Abstract
Background
The emergence of COVID-19 precipitated containment policies (e.g., lockdowns, school closures, etc.). These policies disrupted healthcare, potentially eroding gains for Sustainable Development Goals including for neonatal mortality. Our analysis aimed to evaluate indirect effects of COVID-19 containment policies on neonatal admissions and mortality in 67 neonatal units across Kenya, Malawi, Nigeria, and Tanzania between January 2019 and December 2021.
Methods
The Oxford Stringency Index was applied to quantify COVID-19 policy stringency over time for Kenya, Malawi, Nigeria, and Tanzania. Stringency increased markedly between March and April 2020 for these four countries (although less so in Tanzania), therefore defining the point of interruption. We used March as the primary interruption month, with April for sensitivity analysis. Additional sensitivity analysis excluded data for March and April 2020, modelled the index as a continuous exposure, and examined models for each country. To evaluate changes in neonatal admissions and mortality based on this interruption period, a mixed effects segmented regression was applied. The unit of analysis was the neonatal unit (n = 67), with a total of 266,741 neonatal admissions (January 2019 to December 2021).
Results
Admission to neonatal units decreased by 15% overall from February to March 2020, with half of the 67 neonatal units showing a decline in admissions. Of the 34 neonatal units with a decline in admissions, 19 (28%) had a significant decrease of ≥ 20%. The month-to-month decrease in admissions was approximately 2% on average from March 2020 to December 2021. Despite the decline in admissions, we found no significant changes in overall inpatient neonatal mortality. The three sensitivity analyses provided consistent findings.
Conclusion
COVID-19 containment measures had an impact on neonatal admissions, but no significant change in overall inpatient neonatal mortality was detected. Additional qualitative research in these facilities has explored possible reasons. Strengthening healthcare systems to endure unexpected events, such as pandemics, is critical in continuing progress towards achieving Sustainable Development Goals, including reducing neonatal deaths to less than 12 per 1000 live births by 2030.
Similar content being viewed by others
Key findings
-
1.
What was known?
-
The COVID-19 pandemic disrupted provision, and utilisation of health services. However, few publications have reported primary data from multi-country settings evaluating neonatal care and outcomes associated with this disruption.
-
-
2.
What was done that is new?
-
Data from 67 neonatal units constituting 266,741 admission records from January 2019 to December 2021 across Malawi, Kenya, Nigeria, and Tanzania were analysed to evaluate changes in neonatal admissions, case mix and mortality.
-
The Oxford Stringency Index was used to quantify policy shifts across the four countries to determine interruption time point for an Interrupted Time Series analysis. A mixed effects segmented regression was applied.
-
-
3.
What was found?
-
Implementation of policies appeared more stringent from April 2020. Overall admissions into the NEST360 neonatal units were reduced by 15%, with an average monthly reduction across all the facilities of about 2% after March 2020.
-
Half of the units showed either significant (n = 19) or borderline (n = 15) step reductions. Further, a quarter of the units showed a significant month-to-month decline in admissions by at least 2%.
-
There was no measurable change detected in pooled mortality, although some facilities reported decreases and increases in neonatal mortality.
-
-
4.
What next?
-
Currently, 63 countries are off track to achieve the Sustainable Development Goal 3.2 of reducing neonatal deaths to 12 per 1000 live births by 2030. These efforts may be further derailed by unexpected disruptions to healthcare systems.
-
To mitigate future risks, it is essential to enhance the capacity of health systems to respond quickly and effectively to pandemics, making them more resilient to shocks.
-
Background
Progress has been made in improving child health and survival over the past two decades, with global under-five child mortality reduced by more than half, between 1990 and 2021 [1]. Yet there were an estimated 2.3 million neonatal deaths globally in 2021 [2], representing 47% of under-five deaths, with slower progress made in reducing mortality during the neonatal period (28 days after birth). Reducing neonatal mortality, especially amongst vulnerable newborns [3], is imperative to achieving the Sustainable Development Goal (SDG) target 3.2 for every country to reduce preventable newborn deaths to at least 12 per 1,000 live births and under-five child deaths to at least 25 per 1,000 live births by 2030 [4].
