Abstract
Background
The fundamental mechanism underlying emotional processing in major depressive disorder (MDD) remains unclear. To better understand the neural correlates of emotional processing in MDD, we investigated the role of multiple functional networks (FNs) during emotional stimuli processing.
Methods
Thirty-two medication-naïve subjects with MDD and 36 healthy controls (HCs) underwent an emotional faces fMRI task that included neutral, happy and fearful expressions. Spatial independent component analysis (sICA) and general linear model (GLM) were conducted to examine the main effect of task condition and group, and two-way interactions of group and task conditions.
Results
In sICA analysis, MDD patients and HCs together showed significant differences in task-related modulations in five FNs across task conditions. One FN mainly involving the ventral medial prefrontal cortex showed lower activation during fearful relative to happy condition. Two FNs mainly involving the bilateral inferior frontal gyrus and temporal cortex, showed opposing modulation relative to the ventral medial prefrontal cortex FN, i.e., greater activation during fearful relative to happy condition. Two remaining FNs involving the fronto-parietal and occipital cortices, showed reduced activation during both fearful and happy conditions relative to the neutral condition. However, MDD and HCs did not show significant differences in expression-related modulations in any FNs in this sample.
Conclusions
SICA revealed differing functional activation patterns than typical GLM-based analyses. The sICA findings demonstrated unique FNs involved in processing happy and fearful facial expressions. Potential differences between MDD and HCs in expression-related FN modulation should be investigated further.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is a common and serious mental illness linked to major role impairment, reduced life satisfaction, and high rates of mortality, including suicide [1, 2]. Despite increasing numbers of functional magnetic resonance imaging (fMRI) studies investigating abnormalities in affective processing in MDD, the fundamental mechanism underlying emotional processing in MDD remains unclear and is a major research goal. Abnormal processing of emotional stimuli has been consistently implicated in MDD and is thought to increase risk of disease development, maintenance, and relapse [3,4,5]. Cognitive models of depression suggest that MDD is characterized by mood congruent emotion processing biases, particularly for negative emotional faces [6, 7]. Studies of facial emotion processing provide important information regarding regional brain function abnormalities in MDD and emotional face stimuli are often used in neuroimaging research in MDD.
Neuroimaging studies have made substantial contributions to the understanding of how facial expressions are processed in humans. Some basic emotions can be most reliably recognized from facial expressions (i.e., fear, disgust, anger, happiness, sadness) [8] and have been shown to be universal in their performance and perception [9]. An extensive neural network of areas has been implicated in emotional face processing [10]. For example, during the processing of fearful faces, there is increased neural activation in the amygdala [11, 12], fusiform gyrus [12, 13], inferior frontal gyrus, superior temporal sulcus (STS) [14], and orbitofrontal cortex [15], and deactivation in the ventral anterior cingulate cortex (ACC) [14] in healthy participants. Most of these areas have also been implicated in MDD [16], there is greater activation in amygdala, fusiform gyrus, inferior parietal lobule, inferior frontal gyrus [10], and lower activation in dorsolateral prefrontal cortex (DLPFC) [17], temporal cortex and insula [5] and inferior frontal gyrus [18]. Meanwhile, processing of happy faces has been associated with increased activation in the fusiform gyrus [19] and medial frontal cortex [20], and deactivation in the temporal cortex [14] in healthy participants. MDD has also been associated with increased activation in medial frontal gyrus, insula, middle temporal gyrus and middle occipital gyrus [10], and reduced activation in temporal cortex and insula [21]. These studies were conducted using an hypothesis-driven approach that assesses for differences within several specific networks or between a few regions of interest; such approaches could be biased by a priori definition of regions or networks and do not allow for a broad, discovery-based investigation of neural networks that may contribute to emotional processing differences.
Current neurobiological models for the pathophysiology of MDD postulate that ventral and dorsal subsystems involving multiple functional networks (FNs) are differentially affected in MDD [22,23,24]. An imbalanced functional integration of these subsystems may lead to a heightened response to negative information in ventral regions (bottom–up) on the one hand, and a failure to regulate this response through dorsal regions (top–down) on the other [25]. However, no studies to date have investigated emotional processing FNs from the whole-brain level, instead choosing to focus on predefined regions or networks of interest. An exploratory whole brain approach would allow for a more nuanced study of the relative contributions of FNs that may work in opposition to each other during the processing of emotional face stimuli. Furthermore, inconsistencies in the literature make interpretation of the role of FNs in face processing challenging. For example, aberrant responses to happy face stimuli have been shown in more ventral lateral PFC (VLPFC) areas [17, 26], as well as to fearful face stimuli in the insula [18, 27]. The potential function of overlapping regions among different FNs requires further clarification.
