Abstract
Background
The application of evoked potentials (EPs) to the diagnosis of acute disseminated encephalomyelitis (ADEM ) has not been investigated in detail. The aim of this study, therefore, was to analyze the value of multimodal EPs in the early diagnosis of pediatric ADEM.
Methods
This was a retrospective study in which we enrolled pediatric ADEM patients and controls (Cs) from neurology units between 2017 and 2021. We measured indices in patients using brainstem auditory evoked potentials (BAEPs), visual evoked potentials (VEPs) and somatosensory evoked potentials (SEPs), and then we analyzed their early diagnostic value in ADEM patients.
Results
The mean age of the ADEM group was 6.15 ± 3.28 years (range,1–12 years) and the male/female ratio was 2.1:1 The mean age of the Cs was 5.97 ± 3.40 years (range,1–12 years) and the male/female ratio was 1.3:1. As we used magnetic resonance imaging (MRI) as the diagnostic criterion, the sensitivity, specificity, and accuracy (κ was 0.88) of multimodal EPs were highly consistent with those of MRI; and the validity could be ranked in the following order with respect to the diagnosis of ADEM: multimodal Eps > single SEP > single VEP > single BAEP. Of 34 patients with ADEM, abnormalities in multimodal EPs were 94.12%, while abnormalities in single VEPs, BAEPs and SEPs were 70.59%,64.71%and 85.3%, respectively. We noted significant differences between single VEP/BAEPs and multimodal EPs (χ2 = 6.476/8.995,P = 0.011/0.003).
Conclusions
The combined application of multimodal EPs was superior to BAEPs, VEPs, or SEPs alone in detecting the existence of central nerve demyelination, and we hypothesize that these modalities will be applicable in the early diagnosis of ADEM.
Similar content being viewed by others
Introduction
Multimodal evoked potentials (EPs) include brainstem auditory evoked potentials (BAEPs), visual evoked potentials (VEPs), and somatosensory evoked potentials (SEPs). EPs can indicate lesions in the central nervous system (CNS) in an objective and sensitive manner [1]. BAEPs are recorded in superficial bipolar electrodes (A1or A2, relative to Cz) through the auditory conduction pathway after applying a sound stimulus [2]. A VEP is the evoked response recorded in the visual center after applying a light stimulus, and is the total response of visual signal generation, transduction, and final transmission to the visual center [3]. Furthermore, and an SEP is the evoked response recorded in the corresponding sensory cortex through the deep sensory conduction pathways following electrical stimulation of the limbs [4].
Acute disseminated encephalomyelitis (ADEM) is an immune-mediated idiopathic inflammatory demyelinating disease of the CNS and is characterized by an encephalopathy ranging from behavioral change to alteration in consciousness [5]. Many studies have shown that ADEM is associated with a favorable outcome [6], whereas other studies revealed a mortality rate of 20% and considerable neurologic sequelae in survivors [7, 8]. Therefore, it is critical to identify ADEM through clinical presentations, imaging findings, neurophysiological techniques and immunological testing at the acute stage. Many researchers have investigated multimodal EPs of CNS demyelinating diseases such as multiple sclerosis [9], neuromyelitis optica spectrum disorder [10], and other diseases with clinical features of CNS damage; the latter include chronic HCV infection [11], and primary Sjögren’s Syndrome [12]. However, relatively few studies on multimodal EPs have been conducted on ADEM, particularly in children. Thus, we herein comprehensively investigated the diagnostic value of multimodal EPs in ADEM from auditory, visual, and sensory aspects and thereby retrospectively analyzed the BAEP, VEP, and SEP characteristics of 34 children with ADEM.
Materials and methods
Subjects
The clinical data from children visiting the Neurology Department of our hospital between November 2017 and December 2021 were analysed retrospectively. Inclusion criteria for the ADEM group were ① age ≥ 1 and ≤ 16 years; ② fulfillment of the diagnostic criteria of ADEM based on the International Pediatric MS Study Group (IPMSSG) recommendations [13], in which an acute onset of neurologic disturbance with polysymptomatic presentation and brain MRI changes involving the white matter in the distribution manifest a diffuse disseminated demyelinating disease; and ③ availability of complete and well-documented case records. Exclusion criteria were ① a history of demyelinating disease or other underlying neurological disease before ADEM onset, ②follow-up examination consistent with multiple sclerosis, neuromyelitis optica, or systemic lupus erythematosus, ③connective tissue diseases and tumors complicated by demyelinating diseases of the CNS or inborn errors of metabolism, and ④ lack of consent to follow-up examination. Inclusion criteria for the control group were ① age ≥ 1 and ≤ 16 years ; ② diagnosis with psychiatric disorders, migraine and benign intracranial hypertension, and no clinical features of CNS damage as well as any other autoimmune, or oncological diseases; ③ normal brain magnetic resonance imaging (MRI), and ④ a normal white blood cell count or cerebrospinal fluid (CSF) protein.This study was approved by the Ethics Committee of the Children’s Hospital of Hebei Province.