The COVID-19 pandemic resulted in major public health shifts in all countries, causing millions of deaths worldwide [5]. Governments imposed containment measures such as isolation of infected persons, quarantines, and lockdowns. These measures disrupted provision and utilisation of health services [6, 7], indirectly affecting outpatient and emergency care, especially for the most vulnerable [8].
Newborns and mothers are particularly vulnerable users of any health system [9]. Disruptions of the health system can negatively impact health outcomes for mothers and their newborns, especially those born premature, too small or those who become sick [8]. To achieve SDG target 3.2 of neonatal mortality target of < 12 deaths per 1000 live births, high-quality small and sick newborn care (SSNC) is critical [10].
The Newborn Essential Solutions and Technologies (NEST360) Alliance was formed to support African governments in their commitment to reducing inpatient neonatal deaths through implementation of a sustainable health system strengthening package including innovative devices, training, data systems, and quality improvement with mentorship. The NEST360 Alliance aims to improve the quality of SSNC in 67 facilities across four countries: Kenya, Malawi, Nigeria, and Tanzania.
Whilst disruptions associated with the COVID-19 pandemic have been shown to indirectly affect many aspects of healthcare [8], there is little published multi-country, primary data on the impact of COVID-19 disruption on neonatal admissions and mortality. Some published studies were based on modelling, or surveys, and those with primary data have also shown varied findings [8, 11,12,13,14,15]. This paper is part of a supplement reporting learnings with the NEST360 Alliance.
Objectives
Our aim was to evaluate the indirect impact of COVID-19 containment measures on inpatient neonatal admission and mortality. Specifically, we:
-
1.
Compared stringency of COVID-19 policies in four countries implementing with the NEST360 Alliance (Kenya, Malawi, Nigeria, and Tanzania).
-
2.
Quantified the indirect impact of COVID-19 on neonatal admissions, case-mix, and inpatient neonatal mortality in 67 neonatal units implementing with NEST360 between January 2019 and December 2021.
Methods
Study setting
The 67 facilities affiliated with NEST360 comprised of 69 neonatal units, as some facilities in Nigeria had geographically separated inborn and outborn units. Two units in Malawi did not have neonatal inpatient data and were therefore excluded, resulting in 67 neonatal units as the final total for analysis. Based on the World Health Organization (WHO) level of newborn care [16, 17] and country classifications, of the 67 neonatal units, 33 are District level (31 in Malawi), 24 are Secondary (10 in Kenya), and 10 are Tertiary (5 in Nigeria). We examined data for the period January 2019 to December 2021.
Study design
We applied an Interrupted Time Series (ITS) design to assess the effect of COVID-19 containment measures on neonatal admissions and mortality. ITS evaluates change by two metrics – "step" and "slope" change parameters [18]. Estimation of step and slope change requires prior definition in ITS design. We used the Oxford Stringency Index, which defines the level of stringency of policies in various countries and was computed by the Blavatnik School of Government (University of Oxford) [19].
Admission and mortality data
Newborn admission and mortality data were obtained from the Neonatal Inpatient Dataset (NID). The NEST360 Alliance, with four country governments, co-developed the NID intended to enable and track scale-up of high-quality WHO level-2 SSNC with respiratory support in hospitals [20]. The NID consists of 60 core variables organised into six modules: (1) birth details/maternal history; (2) admission details/identifiers; (3) clinical complications/observations; (4) interventions/investigations; (5) discharge outcomes; and (6) diagnosis/cause-of-death.
In the neonatal units, the data collection takes place post-discharge of the neonates and is carried out by trained data officers. They adhere to well-defined standard operating procedures established by the research team. The patient file, serving as the formal documentation of the clinical condition and management, plays a crucial role in this process. The file contains admission information, treatment sheets, discharge summary forms, laboratory reports, and general clinical notes. In neonatal units where Electronic Health Records (EHRs) are implemented, we have established a linkage between the NID and the EHRs. This integration facilitates seamless data abstraction, contributing to enhanced data quality and efficiency in the overall process.