Independent component analysis (ICA) is a source-blind separation technique that has become a major analysis tool in fMRI studies [28,29,30]. Spatial ICA (sICA) assumes that most of the components generated by sICA and fMRI data are consistent in spatial patterns across different studies and populations [31,32,33]. SICA is more sensitive in detecting task-related and resting state activity changes in fMRI signal than the traditional general linear model-based (GLM) analysis. Because sICA uses a data-driven approach, it can reduce noise in the final analysis by separating artifacts from real fMRI signal. Relevant sICA studies have demonstrated unique findings that are not revealed from a standard GLM approach [29, 34,35,36]. So far, task-based sICA is limited to several tasks that investigate cognitive domains including attention, working memory, and/or response inhibition, finding that the FNs maintain their general spatial patterns but also show task-related modulations, including changes in spatial extent and strength of internal functional connectivity [36, 37]. However, the modulation of FNs during an emotion face paradigm that includes positive, negative and neutral stimuli is unknown. According to previous findings in studies of emotional processing [7, 10,11,12,13,14,15,16,17,18,19,20,21], likely relevant brain regions include prefrontal, fronto-parietal, auditory, and visual networks in sICA [38, 39]. Understanding the functions of these FNs will improve our understanding of brain functional organization in health and in the neuropathophysiology of MDD.
In order to better understand the role of different FNs, sICA was utilized to extract FNs from a fMRI dataset acquired during an emotional face paradigm in healthy and MDD participants. We aimed to characterize these FNs in medication-naive individuals with MDD using sICA. We also applied GLM to explore emotion processing networks using an exploratory, whole brain approach. By using both sICA and GLM, we wished to assess differing findings using these methods. We hypothesized that sICA would reveal greater activation in the amygdala, fusiform gyrus when processing negative emotions, and greater activation in the insula when processing positive emotional faces in MDD. We also hypothesized that facial emotion processing would be associated with multiple FNs, including prefrontal, fronto-parietal, auditory, and visual networks.
Methods
Participants
The Institutional Review Board of the China Medical University approved our study protocol. The participants provided written informed consent after receiving a detailed description of the study.
We recruited 32 medication-naive patients with MDD ages 19–46 years from the outpatient clinic at the Department of Psychiatry, First Affiliated Hospital of China Medical University and the Mental Health Center of Shenyang. MDD diagnosis was confirmed by two trained psychiatrists using the Structured Clinical Interview for DSM-IV. To be included in our study, individuals with MDD had to fulfill the DSM-IV criteria for MDD, have a current depressive episode, have no comorbid Axis I or II diagnoses, have a score indicating clinically significant depressive symptoms on the 17-item Hamilton Rating Scale for Depression (HAMD-17, at least 24) and Hamilton Anxiety Rating Scale (HAM-A), and have no history of psychopharmacological therapy, electroconvulsive therapy, or psychotherapy.
We recruited 36 healthy controls (HC) from Shenyang, China, via advertisement. The absence of DSM-IV Axis I disorders in controls was confirmed by two independent psychiatrists (L.K. and F.W.) using the Structured Clinical Interview for DSM-IV disorders. Individuals with first-degree family members who had a history of DSM-IV Axis I disorders were excluded.
Additional exclusion criteria for both individuals with MDD and controls were the presence of any MRI contraindications, history of head injury or neurologic disorder, history of drug abuse or dependence, and any concomitant medical disorder. All participants were scanned within 24 h of initial contact with the research team. The participants provided written informed consent after receiving a detailed description of the study. The Institutional Review Board of the China Medical University approved our study protocol.
Emotional face paradigm
During the fMRI scan, each participant completed an event-related implicit facial emotion task, which has been described previously [40, 41]. Participants viewed faces from the Ekman series depicting negative (fear), positive (happiness) or neutral emotional expressions, and were instructed to press a button to make a male-female determination. In brief, 5 male and 5 female faces were each presented for 2 s with inter-stimulus-intervals of 4, 8 or 12 s. Each of the 3 expressions was shown for each individual, for a total of 30 facial stimuli and a run time of 5 min, 6 s in randomized order. Instructions were presented on the computer at the beginning of each run, and subjects were asked to respond as quickly and accurately as possible. We introduced the viewing instructions before scanning, and performed emotional recognition testing after the scan. Subjects in both groups performed similarly and with high accuracy.