Clinical data and testing methods
Clinical and laboratory data
We analyzed the clinical features of age, sex, prior infection, clinical manifestations, cranial nerve damage, sensory impairment, autonomic dysfunction, therapeutic drugs, and CSF protein concentration and white cell counts (pleocytosis was defined as white cell counts of more than 10 × 106/L; increased CSF protein concentration was defined as over 0.4 g·L− 1).
MR imaging protocols
Imaging was performed on a 3.0T Signa Hdx MR unit with a head 16-channel coil. The first plain MR sequences were generated as follows: axial, sagittal, and coronal T2-weighted images, axial T1-weighted and fluid-attenuated inversion recovery sequence images. All neuroimaging was reviewed by a neuroradiologist (C.S.) who was unaware of the patient’s identity,clinical presentation, or original radiology report. Brain MRIs with large (> 2 cm axial), hazy, and bilateral lesions were defined as typically MRI-positive.
Evoked potential protocols
The procedures for EPs were conducted according to the International Federation of Clinical Neurophysiology guidelines [14, 15]. ① For BAEP measurements, we performed the examination in a soundproof room using disk-shaped silver chloride electrodes. BAEP clicks were recorded in the A1/Cz and A2/Cz electrodes. The stimulus frequency was 10.7 Hz, the stimulus intensity was 90 dBnHL and the number of sweeps was 1000. The test was repeated ≥ two or three times per ear. and the mean peak latencies of waves I, III, and V were used as primary observational indicators. ② VEP measurements were performed in a dark room, and VEPs to black and white pattern-reversal stimuli or flash stimuli were recorded at the Oz electrode with reference to the Fz electrode. The pattern-reversal VEP was conducted with a chessboard pattern of black and white squares that were aired on a TV monitor from a distance of 1 m. There were stimuli to both the left and right eye at a frequency of 1Hz. A flash VEP was provided monocularly with intermittent red-diode light stimulation that emanated from goggles placed over the eyes. The average number was 100. The stimulation was repeated three times or more per eye and the P100/P1 latency was used as the primary observational indicator. ③ For SEP measurements, the median nerve SEPs of both upper extremities (UEs) were recorded with surface disk-shaped electrodes placed at C3’/C4’ (2 cm posterior to C3/C4), the fifth spinous process, and Erb’s (Erb) points. The reference electrode was placed at Fz. The tibial nerves SEPs of both lower extremities (LEs) were recorded at Cz’ (2 cm posterior to Cz), spinous process of the first lumbar vertebra and the ipsilateral popliteal fossa, the reference electrodes were placed at contralateral C3 or C4, contralateral iliac crest and adjacent knee bone, respectively. The median nerve was stimulated at the wrist with a repetition rate of 5 Hz, and the tibial nerve was stimulated at the ankle with a repetition rate of 2 Hz. The stimulus intensity was above motor threshold and the stimulation of duration was 0.2 msec. The wave N9/N13/N20/P25 latencies of the upper limbs and the wave N21/P40 latencies of the lower limbs were used as the primary observational indicators. Results outside the 97.5 percentiles of values obtained in the age- and sex-matched Cs as well as the absence of waveform were considered anomalies.
Statistical analyses
Categorical data are depicted as proportions, and continuous data are shown as the mean ± SD or medians with IQR; and we applied the SPSS statistical package (SPSS, Inc., version 24, Chicago, IL, USA). Differences in proportions were tested by χ2 tests or Fisher’s exact test. Independent saµples were evaluated with the t test or Wilcoxon rank-suµ test. The sensitivity, specificity, positive and negative predictive value, accuracy, and kappa value (κ value) of single and combined applications of BAEPs, VEPs, and SEPs were calculated. A κ value between 0.4 and 0.75 was considered to be moderately consistent, ≥ 0.75 was highly consistent, and ≤ 0.40 indicated poor consistency. Statistical significance was set at 0.05.