We developed and utilised a novel approach to account for underreporting of data for the smallest babies in neonatal admissions and mortality. Number of admissions and deaths for newborns with either low birthweight (i.e., < 2500g) or gestational age (i.e., < 28 weeks) tend to be underreported in both low- and high-income settings, and especially in routine data systems [21,22,23,24]. Some factors influencing underreporting include: (i) health worker misunderstanding of registration guidance (e.g., applying stillbirth registration threshold of 1000g to neonatal deaths) [25]; (ii) physician perception of viability of the smallest babies, especially considering increased survival of micro-preemies globally [26], and (iii) negative implications associated with reporting (e.g., penalisation of hospitals with higher mortality rates, effort required for more data collection, etc.). The potential impact of COVID-19 disruption is likely to be underestimated if underreporting is not accounted for in the analysis.
The NID was examined for evidence of underreporting for the lowest birthweight categories, using distributions of admissions by birthweight category and the corresponding birthweight-specific mortality (BWSM) curves, also considering time trends. We identified evidence of underreporting, based on implausible birthweight specific mortality curves especially of neonates < 1000g birthweight. A criterion of Neonatal Mortality Rate (NMR) ≥ 700 deaths per 1000 live births for neonates weighing < 1000g was used. This cut-off was considered conservative since mortality in this group without full intensive care was reported to be at least 800 per 1000 live births [24, 27]. Hence using selected neonatal units meeting the criteria for more plausible birthweight-specific mortality for < 1000g, we developed standardised curves. These curves were then applied to the rest of the dataset to adjust for the undercounting of both deaths and admissions by facility (Additional File 1).
Statistical analyses
The neonatal unit was the unit of analysis, and time was defined as calendar months. For the ITS design, we defined a step change as the average modelled change observed between the month when COVID-19 policy stringency increased (i.e., March 2020) as the point of interruption compared to the preceding month (i.e., February 2020). We defined a slope change as the modelled average month-to-month changes observed in the time-series data before the interruption period (January 2019 to February 2020) compared to after the interruption period (i.e., April 2020 to December 2021). Data from January 2019–February 2020 (pre-interruption) was classified as "pre-COVID," and from April 2020–December 2021 as "during COVID". Our ITS impact model evaluated and quantified both immediate step change and month-to-month effects as slope change [18].
The analysis examined overall and unit-level effects, which were estimated using segmented mixed-effects regression in the Frequentist framework, with the primary point of interruption being March 2020. The models for admissions and NMRs data were of the following general form (with differences reflected in the distributions and link functions applied):
where:
-
\({y}_{ij}\) is the adjusted number of either admissions or neonatal mortality rate at ith month for the jth neonatal unit.
-
\({\beta }_{0j}\) denotes pre-interruption intercept for the jth neonatal unit.
-
\({\beta }_{1j}\) denotes pre-interruption slope for the jth neonatal unit.
-
\({\beta }_{2j}\) denotes the step change for the jth neonatal unit after interruption.
-
\({\beta }_{3j}\) denotes slope change for the jth neonatal unit after interruption.
-
\({\mu }_{00}\) denotes pre-interruption average intercept
-
\({\mu }_{01}\) denotes pre-interruption average slope.
-
\({\mu }_{02}\) denotes average step change
-
\({\mu }_{03}\) denotes average slope change
-
\({r}_{0j}\), \({r}_{1j}\), \({r}_{2j}\) and \({r}_{3j}\) are neonatal unit-specific random effects for pre-interruption intercept, pre-interruption slope, step change after interruption, and slope change after interruption, respectively.
-
\({\varvec{\upbeta}}\) denotes a matrix of corresponding parameters for fixed effects including country and non-linear functions of time modelled using harmonic terms [28].
To account for overdispersion, we modelled the monthly number of admissions at the neonatal unit level using a negative binomial distribution with a log link function [29]. NMRs were expressed as proportions and modelled using a beta distribution with a logit link function to restrict adjusted and fitted values to plausible ranges between 0 and 1 [30]. For all models, we smoothed trends using three monthly moving averages. The likelihood ratio test was used to evaluate the importance of including harmonic terms in mean structures and equal tailed uncertainty intervals for random effects were computed using Wald z-distribution approximation. Further, the analysis compared the performance of unstructured and autoregressive variance covariance structures based on how each optimised the likelihood function (Additional File 1 – eTable 1).