MRI data acquisition and preprocessing
The fMRI data were acquired using a GE Signa HDX 3.0 T MRI scanner at the First Affiliated Hospital of China Medical University, Shenyang, China. Head motion was minimized with restraining foam pads. We used a standard head coil for radiofrequency transmission and reception of the nuclear magnetic resonance signal. The fMRI images were acquired using a spin–echo planar imaging sequence, parallel to the AC-PC line with the following scan parameters: repetition time 2000 ms, echo time 40 ms, image matrix 64 × 64, field of view 24 × 24 cm2, 35 contiguous slices of 3 mm and without gap.
Data were analyzed with Statistical Parametric Mapping (SPM8, www.fil.ion.ucl.ac.uk/spm/software/spm8). The first 10 volumes were deleted. Data preprocessing included slice timing, realignment (motion-corrected), spatial normalization and smoothing. Head motion parameters were computed by estimating translation in each direction and the angular rotation about each axis for each volume. We tested for group differences in head motion by using a two-sample t test according to mean framewise displacement Jenkinson measurement [42, 43]. Participants were excluded if their head motion was > 2 mm maximum displacement in any of the x, y or z directions or 2 degrees of any angular motion throughout the course of the scan (no participants were excluded). Spatial normalization was performed using a standard EPI template from the Montreal Neurological Institute (MNI),resampled to 3 × 3 × 3 mm and spatially smoothed with an 8 mm full-width at half-maximum (FWHM) Gaussian kernel.
SPM analysis
We performed single subject analysis first and compared BOLD signal changes using the contrast of [fearful vs. neutral] and [happy vs. neutral]. The contrast maps from each subject were fed into a second level group analysis (i.e., random effect). We used a two sample t-test in SPM to assess for differences in BOLD signal between groups during fearful and happy face conditions. The results were thresholded at cluster level p < 0.05, family-wise error (FWE) corrected for voxel-wise whole brain analysis. Voxel level threshold was set at p < 0.01.
SICA analysis
Group ICA algorithm (GIFT, http://icatb.sourceforge.net/, version1.3 h) was used to extract spatially independent components (ICs) from the whole dataset [44, 45]. Data from all participants from the two groups were concatenated into a single dataset and reduced using two stages of principal component analysis (PCA) [44]. We extracted 75 ICs using Infomax algorithm [46]. The reason for using this high model order ICA is that it generates refined components consistent with known anatomical and functional segmentations of the brain [38, 39, 47,48,49,50,51]. The Infomax algorithm generated a spatial map and a timecourse of BOLD signal changes for each IC. This analysis was repeated 50 times using ICASSO for assessing the repeatability of ICs [52] (Additional file 1: Figure S1). The cluster quality index (Iq) was set greater than 0.9, indicating a highly stable ICA decomposition. Finally, IC timecourses and spatial maps were back-reconstructed for each participant [44, 53, 54].
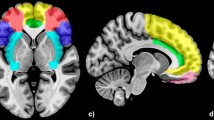
Two investigators (Xu and Li) visually inspected each IC to separate artifacts from FNs. Our diagnostic criteria for artifacts were consistent with the criteria used by Allen and colleagues [38]. ICs with peak voxels at white matter and/or cerebrospinal fluid (CSF) were diagnosed as artifacts. Small dynamic range of power spectra and/or small ratio of the integral of spectral power below 0.10 Hz to the integral of power between 0.15 and 0.25 Hz were used as indicators of artifacts [38, 55]. For defining brain regions associated with each IC, we used the GIFT one sample t test tool to create a group-level t-map for each IC. The significance threshold was set at voxel height p < 0.001, False-Discovery-Rate (FDR) corrected for multiple comparisons of voxel-wise whole-brain analysis. Additional file 2: Figure S2 and Additional file 3: Table S1 show spatial maps of 32 ICs identified as FNs.
Assessing task-related modulation over timecourses
To examine task engagement of each IC, the temporal sorting function from GIFT was used to perform a multiple regression analysis between IC timecourse and the design matrix of each participant created during first level SPM analysis. For each IC, this regression generated a beta-weight value for each trial type. An increase in the beta-weight value for one trial type relative to another indicates a greater task-related activation of related IC at the first trial type. SPSS (version 21.0, IBM Corporation, Armonk, NY, USA) general linear model for repeated measures were used to assess the main effect of trial type, group, and the interaction of trial type x group on the beta-weight of 32 ICs (confidence intervals were 95%). The significance threshold of all statistical analyses were set at p < 0.05, using FDR to correction of multiple comparisons due to multiple ICs. Additional file 4: Table S2 shows the beta-weight and p values of each IC.
Results
The MDD and control groups did not show significant differences in age, gender, and education. The MDD group exhibited a significant higher score on HAMD-17 and HARS relative to the control group (p<0.001, Table 1).
