Abstract
Background
Acute respiratory infection (ARI) is one of the leading causes of morbidity and mortality among children under five globally, particularly in regions like South Asia and sub-Saharan Africa. Bangladesh has made substantial progress in reducing child mortality, yet pneumonia remains a significant contributor to under-five deaths. This study aimed to investigate the association between in-house environmental factors and childhood ARI, considering factors such as household crowding, smoking, and sanitation facilities.
Methods
This case-control study was conducted at a tertiary-level children’s hospital in Dhaka, Bangladesh, from March to September 2019. The study included children aged 6–59 months. Cases were children with ARI symptoms, while controls were children without such symptoms. Rigorous matching by age and gender was employed to ensure comparability. Data were collected through structured questionnaires, and bivariate and conditional logistic regression analyses were performed.
Results
Several household environmental factors were significantly associated with childhood ARIs. Children from overcrowded households (AOR = 2.66, 95% CI = 1.52–4.71; p < 0.001), those using unclean cooking fuels (OR = 2.41, 95% CI: 1.56, 3.73; p = < 0.001), those exposed to in-house smoking (AOR = 1.74, 95% CI = 1.01, 3.05; p = 0.04) and those with unimproved sanitation facilities faced higher odds (AOR = 4.35, 95% CI = 2.14–9.26) of ARIs. Additionally, preterm birth and higher birth order were associated with an increased risk of ARI. In contrast, exclusive breastfeeding was a protective factor.
Conclusion
In-house environmental factors, including sanitation, crowding and in-house smoking, significantly influence childhood ARIs. Additionally, birth order and preterm birth play a crucial role. Promoting exclusive breastfeeding is associated with a lower ARI risk among under-five children in Bangladesh. These findings can guide interventions to reduce ARIs in low-income regions, particularly in South Asia.
Similar content being viewed by others
Introduction
Acute respiratory infections (ARIs) continue to be a leading cause of morbidity and mortality among children under the age of five worldwide [1]. The World Health Organization (WHO) estimates that, in 2022, there were 5 million deaths among children under the age of five caused by ARIs, which could have been prevented or treated. ARI is now a global problem of health policy interests [1, 2], accounting for approximately 20% of children’s deaths globally, with a significant proportion occurring in South Asia and sub-Saharan Africa. Acute respiratory infections (ARIs) account for 3.5% of the worldwide disease burden. They were the cause of 30–50% of all pediatric outpatient visits and resulted in over 30% of pediatric admissions in low- and middle-income countries [1]. According to UNICEF, in 2019, pneumonia alone was responsible for approximately 50% of under-five children’s deaths from all infectious diseases, indicating above 700,000 death tolls globally [2] and 20% of all deaths worldwide, mostly in South Asia and sub-Saharan Africa [3, 4]. Multiple studies have shown that Bangladesh, India, Indonesia, and Nepal are responsible for 40% of children’s deaths due to ARIs [5, 6]. The case fatality of severe pneumonia was found to be increasing while concomitant with measles infection and contributing to approximately 38% of measles deaths [7].
Bangladesh successfully achieved the Millennium Development Goal (MDG) 4 by significantly reducing the child mortality rate to 46/1000 live births by 2015 and it reduced to 45/1000 live births in 2017-18 [8]. According to Bangladesh Sample Vital Statistics 2018, the major cause of death is pneumonia in children under the age of five, accounting for more than one-third of all deaths (34.4%) [9]. To achieve the target set by the Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhea (GAPPD) by 2025, which aims to reduce mortality from pneumonia in under-five children to fewer than 3 per 1000 live births [3].
Several factors increase the risk of contracting and succumbing to acute respiratory infection (ARIs). These factors included poverty, malnutrition, low birth weight, inadequate breastfeeding, complementary food initiation, overcrowding, poor living conditions, insufficient sanitation, exposure to indoor and outdoor pollution, seasonal changes, and limited access to preventive and curative services [5, 6, 10, 11]. Recent observational studies found that household environmental factors, such as smoking, poor water quality, high dwelling density, and a lack of toilet facilities, contributed significantly to childhood acute respiratory infections (ARIs) [12, 13].
Multiple studies explored the role of different determinants of early childhood ARI in Bangladesh [14,15,16,17]. However, most of these studies had limitations due to their cross-sectional designs. Another issue was the need for more data on all potential predictors representing the diverse dimensions. Even though the investigations of multiple studies on whether environmental factors were potential risk factors for ARI [3, 5, 6, 10, 11], there was no clear evidence regarding the association with in-house environmental factors, especially for in-house toilet facilities, indoor smoking and crowding on ARI among children under-five years of age in our country. Hence, the study’s primary aim was to observe the association between in-house environmental factors where the children were nested and ARI. The study’s findings will give us the understanding to focus on the required areas to stem the problem in low-income settings of South Asia by advocating the improvement of behavioral and programmatic intervention programs.