Four sensitivity analyses were conducted to assess the robustness of the ITS modelling of admissions and mortality. First, April 2020 was examined as an interruption time point and segmented mixed effects models re-fitted. Second, data for March and April 2020 were excluded to evaluate their impact on the modelling results, followed by re-fitting of the models using the remaining data. Third, the Oxford Stringency Index was treated as a continuous exposure, with changes in admissions and mortality modelled using an interaction term between the index and time in months. Lastly, the analysis was stratified and segmented regression models fitted to the data for each country.
An exploratory sub-group analysis was performed to investigate any changes in admission by primary diagnosis and referral patterns (i.e., whether neonates were born within the hospital or referred). This subgroup analysis was performed using pooled data and did not include fitting models to assess the statistical significance of changes in primary diagnosis and referral patterns. All the analyses were conducted in R version 4.3.2 [31].
Results
A total of 266,741 newborn records from 67 neonatal units were available from January 2019 to December 2021. Most admissions were in Malawi (N = 36, n = 126,578 (47.5%)), followed by Kenya (N = 13, n = 73,608 (28%)), Tanzania (N = 7, n = 53,558 (20%)), and Nigeria (N = 11, n = 12,997 (5%)) (Table 1).
Interruption time-point(s) based on the Oxford stringency index
Oxford Stringency Index scores were summarised for each of the four countries implementing with NEST360. Variations were observed among countries and over time, with policies appearing stricter in Kenya, Malawi, and Nigeria and less so in Tanzania. Implementation of policies started in March 2020, becoming more stringent by April 2020 (Fig. 1). Data from January 2019–March 2020 accounted for 43% (n = 113,887) of neonatal records (Table 1).
Changes in admission and NMR patterns using March 2020 as interruption time-point
Additional File 1 – eFigures 4a and 4b show good fit for March 2020 interruption models (and for April 2020 interruption), which is demonstrated using data for selected neonatal units from each of the four countries. Fitted admission and mortality trends for all neonatal units using the March 2020 interruption models are presented in Additional File 1 – eFigures 5a and b. Similar trends were obtained using the April 2020 interruption models and are presented in Additional File 1 – eFigures 6a and b.
Changes in admission trends
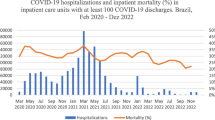
In March 2020, there was a significant 15% (Rate Ratio = 0.85; 95% Confidence Interval (CI): 0.78–0.93) decline in overall number of admissions to all neonatal units compared to February 2020 (Table 2). Half of the neonatal units (N = 34/67) reflected this decrease, with 19 of them experiencing a significant reduction by ≥ 20%. The remaining 15 demonstrated borderline effects (Fig. 2a). In each country, among the 34 neonatal units, 5 of 7 units in Tanzania, 8 of 13 units in Kenya, 3 of 11 units in Nigeria, and 18 of 36 units in Malawi showed either significant or borderline step reductions in admissions between February and March 2020.
From March 2020 onwards, the overall month-to-month (slope) decrease in admissions remained at approximately 2% (Rate Ratio = 0.98; 95% CI: 0.97–0.99) (Table 2). Of the 67 neonatal units, 15 (12 of 36 units in Malawi, 2 of 7 units in Tanzania and 1 of 13 units in Kenya) showed a significant month-to-month decrease in admissions by ≥ 2%. In contrast, four neonatal units (2 of 36 units in Malawi, 1 of 7 units in Tanzania, and 1 of 13 units in Kenya) had a significant month-to-month increase in admissions by ≥ 3%. No measurable month-to-month changes were observed in neonatal units in Nigeria (Fig. 2b). Similar overall and neonatal unit step and slope changes were observed for the April 2020 interruption models (see Additional File 1 – eFigure 7). Additionally, the analysis that excluded data for March and April 2020 also showed approximately similar findings (Table 2).