In sICA, among the 32 ICs classified as FNs, five showed significant main effects of task condition. There was no significant IC for main effect of group, or two-way interaction of group and task conditions. Therefore, we combined HCs and patient data together as one group for describing task-related modulations in IC timecourses as described below.
IC3 [F(2,65) = 6.017, p = .004, η2 = .156] mainly involves ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC) and bilateral orbital frontal cortex (OFC) and is consistent with the vmPFC network [56,57,58]. It showed significant opposing modulation between happy vs. fearful faces (Fig. 1). Relative to neutral face, it showed positive and negative modulations during happy and fearful faces, respectively. IC34 [F(2,65) = 7.718, p = .002, η2 = .181] mainly involves the right lateral frontal and parietal cortex and is consistent with the right fronto-parietal network (RFPN) [24, 35, 37, 39, 56]. IC54 [F(2,65) = 7.295, p = .001, η2 = .183] mainly involves the fusiform gyrus and middle occipital gyrus, bilaterally, and is consistent with the visual network [24, 39, 56]. Both of these ICs showed a negative modulation during happy and fearful relative to neutral face conditions (Figs. 2 and 3). IC1 [F(2,65) = 8.656, p = .000, η2 = .210] mainly involves the inferior frontal gyrus (IFG) and precentral gyrus, bilaterally, and is consistent with the sensorimotor network [37, 38, 47]. IC9 [F(2,65) = 7.295, p = .001, η2 = .183] mainly involves the temporal cortex and posterior insula, bilaterally, and is consistent with the auditory network [24, 39, 56]. Both ICs showed positive modulation during fearful relative to happy and neutral faces (Figs. 4 and 5).
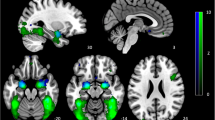
Task-related modulation of IC3 (vmPFC network). a Red-yellow color on T1 template in MNI space shows the spatial distribution of IC3. Color bar indicates t values. The number to the bottom right of the axis indicates z coordinates. b Line graph shows the beta weight (Y-axis) of IC3 at each task condition (X-axis)
Task-related modulation of IC34 (RFPN). a Red-yellow-blue color on T1 template in MNI space shows the spatial distribution of IC34. Color bar indicates t values. The number to the bottom right of the axis indicates z coordinates. b Line graph shows the beta weight (Y-axis) of IC34 at each task condition (X-axis)
Task-related modulation of IC54 (visual network). a Red-yellow-blue color on T1 template in MNI space shows the spatial distribution of IC54. Color bar indicates t values. The number to the bottom right of the axis indicates z coordinates. b Line graph shows the beta weight (Y-axis) of IC54 at each task condition (X-axis)
Task-related modulation of IC1 (sensorimotor network). a Red-yellow color on T1 template in MNI space shows the spatial distribution of IC1. Color bar indicates t values. The number to the bottom right of the axis indicates z coordinates. b Line graph shows the beta weight (Y-axis) of IC1 at each task condition (X-axis)
Task-related modulation of IC9 (auditory network). a Red-yellow-blue color on T1 template in MNI space shows the spatial distribution of IC9. Color bar indicates t values. The number to the bottom right of the axis indicates z coordinates. b Line graph shows the beta weight (Y-axis) of IC9 at each task condition (X-axis)
We did not observe significant group difference for task conditions, or two-way interaction of group and task conditions in SPM analysis.
Discussion
To the best of our knowledge, this is the first study to investigate the modulation of FNs during an emotional face task using sICA. SICA is more sensitive in detecting different emotional correlations with the task-positive FNs than GLM, because sICA allows for the detection of both opposing and coincident correlations of fearful and happy conditions, which is not possible in GLM-based analysis. According to our sICA findings, there were significant differences across emotional conditions in 5 ICs, including the vmPFC network, RFPN, visual network, sensorimotor network, and auditory network. The spatial patterns of these FNs are consistent with reported FNs by sICA and fMRI data acquired either during rest or task performance, including a number of visual areas, temporal areas, parietal areas and prefrontal areas [10]. Based on the modulation of emotional face stimuli, the 5 ICs presented were divided into three types. Although we report modulation of multiple FNs, suggesting that extensive neural networks are implicated in face processing, there were not significant differences in diagnostic groups or any two-way interaction effects. This unexpected finding will be discussed in more detail below.