Methods
Study design and sampling technique
We conducted a hospital-based matched case-control study at a specialized government-run tertiary children’s hospital (Dhaka Shishu Hospital) in Dhaka, Bangladesh, between March and September 2019. The study population consisted of children aged 6–59 months. We employed a rigorous matching process to ensure the comparability of cases and controls. Specifically, for each case (children with ARI), we selected a control from the same hospital, individually matched by age and gender. This matching process involved identifying a control who belonged to the same age category and shared the same gender as the corresponding case. In essence, each case was precisely paired with a control of the same age and gender, thus minimizing potential confounding variables. This stringent matching strategy was crucial to ensuring the comparability of cases and controls throughout the study. Study population.
Cases were defined as children aged 6–59 months who were hospitalized in the pediatric department with specific symptoms, including mild running nose, cough, chest in-drawing, and fast and difficult breathing (11) and diagnosed by the physicians of the hospital using the ‘Integrated Management of Childhood Illness protocol’ [18]. In contrast, controls consisted of the children aged 6–59 months who had attended the outpatient department without ARI symptoms, and the healthy children accompanying the patients to the same hospital and having no ARI symptoms in the previous 30 days were defined as the control population. The children with compromised immune systems diagnosed in the past were not involved in the study to minimize any potential effect on the study results.
Sample size estimation
The sample size of the study was calculated using the following formula, assuming a 5% significance level (i.e., \({Z}_{\raisebox{1ex}{$\alpha $}\!\left/ \!\raisebox{-1ex}{$2$}\right.}\)=1.96), 80% power (i.e., \({Z}_{\beta }\) = 0.84) of the study, and a case-control ratio of 1:1 (r=1). The percentage of control under five years of age exposed to overcrowding was assumed to be 30.5% (i.e., \({p}_{2}\)=0.305) with an odds ratio (OR) of 2.06 based on an analytical cross-sectional study done in India [19].
Therefore, based on the standard statistical parameters indicated above, our study was initially planned with a minimum sample size of 141 cases and an equal number of controls. However, the availability of facilities and resources allowed us to expand our sample size to 348, comprising 174 cases and 174 controls. This increase in the sample size was a strategic decision taken to enhance the statistical power of our study. A larger sample size improves the reliability of our results and increases our sensitivity to identifying relationships.
Data collection technique and quality control
Once the parents or other caregivers of the children had provided written informed consent, the skilled and widely trained data collectors administered a semi-structured questionnaire (described in both English and Bengali) for data collection through frontal interviews. For both the case and control groups, we utilized a single questionnaire utilizing national standard tools [20, 21] and maintained WHO-recommended procedures, [22] and the data collected were checked and rechecked for their reliability and validity.
Outcome variable
Acute respiratory infection (ARI) was defined as the specific symptoms that were onset in the preceding 10 days from the day of the visit, including a mild running nose, cough, chest in-drawing, and fast and difficult breathing.
Independent variables
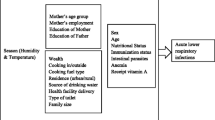
Data were collected on three sets of predictors: household environmental (exposure variables), sociodemographic, and mother-child characteristics.
Household environmental (exposure variables)
A group of variables was set for analysis to determine the effect of the household environment on ARIs among children under five. It included the place of residence (urban, rural), in-house crowding, in-house smoking habits by family members, cooking fuel, source of drinking water, shared toilets, and toilet facilities. The child’s residence had been identified as whether he/she was part of urban or rural living. In-house crowding was described as the total number of household members divided by the total number of bedrooms [23]. The number of members of the household was counted, asking, “How many members stay with the child in your house?” We categorized the in-house crowding into two following groups: >3 and ≤ 3 people per bedroom [13]. We assumed the cut point of 3.0 for in-house crowding categorization because we thought the child was staying with his/her parents.
We further investigated several household environmental variables: the type of cooking fuel used and the in-house smoking habits of the family members in the house. The use of cooking fuel was classified into two groups: clean (natural gas, LPG or electricity) or unclean (biogas, wood, agricultural products, solid waste or coal) [24]. In-house smoking was determined by asking, “Do any of the adult members of your family smoke on the home premises?” We also took data on their source of drinking water, which was recorded as either tap or piped water or tube-well or surface water. In addition, two kinds of data were obtained from the provision of sanitation facilities in the house in which the children stayed. First, whether or not they used a communal toilet (more than five people use the same toilet) and second, the type of toilet they were using. The toilets were divided into two categories: improved (safe disposal of excreta without human contact, e.g. flush to the piped sewage system, septic tanks or pit latrines) and unimproved facilities (pit latrine without slab/platform, hanging/bucket toilet or open) [25].
Other covariates
The sociodemographic variables consisted of the child’s age (in months) and gender, the level of education for both parents, the mother’s occupation, and the family’s monthly income. Parental education was categorized by years of schooling (illiterate, 1–5, 6–12, and 12 + years). The mother’s occupation was classified as either a housewife or employed. We defined the employed mothers as those who received a monthly salary from their occupation. Family income was classified into three groups ( < = 10000, 10,001–25000, > 25000 BDT/month).