Changes in neonatal mortality trends
No measurable change in overall neonatal mortality was observed between February and March 2020 (Odds Ratio = 0.95; 95% CI: 0.89–1.03) (Table 2). However, specific neonatal units experienced significant step changes in neonatal mortality. Of the 67 neonatal units, 6 had a significant step increase (5 of 36 units in Malawi and 1 of 11 units in Nigeria) in neonatal mortality by ≥ 30%, while 6 units (3 of 36 units in Malawi, 1 of 7 units in Tanzania, and 2 of 11 units in Nigeria) had a step decrease by ≥ 25% (Fig. 2c).
Further analysis of month-to-month changes across all units showed no slope change in neonatal mortality (Odds Ratio = 1.00; 95% CI: 0.99–1.01) (Table 2). A significant month-to-month increase in neonatal mortality by ≥ 5% was observed in 13 units (9 of 36 units in Malawi and 4 of 11 units in Nigeria). In contrast, 7 of 36 neonatal units in Malawi reported a significant month-to-month decrease in NMR by ≥ 4% (Fig. 2d).
Changes in neonatal admission case mix
When analysing trends of neonates admitted with specific conditions, there were minimal changes in trends for neonates primarily admitted due to congenital malformations, intrapartum-related illnesses, and jaundice. Neonates admitted for prematurity showed a slight increase over time (from ~ 25% in January 2019 to ~ 30% in December 2021), while those admitted for infections showed a decrease, particularly between April (~ 25%) and August 2020 (~ 12%) (Additional File 1 – Fig. 9a). From March 2020 onwards, there was a decrease in the number of neonates born both within and outside the facilities. The decline was slightly more pronounced for neonates born within the facilities compared to those born outside (Additional File 1 – Fig. 9b).
Analysis using Oxford Stringency Index as a continuous exposure
The interaction term between time in months and the Oxford Stringency Index showed an overall significant reduction in admission numbers over time. However, a similar analysis did not show any significant change in overall mortality (Additional File 1 – eTable 2). See the fitted trends for admissions and NMR in Additional File 1 – Figs. 8a and b.
Stratified analysis for each country with March as an interruption time-point
In the country-specific models, 29 out of 67 neonatal units showed either borderline or significant reductions in admission numbers compared to 34 out of 67 we obtained in the pooled analysis for all countries (see Additional File 1 – eTable 3 and 4). The difference of seven units were mostly in Malawi (six of the seven units) and one in Tanzania. The six neonatal units in Malawi are small; hence, the differences could potentially be attributed to reduced sample sizes.
Discussion
This study used a large dataset of 266,741 neonatal admissions to examine indirect effects of COVID-19 containment measures on admissions and mortality trends in 67 neonatal units implementing with NEST360 in Kenya, Malawi, Nigeria, and Tanzania. We used the Oxford Stringency Index to define the month of interruption and found a 15% decline in neonatal admissions overall between February and March 2020 and an average month-to-month reduction in admissions by 2% between March 2020 and December 2021. Despite the decline in admissions, we found no significant changes in overall neonatal mortality (Odds Ratio = 0.95; 95% CI: 0.89–1.03). However, results varied across individual neonatal units, i.e., between February and March 2020, six neonatal units showed a significant increase in neonatal mortality by ≥ 30%, whereas six neonatal units showed a decrease by ≥ 25%. Similarly, between March 2020 and December 2021, a significant month-to-month increase (≥ 5%) in neonatal mortality was observed in 13 units compared to seven units that reported a significant decrease.
Our analysis showed a substantial effect on neonatal admissions, aligning with findings from other studies evaluating neonatal admissions during the pandemic [8, 11, 32]. These results are unsurprising as COVID-19 containment measures caused disruption of health service delivery and demand across various levels of healthcare, including prenatal, maternal, and paediatric care [33]. COVID-19 reported effects on neonatal mortality have been mixed. For example, some studies found no significant mortality change, whereas others reported increases [8, 11,12,13,14,15, 34]. Specifically, studies in Zimbabwe, Malawi, Ireland and Botswana found no impact of COVID-19 on neonatal mortality [12, 13, 34], whereas a study conducted in Nepal reported a significant increase in institutional neonatal mortality, from 13 to 40 deaths per 1000 live births, noting that this was on labour ward [11]. A study in Turkey found little change [25], while two studies in Uganda reported a relative increase in neonatal mortality by 25–30% [8, 14]. Studies which reported increased mortality during COVID-19 might be related to reprioritisation of health resources, delayed presentation to the facilities, late diagnosis, and partial immunisation coverage, among other reasons [8, 14].