Fearful<happy
The vmPFC network (IC3) demonstrated a negative correlation with the fearful face condition and a positive correlation with the happy face condition, suggesting opposing modulation of the vmPFC network by emotional valence. As a major region in this network, the vmPFC is known to be involved in emotional processing and affect regulation. The vmPFC has been implicated in human and animal studies of conditioned fear extinction [59, 60]. Coupling between the vmPFC and the amygdala has previously been found during down-regulation of negative affect in healthy controls [61], as reflected by decreasing amygdala activation with increasing vmPFC activation. The inverse functional coupling suggests that vmPFC exerts a top-down inhibitory influence on the activity of the amygdala to transform and down-regulate the experience of negative stimuli [61]. In a similar study in depression, MDD patients showed a positive association between the vmPFC and the amygdala, potentially reflecting impaired top-down control over amygdala responses and inability to down-regulate negative affect [62]. On the other hand, the vmPFC activity is associated with happy faces positively. Previous research has shown that both younger and older adults demonstrate greater vmPFC activity to happy compared to angry or neutral faces [63]. There is evidence that patient with MDD process and interpret positive stimuli as negative [64]. These results suggest different roles for aberrant vmPFC activity in response to negative and positive valence stimuli.
Fearful and happy<neutral
The RFPN (IC34) and visual network (IC54) demonstrated negative correlation with fearful and happy conditions, and were significant decreased in fearful and happy relative to neutral face conditions. To our knowledge, the overlapping modulation of these two networks in fearful and happy face conditions has not been reported before.
The FPN is commonly engaged in supporting memory, attention, cognitive control and decision-making processes [65]. The laterality of FPN is likely related to different aspects in regulation of emotional processing. Disparate behavior of these lateralized networks has been noted previously [31, 39] and corresponds well with task-based findings. The LFPN is more implicated in explicit memory retrieval related with recollection, whereas the RFPN is likely to be influenced by familiarity [66]. Although the FPN comprises both frontal and parietal regions, most studies have primarily investigated the DLPFC, mainly because the DLPFC plays an important role in the top-down regulation of emotional processing [67,68,69]. DLPFC has been associated with cognitive or executive functions [70], as well as emotion [71, 72]. Our findings of down modulation for both fearful and happy conditions suggests the RPFN participates in the regulation of emotion generally.
The visual network has been implicated in the perceptual processing of facial expressions [7], including primary, lateral and medial visual cortex [24]. In particular, the face-selective areas in the fusiform gyrus exhibit greater activation to emotional than to neutral faces in both PET and fMRI studies [73, 74], this phenomenon is more common for fearful faces than for happy faces [75, 76]. In the current study, there was similar modulation patterns of the visual network in the processing of both emotional face conditions. These results would be consistent with the existence of direct connections between visual cortex and the amygdala, direct feedback signals from the amygdala [77]. An early mechanism for identifying emotional stimuli would be adaptive; rapid identification and processing of emotional stimuli can signify both potential threat and potential reward [78]. However, we found similar modulation in response to positive and negative emotional stimuli in current study. The unusual finding could be derived from the potential function of overlapping regions within visual network. Whether the mechanism of modulation in positive emotional face stimuli is the same as in negative stimuli warrants further exploration.
Fearful>happy
The sensorimotor network (IC1) and auditory network (IC9) showed significantly increased activation in the fearful relative to happy and neutral conditions. There was no significant difference between the happy and neutral conditions. Previously, these two networks have not been considered key components concerning emotional stimuli processing and have been given little attention.
The available evidence suggests a role of the IFG in general inhibitory processes [79], and an index of successful inhibition of distracting emotional stimuli in HCs [80, 81]. Emotional distraction often interferes with cognitive processing. Several fMRI studies [82,83,84] suggest that during attention tasks, enhanced IFG response is associated with the impaired ability to divert attention from task-irrelevant negative emotional information in MDD. We observed that the bilateral IFG within the sensorimotor network showed greater activation in the fearful relative to happy and neutral conditions. Our findings regarding negative emotional stimuli are in line with previous studies.
Early perceptual processing of faces has been suggested to draw on the temporal cortex that construct detailed representations from the configuration of facial features [9], although the exact functional interplay between these areas is not clear. Our findings in the auditory network showed significant activation in response to fearful stimuli. However, deactivation during viewing of fearful faces has been found in the bilateral temporal cortex in adolescents [14]. In another study, whole-brain analyses revealed lower activation in that right superior/middle temporal gyrus in MDD when viewing fearful faces [5]. More research is needed to determine whether such inconsistencies that may be related to differences in subjects, task design, or analytic methodology. The mechanism underlying differences in network modulation requires further investigation.
