as a covariate, no relationship between neutrophils count, tobacco consumption, CLZ and DMC plasma levels were found, p = 0.893 and p = 0.905, respectively.
Discussion
The method developed and validated here for CLZ and DMC plasma quantification showed to be selective, precise, with excellent recovery percentage. We showed that the method was well-suited for determining the drug and the metabolite, not only for the purpose of the present study, but to be used in clinical pharmacovigilance of this drug or to use it for other mechanistic studies.
The CLZ plasma levels observed in the sample of patients are in the rank positively associated with CLZ efficacy and below those related to overt toxicity observed in other studies [17, 18]. In this regard, we consider that the main result of the present study is the significant correlation observed between CLZ plasma levels and the neutrophils and leucocytes counting of the patients. In our opinion, this relationship is important, because the mechanism of clozapine-induced agranulocytosis, although unclear, has been suggested to be the result of the metabolism of the drug. In this regard, the metabolic transformation of CLZ to produce reactive oxygen species and nitrenium ion, is performed by NADPH oxidase/myeloperoxidase system, and CYP3A4, CYP2D6 and CYP1A2, mainly [19] and this biotransformation might be responsible for the decrease in the leukocytes count [20]. Other possibility is that CLZ itself may act at leucocytes to promote cells’ death by apoptosis, as reported by Bergemann et al. [21]. They found an 8-times increase of CLZ intracellular leucocytes concentration in patients suffering leukocytopenia, as compared to control patients without the adverse effect. Thus, CLZ may directly activate the nitrenium ion through two isoenzymes, then promoting hemotoxicity. The results presented here are in disagreement with those of Smith, et al. [22]. They found a positive correlation between neutrophils count and plasma DMC concentrations. Other authors have found no correlation between plasma CLZ and cells‘counting [23, 24]. Two factors may explain the differences between the results presented here and previous studies exploring the subject: 1) Methodological differences, as some studies are case reports of patients with agranulocytosis or longitudinal short-term studies 2) Obvious differences in populations’ genetics and a higher percentage of smokers in the study by Smith et al. may account for the different results obtained. As that study was retrospective, they recorded no data on the time the patients were taking the medication, a significant covariate in our study. They also employed two different methods to analyse plasma CLZ and DMC, making difficult to assess the reliability of the results. In contrast, our data were obtained after a careful validation of the analytical method employed. In the case of studies showing no correlation between plasma CLZ. In the case of.
It is important to remark that the dose of CLZ taken by patients was not a strong determinant of leucocyte counting in our study, as was plasma CLZ. This suggests that metabolism of CLZ, due to differences in CYP isoforms, may influence the effect of CLZ on neutrophils and leucocytes toxicity [25]. As a result of that, monitoring plasma CLZ itself may help in detecting a CLZ overdose with consequent decrease of neutrophil count. Drug monitoring of CLZ may also help psychiatrists to decision-making on CZP drug optimization and compliance.
The intensive pharmacovigilance must aim to monitor adverse reactions at any level, since a fatal adverse reaction can occur, such as the QT prolongation observed in this study. In this case, the patient’s levels of CLZ and DMC were, 152.90 ng/ml and 93.54 ng/ml, respectively, values above the average values of the study sample.
Conclusion
In this study, we validated an analytical method by high performance liquid chromatography to measure plasma levels of CLZ, which allowed us to correlate plasma CLZ levels with the number of neutrophils and leukocytes. Likewise, the results showed that there is a negative correlation between the plasma levels of CLZ and the counting of blood neutrophils and leukocytes but not with DMC, suggesting that neutrophils and leukocytes toxicity is associated to CLZ metabolism. As the dose of CLZ taken by the patient was a poor determinant of leucocyte counting, it is of great importance to adjust CLZ doses by using CLZ monitoring, in order to maintain safe therapeutic dosing without the presence of adverse effects.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CLZ:
-
Clozapine
- DMC:
-
N-desmethyl-clozapine
- FGA:
-
First-generation agents
- SCID:
-
Structured Clinical Interview
- SGA:
-
Second-generation antipsychotics
References
Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. 2015;15:193–207.
Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76.
Howes O. When the drugs don’t work: the neurobiology of TRS and implications for new therapies. Eur Neuropsychopharmacol. 2017;27:S1135–6.
Crilly J. The history of clozapine and its emergence in the US market: a review and analysis. Hist Psychiatry. 2007;18:39–60.
Essali A, Al-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009;21:CD000059.
IBM Micromedex DRUGDEX (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA. Available at https://www.micromedexsolutions.com. ).
Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with Clozaril National Registry. J Clin Psychiatry. 1998;59(3):3–7.
Smith RL, Haslemo T, Andreassen OA, Eliasson E, Dahl ML, Spigset O, Molden E. Correlation between serum concentrations of N-Desmethylclozapine and granulocyte levels in patients with schizophrenia: a retrospective observational study. CNS Drugs. 2017;57–64.
Berk M, Copolov D, Dean O, Lu D, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Ording-Jespersen S, Litlle J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia – a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8.