Based on this quantitative analysis, the NEST360 Alliance conducted a separate qualitative study in these neonatal units to better understand barriers and protective factors, as well as learn from pivots during the COVID-19 pandemic [35]. This qualitative study suggested several ways that SSNC was protected during the pandemic. One theme that emerged was around COVID-specific opportunities from the pandemic including more focus on infection control measures to prevent the spread of COVID-19, which may also have helped reduce other infections in the units. Another protective mechanism was utilising technology like telemedicine to facilitate patient-provider communication and monitor patient health. Additionally, access to oxygen and routine maintenance of medical devices received new investment. This qualitative study also provided valuable insights into the gaps for SSNC, which is still new on the global health agenda, and the need for increased investment in infrastructure and workers to enable more resilience to future shocks.
Our ITS study has strengths and limitations. One obvious strength is the large dataset of more than a quarter of a million records from 67 neonatal care units across four countries, being the largest primary neonatal analyses published to date on the indirect impact of COVID-19. The rigour of the analyses included robust ITS modelling with three different sensitivity analyses approaches, still resulting in consistent findings. Limitations include reliance on the Oxford Stringency Index which was based on national policy and contingency measures that were put in place in response to COVID-19 and may not be reflective of what actually happened in practice. Additionally, we were not able to consistently account for sub-national variation in implementation of COVID-19 containment measures, which would be particularly relevant in large, decentralised countries such as Nigeria. The use of routine facility-reported data may miss admissions and deaths, especially of the smallest neonates, and even though we attempted to adjust for underreporting, it is difficult to judge how representative the adjusted estimates are from the “truth” in the absence of a gold standard. The NEST360 Alliance is working with these facilities and the government to improve neonatal data quality in routine national systems.
Conclusion
We found an overall decrease in neonatal admissions associated with COVID-19 containment measures, but without measurable change in newborn mortality across 67 neonatal units. There is a need to protect all care, including newborn care, by allocating sufficient resources [36]. Healthcare systems need practical approaches to resilience, including policies that can adapt to rapidly changing circumstances [37, 38]. The linked qualitative research found remarkable examples of local leadership by healthcare providers, government, and partners. Importantly many stakeholders need to be more aware of the vulnerability of newborns and their families at times of crises and use their voices and resources to protect them [39].
Availability of data and materials
All partners in the NEST360 alliance collaborated to create and sign data sharing and transfer agreements. The dataset from this study will be accessible upon request, pending approval from the NEST360 learning network and collaborating parties.
Abbreviations
- COVID-19:
-
Coronavirus Infectious Disease Pandemic
- CI:
-
Confidence Interval
- ITS:
-
Interrupted Time Series
- NEST360:
-
Newborn Essential Solutions and Technologies
- NID:
-
Neonatal Inpatient Dataset
- NMR:
-
Neonatal Mortality Rate
- SDGs:
-
Sustainable Development Goals
- SSNC:
-
Small and Sick Newborn Care
- WHO:
-
World Health Organization
References
UNICEF. Monitoring the situation of children and women. 2023 January 2023 [cited 2023 4th Dec 2023]; Available from: https://data.unicef.org/topic/child-survival/under-five-mortality/.
Hug L, et al. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. 2019;7(6):e710–20.
Lawn JE, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. The Lancet. 2023;401(10389):1707–19.
UNDESA. Goal 3: Ensure healthy lives and promote well-being for all at all ages. 2023 [cited 2023; Available from: https://sdgs.un.org/goals/goal3.
WHO. Weekly epidemiological update on COVID-19 - 21 December 2021. 2021 [cited 2023; Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-december-2021.
Roberton T, et al. Early estimates of the indirect effects of the COVID-19 pandemic on maternal and child mortality in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(7):e901–8.