Negative findings
There was no significant IC for main effect of group, or two-way interaction of group and task conditions in this study, which may be ascribed to several possible factors. Unlike many previous studies, all participants with MDD in the present study were experiencing acute depressive episodes; therefore our findings may not necessarily be generalized to the entire MDD population. Future studies of participants in the euthymic state or individuals at risk for MDD are warranted to elucidate connectivity abnormalities associated with MDD neuropathophysiology more generally. Likewise, previous studies have typically included participants who have experienced multiple depressive episodes and therapies for depression, including pharmacological therapies. It could be that our negative finding is due to the fact that our participants were unmedicated, first episode, and had never received treatment of any kind for depression. A previous longitudinal study of duloxetine treatment in MDD found that treatment normalized resting state activity relative to healthy controls, but that there were no differences in activity during an emotional face task at baseline or group x time effects of treatment [85]. Other longitudinal studies of medication effects have found activity differences in the amygdala in response to emotional faces; however, these studies are not of first episode populations [86]. It is likely that our sample includes individuals that will differ in their clinical outcome; some may remit and some may have a more chronic illness course. Future studies are needed to conduct longitudinal assessments of treatment and illness course impact on FN modulation during emotion processing. Additionally, fearful faces were included as a negative emotion in the study. The most frequently observed negative emotional biases in MDD are toward sad and/or angry faces. Future studies including more negative emotions are necessary to better understand the neural correlates of emotion processing in MDD. Negative findings could also be due to the scan duration used here (one run),which might be insufficient to detect significant FN differences. Future studies including more runs and more emotional conditions are needed to fully understand the neuropathophysiology of MDD.
Limitations
All participants in this study simply watched the emotional stimuli passively without being asked to respond; no behavioral data (subjective ratings of the emotional value of the stimuli) were collected during the fMRI session. Although passive viewing tasks are useful for engaging implicit emotional processing circuity, passive viewing makes it difficult to control for possible differences in attention or emotion recognition accuracy between the two groups, although we introduced viewing instructions before scanning, and performed emotional recognition testing after the scan that did not show group differences. Subjects in both groups performed similarly and with high accuracy. Additionally, Western faces from the Ekman’s emotional expressions were viewed by participants in emotion paradigm. Although facial expressions of primary emotions are universal, cross-cultural differences in emotion expression intensity could have influenced the way in which participants processed these stimuli, particularly negative emotions. Assessing the validity in Han Chinese populations of standardized emotional face stimuli designed with an assumed Western audience is an important goal for future studies. Finally, although performed using an exploratory approach, sICA is still associated with methodological constraints. The spatial pattern of each IC may be differ dependent on the numbers of ICs extracted. Therefore, the number and location of FN overlap may change based on the number of extracted ICs. Because of this, we have to infer each network’s modularity and hierarchical order based on existing knowledge and available literature. However, it has been demonstrated that ICs remain accurate across a large range of numbers of selected ICs, and many ICs generated by sICA and fMRI data are very consistent in spatial patterns across different studies and populations. Network homogeneity provides a potential alternative approach to assess the homogeneity of specific large-scale networks, which should be used to validate the results in the future.
Conclusion
This is the first study to investigate the modulation of FNs during implicit processing of face emotions using sICA. Our results demonstrated unique FNs involved in processing happy and fearful facial expressions. These findings suggest that the sICA method may be more sensitive than a GLM approach; sICA is an important complementary tool for assessment of FN modulation, in addition to the GLM method. Although we did not report differences between patients with MDD and controls in this study, future research should assess for effects of mood state, treatment history, and illness course on the emotional modulation of FNs.
Abbreviations
- ACC:
-
anterior cingulate cortex
- CSF:
-
cerebrospinal fluid
- DLPFC:
-
dorsolateral prefrontal cortex
- FDR:
-
False-Discovery-Rate
- fMRI:
-
functional MRI
- FNs:
-
functional networks
- FWE:
-
family-wise error
- FWHM:
-
full-width at half-maximum
- GLM:
-
general linear model
- HAM-A:
-
Hamilton Anxiety Rating Scale
- HAMD:
-
Hamilton Rating Scale for Depression
- HCs:
-
healthy controls
- ICA:
-
Independent component analysis
- ICs:
-
independent components
- IFG:
-
inferior frontal gyrus
- Iq:
-
quality index
- MDD:
-
major depressive disorder
- MNI:
-
Montreal Neurological Institute
- N/A:
-
not applicable
- OFC:
-
orbital frontal cortex
- PCA:
-
principal component analysis
- RFPN:
-
right fronto-parietal network
- SD:
-
standard deviation
- sICA:
-
Spatial independent component analysis
- VLPFC:
-
ventral lateral PFC
- vmPFC:
-
ventromedial prefrontal cortex
References
Nes RB, Czajkowski NO, Roysamb E, Orstavik RE, Tambs K, Reichborn-Kjennerud T. Major Depression and life satisfaction: a population-based twin study. J Affect Disord. 2013;144:51–8.
Zhang J, Wang J, Wu Q, Kuang W, Huang X, He Y, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry. 2011;70:334–42.
Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–95.
Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front Psychol. 2012;3:489.
Hall LM, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, et al. An fMRI study of emotional face processing in adolescent major depression. J Affect Disord. 2014;168:44–50.
Dannlowski U, Kersting A, Lalee-Mentzel J, Donges US, Arolt V, Suslow T. Subliminal affective priming in clinical depression and comorbid anxiety: a longitudinal investigation. Psychiatry Res. 2006;143:63–75.
Leppanen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–9.
Ekman P, Friesen WV, O'Sullivan M, Chan A, Diacoyanni-Tarlatzis I, Heider K, et al. Universals and cultural differences in the judgments of facial expressions of emotion. J Pers Soc Psychol. 1987;53:712–7.
Adolphs R. Neural Systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–77.
Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–32.
Sauder CL, Hajcak G, Angstadt M, Phan KL. Test-retest reliability of amygdala response to emotional faces. Psychophysiology. 2013;50:1147–56.
Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat Neurosci. 2004;7:1271–8.
Campanella S, Bourguignon M, Peigneux P, Metens T, Nouali M, Goldman S, et al. BOLD response to deviant face detection informed by P300 event-related potential parameters: a simultaneous ERP-fMRI study. NeuroImage. 2013;71:92–103.
Rahko J, Paakki JJ, Starck T, Nikkinen J, Remes J, Hurtig T, et al. Functional mapping of dynamic happy and fearful facial expression processing in adolescents. Brain Imaging Behav. 2010;4:164–76.
Satterthwaite TD, Wolf DH, Gur RC, Ruparel K, Valdez JN, Gur RE, et al. Frontolimbic responses to emotional face memory: the neural correlates of first impressions. Hum Brain Mapp. 2009;30:3748–58.
Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biol Mood Anxiety Disord. 2011;1:10.
Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–87.
Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, et al. Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol. 2011;88:233–42.
Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–9.
Kessler H, Doyen-Waldecker C, Hofer C, Hoffmann H, Traue HC, Abler B. Neural Correlates of the perception of dynamic versus static facial expressions of emotion. Psychosoc Med. 2011;8
Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, et al. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry. 2010;67:155–60.
Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207.
Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118.
Veer IM, Beckmann CF, van Tol MJ, Ferrarini L, Milles J, Veltman DJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010; 4. pii: 41.
Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–28.
Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, et al. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–4.
Townsend JD, Eberhart NK, Bookheimer SY, Eisenberger NI, Foland-Ross LC, Cook IA, et al. fMRI activation in the amygdala and the orbitofrontal cortex in unmedicated subjects with major depressive disorder. Psychiatry Res. 2010;183:209–17.
McKeown MJ, Sejnowski TJ. Independent component analysis of fMRI data: examining the assumptions. Hum Brain Mapp. 1998;6:368–72.
Calhoun VD, Adali T, McGinty VB, Pekar JJ, Watson TD, Pearlson GD. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. NeuroImage. 2001;14:1080–8.
Kiviniemi V, Kantola JH, Jauhiainen J, Hyvarinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. NeuroImage. 2003;19:253–60.
Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–38.
Domagalik A, Beldzik E, Fafrowicz M, Oginska H, Marek T. Neural networks related to pro-saccades and anti-saccades revealed by independent component analysis. NeuroImage. 2012;62:1325–33.
Raichle ME. The restless brain. Brain Connect. 2011;1:3–12.
Beldzik E, Domagalik A, Daselaar S, Fafrowicz M, Froncisz W, Oginska H, et al. Contributive sources analysis: a measure of neural networks' contribution to brain activations. NeuroImage. 2013;76:304–12.
Xu J, Calhoun VD, Pearlson GD, Potenza MN. Opposite modulation of brain functional networks implicated at low vs. high demand of attention and working memory. PLoS One. 2014;9:e87078.
Xu J, Zhang S, Calhoun VD, Monterosso J, Li CS, Worhunsky PD, et al. Task-related concurrent but opposite modulations of overlapping functional networks as revealed by spatial ICA. NeuroImage. 2013;79:62–71.
Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp. 2012;33:89–104.
Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2.
Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–5.
Kerestes R, Bhagwagar Z, Nathan PJ, Meda SA, Ladouceur CD, Maloney K, et al. Prefrontal cortical response to emotional faces in individuals with major depressive disorder in remission. Psychiatry Res. 2012;202:30–7.
Shah MP, Wang F, Kalmar JH, Chepenik LG, Tie K, Pittman B, et al. Role of variation in the serotonin transporter protein gene (SLC6A4) in trait disturbances in the ventral anterior cingulate in bipolar disorder. Neuropsychopharmacology. 2009;34:1301–10.
Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, et al. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201.
Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–41.
Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51.
Calhoun VD, Liu J, Adali T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–72.
Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–59.
Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, et al. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30:3865–86.
Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, et al. Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp. 2009;30:615–24.
Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31:1207–16.
Ystad M, Eichele T, Lundervold AJ, Lundervold A. Subcortical functional connectivity and verbal episodic memory in healthy elderly--a resting state fMRI study. NeuroImage. 2010;52:379–88.
Balsters JH, Robertson IH, Calhoun VD. BOLD frequency power indexes working memory performance. Front Hum Neurosci. 2013;7:207.
Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–22.
Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–95.
Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS One. 2009;4:e7911.
Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, et al. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 2009;10:137.
Wei M, Qin J, Yan R, Li H, Yao Z, Lu Q. Identifying major depressive disorder using Hurst exponent of resting-state brain networks. Psychiatry Res. 2013;214:306–12.
Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–77.
Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav Brain Res. 2009;201:239–43.
Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4.
Phelps EA, Delgado MR, Nearing KI, JE LD. Extinction Learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905.
Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–25.
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–84.
Ebner NC, Johnson MK, Fischer H. Neural Mechanisms of reading facial emotions in young and older adults. Front Psychol. 2012;3:223.
Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, et al. Neural responses to happy facial expressions in major depression following antidepressant treatment. Am J Psychiatry. 2007;164:599–607.
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42.
Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. Frontoparietal network involved in successful retrieval from episodic memory. Spatial and temporal analyses using fMRI and ERP. Cereb Cortex. 2006;16:1349–60.
Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9.
Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99.
Fitzgerald PB, Srithiran A, Benitez J, Daskalakis ZZ, Oxley TJ, Kulkarni J, et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum Brain Mapp. 2008;29:490–501.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202.
Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–9.
Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28:409–23.
Winston JS, O'Doherty J, Dolan RJ. Common and distinct neural responses during direct and incidental processing of multiple facial emotions. NeuroImage. 2003;20:84–97.
Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121(Pt 1):47–57.
Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87.
Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–94.
Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: what is magic and what is not. Biol Psychol. 2013;92(3):492–512.
Kim MJ, Solomon KM, Neta M, Davis FC, Oler JA, Mazzulla EC, et al. A face versus non-face context influences amygdala responses to masked fearful eye whites. Soc Cogn Affect Neurosci. 2016;11(12):1933–41.
Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7.
Johnson MK, Raye CL, Mitchell KJ, Greene EJ, Cunningham WA, Sanislow CA. Using fMRI to investigate a component process of reflection: prefrontal correlates of refreshing a just-activated representation. Cogn Affect Behav Neurosci. 2005;5:339–61.
Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26:2072–9.
Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry. 2009;66:886–97.
Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, et al. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–55.
Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604.
Fu CH, Costafreda SG, Sankar A, Adams TM, Rasenick MM, Liu P, et al. Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry. 2015;15:82–92.
Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology. 2015;40:2398–408.
Acknowledgements
Not applicable.
Dedication
This paper is dedicated to the memory of the senior author, Jiansong Xu, who passed away during preparation of this paper. Dr. Xu was a beloved mentor, colleague, and friend whose dedication to psychiatric neuroscience was an inspiration to all who knew him. His death is a loss to the field and he will be greatly missed.
Funding
National Natural Science Foundation of China (81571311, 81071099 and 81271499 to Y.T., 81571331 to F.W.), Liaoning Pandeng Scholar (to F.W.), the National Institution of Health (K01MH086621 to F.W.). The funders had no role in design, conduct, or reporting of the study.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: JL FW. Performed the experiments: JL. Analyzed the data: JL JSX FW. Contributed reagents/materials/analysis tools: JL JSX FW. Wrote the paper: JL EKE YQT GGF KX FW JSX.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study will be conducted in accordance with the Helsinki Declaration, meaning that participation is voluntary and written informed consent will be obtained.
The study has been approved by the medical ethical committee of the China Medical University Centre, Shenyang, China (registration number [2016]2016–47-2).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. ICASSO results. (PDF 125 kb)
Additional file 2:
Figure S2. Matched ICs from different datasets. (PDF 2121 kb)
Additional file 3:
Table S1. Brain regions showing task-related changes in BOLD signal assessed by SPM8. (PDF 106 kb)
Additional file 4:
Table S2. Betaweights at each task condition and related p values. (PDF 99 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, J., Kale Edmiston, E., Tang, Y. et al. Shared facial emotion processing functional network findings in medication-naïve major depressive disorder and healthy individuals: detection by sICA. BMC Psychiatry 18, 96 (2018). https://doi.org/10.1186/s12888-018-1631-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-018-1631-0