Gibson S, Brand SL, Burt S, Boden ZVR, Benson O. Understanding treatment non-adherence in schizophrenia and bipolar disorder: a survey of what service users do and why. BMC Psychiatry. 2013;13:153.
Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, Fusté AC, Furu K, Garuoliené D, Hoffmann F, Hollingworth S, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, López SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Piovani D, Reutfors J, Saastomoinen LK, Sato I, Shuiling-Veninga CCM, Shyu YC, Siskind D, Skurtveit S, Verdoux H, Wang LJ, Yahni CZ, Zoëga H, Taylor D. Internacional trends in clozpine use: a study in 17 countries. Acta Psychiatr Scand. 2017;36:37–51.
Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102–6.
Volpicelli SA, Centorrino F, Puopolo PR, Kando J, Frankenburg FR, Baldessarini RJ, Flood JG. Determination of clozapine, norclozapine, and clozapine-N-oxide in serum by liquid chromatography. Clin Chem. 1993;39(Suppl 8):1656–9.
Guidence for Industry, Bioanalytical method validation, US Department of Health and Human Services, Food and Drug Administration, CDER, CVM, (May 2001).
Glimer TP, Dolder CR, Lacro JP, Folsom DP, Lindamer L, García P, Jeste DV. Adherence to treatment with antipsychotic medication and health care cost among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161:692–9.
de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiologic survey in a state hospital. Am J Psychiatry. 1995;152:453–5.
Buur-Rasmussen B, Brøsen K. Cytochrome P450 and therapeutic drug monitoring with respect to clozapine. Eur Neuropsychopharmacol. 1999;9:453–9.
Spina A, Avenoso G, Facciolà MG, Scordo MG, Ancione M, Madia AG, Ventimiglia A, Perucca E. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology. 2000;148:83–9.
Wiciński M, Weclewicz MM. Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr Opin Hematol. 2018;25:22–8.
Winnie N, Rossoune K, Uetrecht J. Effect of clozapine and olanzapine on neutrophil kinetics: implications for drug-induced agranulocytosis. Chem Res Toxicol. 2014;27:1104–8.
Bergemann N, Abu-Tair F, Aderjan R, Kopitz J. High clozapine concentrations in leukocytes in a patient who developed leukocytopenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2007;31:1068–71.
Smith RL, Haslemo T, Andreassen OA, Eliasson E, Dahl ML, Spigset O, Molden E. Correlation between serum concentrations of N-Desmethylclozapine and granulocyte levels in patients with schizophrenia: a retrospective observational study. CNS Drugs. 2017;31:991–7.
Oyewumi L, Cernovsky Z, Freeman D, Streiner D. Relation of blood counts during clozapine treatment to serum concentrations of clozapine and nor-clozapine. Can J Psychiatr. 2002;47(3):257–61.
Hasegawa M, Cola P, Meltzer H. Plasma clozapine and desmethyl clozapine levels in clozapine-induced agranulocytosis. Neuropsychopharmacology. 1994;11:45–7.
Maggs J, Williams D, Pirmohamed M. The metabolic formation of reactive intermediates from clozapine, a drug associated with agranulocytosis in man. J Pharmacol Exp Ther. 1995;275(3):1463–75.
Acknowledgements
The authors thank Psicofarma S.A. of C.V. for financial support to perform the CLZ and DMC determinations.
Funding
The present research was partially supported by FONSEC SSA-261721, from the Consejo Nacional de Ciencia y Tecnologia de Mexico (CONACYT). CONACYT grant 261721 was employed to fund the analyses of the drugs in plasma from patients. Additional CONACyT (241787) funding was granted as a Ph. D. fellowship stipend to AM V-B.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the study design, ethical committee approval and manuscript. MVB did the experimental part, did the pharmacotherapy follow-up and wrote the first draft of the manuscript. ADR reviewed relevant literature and contributed with expertise in statistics, analyses and epidemiological issues. LTL contributed with expertise in the analytical method to determine the plasma levels of CLZ and DMC. CAC contributed with the psychiatric evaluation. CT contributed with pharmacological expertise to the design and further analyses of the clinical data from patients; JRB contributed with pharmacological expertise to the clinical design and analyses of the data. He was also in charge of the clinical characterization of the patients. SM contributed with pharmacological expertise, and with expertise in the analytical method to determinate oxidative stress; and CR contributed with pharmacological, pharmacoepidemiologic and analytical issues. All authors critically reviewed the article and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the National Institute of Neurology and Neurosurgery Bioethics Committee. Written informed consent to participate was obtained from all subjects before enrollment in the study, from a relative or guardian for each patient and two witnesses, according to Mexican Code of Health Regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest and no competing interests associated with the present study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Vaquero-Baez, M., Díaz-Ruíz, A., Tristán-López, L. et al. Clozapine and desmethylclozapine: correlation with neutrophils and leucocytes counting in Mexican patients with schizophrenia. BMC Psychiatry 19, 295 (2019). https://doi.org/10.1186/s12888-019-2286-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-019-2286-1