Rao, S.A.-O., et al., Small and sick newborn care during the COVID-19 pandemic: global survey and thematic analysis of healthcare providers' voices and experiences. LID - https://doi.org/10.1136/bmjgh-2020-004347 LID - e004347. (2059–7908 (Print)).
Hedstrom, A.A.-O., et al., Impact of the early COVID-19 pandemic on outcomes in a rural Ugandan neonatal unit: A retrospective cohort study. (1932–6203 (Electronic)).
Dickson KE, et al. Every Newborn: health-systems bottlenecks and strategies to accelerate scale-up in countries. The Lancet. 2014;384(9941):438–54.
Rosa-Mangeret, F.A.-O., et al., 2.5 Million Annual Deaths-Are Neonates in Low- and Middle-Income Countries Too Small to Be Seen? A Bottom-Up Overview on Neonatal Morbi-Mortality. LID - https://doi.org/10.3390/tropicalmed7050064 LID - 64. (2414–6366 (Electronic)).
Kc A, et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8(10):e1273–81.
McDonnell S, et al. The impact of the Covid-19 pandemic on maternity services: A review of maternal and neonatal outcomes before, during and after the pandemic. European Journal of Obstetrics and Gynecology and Reproductive Biology. 2020;255:172–6.
Caniglia EC, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. 2021;224(6):615.e1-615.e12.
Burt JF, et al. Indirect effects of COVID-19 on maternal, neonatal, child, sexual and reproductive health services in Kampala, Uganda. BMJ Glob Health. 2021;6(8): e006102.
Hekimoğlu, B. and F. Aktürk Acar, Effects of COVID-19 pandemic period on neonatal mortality and morbidity. Pediatrics & Neonatology, 2022. 63(1): p. 78–83.
UNICEF. Survive and thrive: Transforming care for every small and sick newborn. 2018; Available from: https://apps.who.int/iris/bitstream/handle/10665/276655/WHO-FWC-MCA-18.11-eng.pdf?ua=1.
Jamison, D.T., et al., Disease Control Priorities in Developing Countries (Second Edition). Disease Control Priorities. 2006: The World Bank. 1452.
Bernal, J.L., S. Cummins, and A. Gasparrini, Interrupted time series regression for the evaluation of public health interventions: a tutorial. (1464–3685 (Electronic)).
Hale T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5(4):529–38.
Cross JH, et al. Neonatal inpatient dataset for small and sick newborn care in low- and middle-income countries: systematic development and multi-country operationalisation with NEST360. BMC Pediatr. 2023;23(2):567.
Masaba BB, Mmusi-Phetoe RM. Neonatal Survival in Sub-Sahara: A Review of Kenya and South Africa. J Multidiscip Healthc. 2020;13:709–16.
WHO. True magnitude of stillbirths and maternal and neonatal deaths underreported. 2016 [cited 2022 22–04–2022]; Available from: https://www.who.int/news/item/16-08-2016-true-magnitude-of-stillbirths-and-maternal-and-neonatal-deaths-underreported.
McCarthy Bj Fau - Terry, J., et al., The underregistration of neonatal deaths: Georgia 1974--77. (0090–0036 (Print)).
Blencowe, H., et al., National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. (1474–547X (Electronic)).
Merli MG. Underreporting of Births and Infant Deaths in Rural China: Evidence from Field Research in One County of Northern China. The China Quarterly. 1998;155:637–55.
Morgan, M.A., J. Goldenberg Rl Fau - Schulkin, and J. Schulkin, Obstetrician-gynecologists' practices regarding preterm birth at the limit of viability. (1476–7058 (Print)).
Carlo, W.A., et al., Newborn-care training and perinatal mortality in developing countries. (1533–4406 (Electronic)).
Bhaskaran, K., et al., Time series regression studies in environmental epidemiology. (1464–3685 (Electronic)).
Ross, G.J.S. and D.A. Preece, The Negative Binomial Distribution. Journal of the Royal Statistical Society. Series D (The Statistician), 1985. 34(3): p. 323–335.
Ferrari S, Cribari-Neto F. Beta Regression for Modelling Rates and Proportions. J Appl Stat. 2004;31(7):799–815.