Results
Baseline clinical characteristics
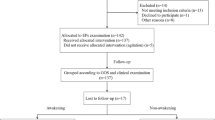
There were 34 children in the ADEM group, comprising 23 boys and 11 girls, with a mean age of 6.15 ± 3.28 years; the control group was composed of 30 children, 17 boys and 13 girls, with a mean age of 5.97 ± 3.40 years. There was no significant difference in sex or age between the Cs and the ADEM group at the initial medical examination. After disease onset, all children were treated with methyl-prednisolone combined with an intravenous globulin and all underwent cranial and spinal imaging (Fig. 1). Other clinical features of ADEM were summarized in Table 1.
When investigating the multimodal EPs, 64.71% (22/34) of the children with ADEM exhibited an abnormal BAEP, including 18 cases with prolonged latency of waves III/V and four cases with unelicited III/V waveforms (Fig. 2; Table 2). In addition, 70.59% (24/34) of children showed abnormal VEPs, comprising nine cases with prolonged P1 latency and 15 cases with prolonged P100 latency (Fig. 3;Table 2). Additionally, 82.4% (28/34) of ADEM children had abnormal UE SEPs, comprising 13 cases with prolonged N20 and P25 latencies and 15 cases with unelicited N20 and P25 waveforms (Fig. 4; Table 2); and 85.3% (29/34) of ADEM children had abnormal LE SEPs, comprising 13 cases with prolonged P40 latencies and 16 cases with unelicited P40 waveforms.
Application of single EPs and multimodal EPs in the diagnosis of ADEM
As MRI constituted the diagnostic criteria, the sensitivity, specificity, and accuracy (κ was 0.88) of multimodal EPs were highly consistent with those of MRI; and the validity was ranked in the following order with respect to the diagnosis of ADEM: multimodal EPs > single SEP > single VEP > single BAEP (Table 3).
We observed abnormalities in multimodal EPs in 94.12% of the 34 patients with ADEM: these were in single VEPs, BAEPs, and SEPs at proportions of 70.59%, 64.71%, and 85.3%, respectively. While there were significant differences between the single VEPs/BAEPs and multimodal EPs (χ2= 6.476/8.995, P = 0.011/0.003), there were no differences between the single SEPs and multimodal EPs (P = 0.427).
Discussion
ADEM is a multifocal monophasic inflammatory disease of the CNS that tends to primarily affect children and young adults [16]. In the present study, 23.5% of our patients reflected a history of infection or vaccination, and the proportion of men was higher, but not significant. ADEM manifests a rapid onset and presents with diverse clinical symptoms, with a small subset of children with ADEM experiencing more than one event, making the diagnosis of pediatric ADEM relatively difficult. There is a paucity of biomarkers for the early diagnosis of ADEM other than typical imaging [17]; and as brain and spinal imaging are normal in the early course of the disease, it is essential to seek sensitive and objective neurophysiological markers that can be applied to identify ADEM at the acute stage.
Recording EPs can detect clinical or subclinical lesions in visual, auditory, sensory, and motor pathways; and they are particularly suitable for young children who cannot cooperate fully with the examination. Characteristic findings of BAEPs are primarily manifested as absent or delayed latencies in CNS demyelinating disorders [18],such as MS/NMOSD,or other diseases with clinical features of CNS damage [19]. In addition, prolonged latency and interpeak intervals of waves were expressions of demyelinating damage and are highly accurate in identifying demyelinating diseases [20]. Another finding of BAEPs encompasses amplitudes and morphological abnormalities, which are expressions of axonal damage that is considered the most important factor in determining disability [21]. The BAEPs of children with ADEM in the present study exhibited prolonged latencies of waves III and V, and a significantly higher rate of abnormalities, whereas the latency of wave I did not change significantly. This indicates that the damage to the auditory conduction pathway in children with ADEM was primarily at the level of the superior olivary complex and inferior colliculus, abnormalities observed corresponded to the clinical localization of the lesion, consistent with most studies [17].
In routine practice, VEPs are proven to be useful in the diagnosis of neurological disorders affecting the visual pathways, including optic neuritis [22], multiple sclerosis, Leber’s hereditary optic neuropathy [23], and central nervous system tumors [24].The P100 latency of pattern VEPs indicated myelin damage while flash VEPs indicated axonal pathology; pattern VEPs were also more sensitive than flash VEPs in detecting optic nerve demyelination [25].Abnormal VEP patterns with preserved normal flash VEPs indicated isolated myelin damage, and abnormalities in both pattern VEPs and flash VEPs indicated myelin and axonal damage in the optic nerve [26].Among 34 children with ADEM, there were five patients with optic neuritis, of whom four showed abnormal pattern VEPs and one manifested abnormal flash VEPs; 19 cases showed abnormal subclinical VEPs. These results agree with a previous report showing that the characteristic VEP finding of patients with ADEM was a delay or a loss of P100/P1, indicating that ADEM primarily affected the central portion of the visual conduction pathway.
With respect to SEPs, (which are used to evaluate the function of afferent sensory pathways), the N20 of upper-limb SEPs originates in the primary sensory cortex of the contralateral parietal lobe, while the N9 notch is generated in the brachial plexus and N13 is a synaptic potential generated in the cervical spinal cord where sensory afferents connect with the posterior spinal cord horn [27], Higher abnormal SEPs thus indicated that the afferent sensory conduction pathway in CNS demyelinating diseases, (such as MS and ADEM) primarily involved the central part of the sensory nerve [28].The high sensitivity of SEPs in this study was similar to that of other demyelinating diseases and indicated the poor differentiation of cortical waves and nearly normal latency in peripheral recording sites.
The present results also supported the diagnostic value of BAEPs/VEPs/SEPs in ADEM. In addition, the combined application of multimodal EPs exhibited statistically significant differences compared with BAEPs or VEPs alone; thus, the combination of multimodal EPs can increase the early diagnostic value of children with ADEM.
Our study was also subject to some limitations. First, we only analyzed the latencies of EPS in this study, and did not evaluate wave amplitudes. Second, this was a small retrospective study that only comprised 34 pediatric ADEM patients. In the future, we will also conduct some prospective clinical studies on the prognosis of children with ADEM based on the present study.
In conclusion, we herein analyzed the EP characteristics of pediatric patients with ADEM and demonstrated that the combined application of multimodal EPs was superior to BAEPs, VEPs, or SEPs alone in detecting central nervous system involvement. We recommend that these EPs would be conducted in all patients with ADEM.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the data protection and privacy of the patients hospitalized at the Children’s Hospital of Hebei province, but are available from the corresponding author on reasonable request.
References
lnvernizzi P, Bertolasi L, Bianchi MR, Turatti M, Gajofatto A, Benedetti MD. Prognostic value of multimodal evoked potentials in multiple sclerosis:the EP score. J Neurol 2011,258(11): 1933–9.
Barbosa DAN, Samelli AG, Patriota de Oliveira D, da Paz JA, Matas CG. Auditory evoked potentials in children and adolescents with multiple sclerosis and neuromyelitis optica spectrum disorders. Int J Pediatr Otorhinolaryngol. 2022;153:111013.
Odom JV, Bach M, Brigell M, et al. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol. 2016;133(1):1–9.
Cruccua G, Aminoff MJ, Curioc G, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119(8):1705–19.
Boesen MS, Blinkenberg M, Koch-Henriksen N, et al. Implications of the International Paediatric Multiple Sclerosis Study Group consensus criteria for pediatric acute disseminated encephalomyelitis: a nationwide validation study. Dev Med Child Neurol. 2018;60(11):1123–31.
Koelman DL, Chahin S, Mar SS, et al. Acute disseminated encephalomyelitis in 228 patients: a retrospective, multicenter US study. Neurology. 2016;86(22):2085–93.
Absoud M, Parslow RC, Wassmer E, et al. Severe acute disseminated encephalomyelitis: a paediatric intensive care population-based study. Mult Scler. 2011;17(10):1258–61.
Tenembaum S, Chamoles N, Fejerman N. Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology. 2002;59:1224–31.
Schlaeger R, Hardmeier MD’, Souza M et al. Monitoring multiple sclerosis by multimodal evoked potentials:numerically versus ordinally scaled scoring systems. Clin Neurophysiol 2016,127:1864–71.
Neto SP, Alvarenga RM, Vasconcelos CC, Alvarenga MP, Pinto LC, Pinto VL. Evaluation of pattern-reversal visual evoked potential in patients with neuromyelitis optica. Mult Scler. 2013;19(2):173–8.
Waliszewska-Prosół M, Bladowska J, Ejma M, et al. Visual and brainstem auditory evoked potentials in HCV-infected patients before and after interferon-free therapy- A pilot study. Int J Infect Dis. 2019;80:122–8.
Gawron W, Pośpiech L, Noczyńska A, Orendorz-Fraczkowska K. Electrophysiological tests of the hearing organ in Hashimoto’s disease. J Pediatr Endocr Met. 2004;17(1):27–32.