R Core Team (2023). _R: A Language and Environment for Statistical Computing_. R Foundation for Statistical Computing, Vienna,Austria. <https://www.R-project.org/>.
Delamou, A., et al., Effect of COVID-19 on maternal and neonatal healthcare services in Guinea: a hospital based study. European Journal of Public Health, 2021. 31(Supplement_3): p. ckab164.158.
Adu PA, et al. The direct and indirect impact of COVID-19 pandemic on maternal and child health services in Africa: a scoping review. Global Health Research and Policy. 2022;7(1):20.
Simbarashe C, et al. Indirect impacts of the COVID-19 pandemic at two tertiary neonatal units in Zimbabwe and Malawi: an interrupted time series analysis. BMJ Open. 2022;12(6): e048955.
Steege R, et al. Protecting small and sick newborn care in the COVID-19 pandemic: multi-stakeholder qualitative data from four African countries with NEST360. BMC Pediatr. 2023;23(2):572.
Bhutta, Z.A., et al., Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? (1474–547X (Electronic)).
World Health, O., Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages: interim guidance, 6. 2020. Geneva: World Health Organization; April 2020.
CDC. Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. 2019; Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html.
CDC. Evaluation and Management Considerations for Neonates At Risk for COVID-19. 2020 [cited 2023; Available from: https://public4.pagefreezer.com/browse/CDC%20Covid%20Pages/11-05-2022T12:30/https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-newborns.html.
Acknowledgements
Firstly, and most importantly, our gratitude goes to the newborns and their mothers, as their data form the core of NEST360. We express appreciation to the participants in the NEST360 Neonatal Inpatient Dataset Learning Group, along with the dedicated data collectors, healthcare professionals, and quality improvement teams responsible for inputting and utilising the data. Special thanks to the supportive administrative staff. Our sincere thanks extend to fellow researchers, guest editors, and the peer-reviewers who contributed to refining this paper. We also acknowledge the valuable insights from managing editors at BMC and within NEST360, including Caroline Noxon, Sarah Murless-Collins, and Joy E. Lawn.
About this supplement
This article has been published as part of BMC Pediatrics, Volume 23 Supplement S2, 2023: NEST360 Small and sick newborn care: learning for implementation across Africa and beyond. The full contents of the supplement are available at https://bmcpediatr.biomedcentral.com/articles/supplements/volume-23-supplement-2.
Funding
This work is funded through the NEST360 Alliance with thanks to John D. and Catherine T. MacArthur Foundation, the Bill & Melinda Gates Foundation, ELMA Philanthropies, The Children’s Investment Fund Foundation UK, The Lemelson Foundation, The Sall Family Foundation, and the Ting Tsung and Wei Fong Chao Foundation under agreements to William Marsh Rice University.
Author information
Authors and Affiliations
Contributions
The NEST360 Complex Evaluation was conceptualised by the NEST360 Alliance Team, facilitated by JEL. All collaborators contributed to the design of the study protocol. LM and EOO with JEL developed the detailed research questions and overall analysis plan for this paper. These were refined with inputs from the wider NEST360 Implementation Research Group and the Multi-country neonatal mortality working group. Analysis was undertaken by LM with oversight from EOO. The manuscript was drafted by LM with input from JS, SN, OD, JW, JA, IK, JHC, NS, EZ, CE, REP, DG, RT, MC, OO, NL, KK, GI, OT, SMC, CB, RRK, MO, EOO and JEL. All authors reviewed the manuscript and agreed on the final version for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was received in each country from a local institutional review board (Additional File 2) and the London School of Hygiene & Tropical Medicine ethics committee (no. 21892).
Consent for publication
Not applicable as this was secondary analysis of routine data.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12887_2024_4873_MOESM1_ESM.docx
Additional file 1. The file highlights the adjustment approach in detail and also provides additional results referenced in the main paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Malla, L., Ohuma, E.O., Shabani, J. et al. COVID-19 pandemic effects on neonatal inpatient admissions and mortality: interrupted time series analysis of facilities implementing NEST360 in Kenya, Malawi, Nigeria, and Tanzania. BMC Pediatr 23 (Suppl 2), 657 (2023). https://doi.org/10.1186/s12887-024-04873-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04873-1