Krupp LB, Tardieu M, Amato MP, et al. International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders:revisions to the 2007 definitions. Mult Scler J. 2013;19(10):1261–7.
Celesia GG, Bodis-Wollner I, Chatrian GE, Harding GF, Sokol S, Spekreijse H. Recommended standards for electroretinograms and visual evoked potentials. Report of an IFCN committee. Electroenphalogr Clin Neurophysiol. 1993;87(6):421–36.
Nuwer MR, Aminoff M, Goodin D, et al. IFCN recommended standards for brain-stem auditory evoked potentials. Report of an IFCN committee. Electroen Clin Neuro. 1994;91(1):12–7.
Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology. 2016;87:38–45.
Murthy JM. Yangala R,Meena AK,Reddy JJ.Clinical,electrophysiological and magnetic resonance imaging study of acute disseminated encephalomyelitis. J Assoc Physicians India. 1999;47(3):280–3.
Kallmann BA, Facklmann S, Toyka KV, Reiners K, Reiners. Early abnormalities of evoked potentials and future disability in patients with multiple sclerosis. Mult Scler J 2006,12(1), 58–66.
Mihaylova VM, Kosseva OR, Kotzev IA, et al. Evoked potentials in patients with Wilson Disease. J Clin Neurophysiol. 2022;39(6):510–2.
Ohnari K, Okada K, Takahashi T, Mafune K, Adachi H. Evoked potentials are useful for diagnosis of neuromyelitis optica spectrum disorder. J Neurol Sci. 2016;364:97–101.
Srinivasan VS, Krishna R, Munirathinam BR. Effectiveness of brainstem auditory evoked potentials scoring in evaluating brainstem dysfunction and disability among individuals with multiple sclerosis. Am J Audiol. 2021;30(2):255–65.
Andrade EP, Sacai PY, Berezovsky A, Salomao SR. Pattern-reversal visual evoked potential abnormalities in patients with defined multiple sclerosis. Arq Bras Oftalmol. 2007;70:943–8.
Sacai PY, Salomao SR, Carelli V, et al. Visual evoked potentials findings in non-affected subjects from a large Brazilian pedigree of 11778 Leber’s hereditary optic neuropathy. Doc Ophthalmol. 2010;121(2):147–54.
Brecejl J. Visual electrophysiology in the clinical evaluation of optic neuritis, chiasmal tumours, achiasmia, and ocular albinism: an overview. Doc Ophthalmol. 2014;129(2):71–84.
Chilinska A, Ejma M, Turno-Krecicka A, Guranski K, Misiuk-Hojlo M. Analysis of retinal nerve fibre layer, visual evoked potentials and relative afferent pupillary defect in multiple sclerosis patients. Clin Neurophysiol. 2016;127(1):821–6.
Moussa H, Sawaya RA, Deeb R, El Ayoubi N. .Correlation of pattern reversal and Flash Visual evoked potential latencies with Optical Coherence Tomography measures in patients with Optic Neuropathy and patients with multiple sclerosis without Optic Neuropathy. J Clin Neurophysiol. 2022;39(7):637–42.
Schmitt B, Thun-Hohenstein L, Molinari L, SupertiFurga A, Boltshauser E. Somatosensory evoked potentials with high cortical amplitudes: clinical data in 31 children. Neuropediatrics. 1994;25(2):78–84.
Margaritella N, Mendozzi L, Garegnani M, et al. Sensory evoked potentials to predict short-term progression of disability in multiple sclerosis. Neurol Sci. 2012;33(4):887–92.
Acknowledgements
The authors appreciate the time and effort given by participants during the data collection phase of the study. We thank LetPub (www.letpub.com) and Editage (www.editage.cn) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by grants from the Medical Science Research Key Project Plan of Hebei Province in 2019(20190806).
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported. JL acquired the electrophysiological data. YW and MZ acquired clinical data and completed the statistical analysis. MJ designed the experiments, interpreted the results, and drafted the initial manuscript. SS revised the initial draft and wrote the final manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Legal guardians of all children provided written informed consent before inclusion. The study was granted ethical approval by the Ethics Committee of Children’s Hospital of Hebei province. The study was carried out following the ethical standards of the responsible committee on human experimentation and with the 1975 Helsinki Declaration and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, J., Jin, M., Zhang, M. et al. Multimodal evoked potentials are useful for the diagnosis of pediatric acute disseminated encephalomyelitis. BMC Pediatr 24, 92 (2024). https://doi.org/10.1186/s12887-024-04576-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04576-7