Last, the maternal-child characteristics included birth order, gestational age (preterm/full-term), mode of delivery (cesarean section/normal), exclusive breastfeeding, nutritional status, and vaccination status (based on EPI schedule; completeness for age or no/incomplete). Preterm birth is defined as the birth of a child before completing 37 weeks of gestation, and full-term birth refers to a birth after 37 weeks [26]. Nutritional status was assessed by the mid-upper arm circumference (MUAC) and categorized into two groups: healthy (> 13.5 cm) and malnourished ( < = 13.5 cm). It was measured by MUAC (mid-upper arm circumference) tape at the midpoint between the tip of the shoulder and the tip of the elbow. The measurements were meticulously recorded with a precision of 0.1 cm. The tape fitted firmly but did not create any pit in the upper arm.
Statistical analysis
The questionnaires analyzed were checked for completeness, precision, and internal consistency, and the exclusion of incomplete or inaccurate data. The analytical software STATA was used to analyze the data. The descriptive statistics were presented as percentages and frequencies. We conducted bivariate and multivariate conditional logistic regression analyses to examine the association between in-house environmental factors and childhood ARI. The study employed a matched case-control design, where each case (children with ARI) was matched with a control (children without ARI) based on age and gender. This matching was essential to control for potential confounding variables and to ensure that cases and controls were comparable. Conditional logistic regression was well-suited for analyzing matched data because it takes into account the matched pairs and their characteristics. Furthermore, the outcome variable in this study was binary (ARI present or not present), which was suitable for logistic regression analysis. Conditional logistic regression further extended logistic regression to matched data, allowing researchers to examine the relationship between the predictors (in-house environmental factors, sociodemographic variables, etc.) and the binary outcome while accounting for the matching.
We built our regression models iteratively, considering variables’ significance and potential confounding effects. Collinearity among all independent variables included in the study was assessed using the Variance Inflation Factor (VIF). Apart from “residence”, no other variable exhibited significant collinearity. As a result, these were retained and included in the subsequent conditional logistic regression model. This allowed us to accurately examine the relationship between these variables and the binary outcome. In both models, odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength and direction of associations and a p-value < 0.05 was considered to determine statistical significance.
Result
The analysis considered three hundred and forty-eight children aged 6 to 59 months, with 174 cases and 174 controls. As a matched case-control study by age and gender, the distribution of boys (60.34%) and girls (39.66%) was similar in both the case and control groups. Most participants in each group were between the ages of 25–36 months (39.66% of cases and 39.08% of controls).
Association of sociodemographic characteristics with childhood ARI
We examined the impact of various sociodemographic characteristics on childhood ARI (acute respiratory infections) in the bivariate logistic regression model [Table 1]. Approximately half of the mothers (47.13% for cases and 50.57% for controls) completed 6–12 years of schooling. The correlation between maternal education and childhood ARI was almost significant (p = 0.051), with children of mothers who completed 6–12 years of schooling having 39% lower odds of ARI compared to those with less-educated mothers. Almost 40% of the fathers and 23% of mothers completed more than 12 years of schooling. However, a closer examination revealed that children of more highly educated mothers (12 + educational years) and fathers with secondary education (6–12 years of schooling) demonstrated substantial decreased odds of childhood ARI by 66% (OR = 0.44, 95% CI: 0.24, 0.81; p = 0.008) and 76% (OR = 0.34, 95% CI: 0.15, 0.72; p = 0.006) respectively, relative to illiterate parents (reference category).
Furthermore, Approximately 52% of children were nested in households with monthly family income between BDT 10,001 and BDT 25000 in both cases (50.57%) and control (52.87%)groups. Children from families with a monthly income exceeding 25,000 BDT per month displayed 50% lower odds (OR = 0.50, 95% CI: 0.26, 0.94; p = 0.033) of ARIs than those from families with monthly incomes 10,000 BDT or less. However, the mother’s occupation did not exhibit any significant association with childhood ARI [Table 1].
Association of in-house environmental factors with childhood ARI
We also investigated the influence of household environmental factors on ARIs among children aged 6–59 months, as demonstrated in the unadjusted model [Table 2]. The residence of the children exhibited a significant association with childhood ARI. Specifically, children residing in rural areas were more than twice as likely (OR = 2.12, 95% CI: 1.38, 3.28; p = 0.001) to experience ARIs compared to their urban counterparts (reference category). In particular, our data revealed that 53.45% of households in the case group had more than three individuals per bedroom, which was significantly higher than the 24.13% observed in the control group. Children living in overcrowded households with more than three people per bedroom faced 3.61 times higher odds of developing acute respiratory infections (ARIs) when compared to those in less crowded households with three people or fewer per bedroom (OR = 3.61, 95% CI: 2.29, 5.74; p = < 0.001) in the unadjusted model. Furthermore, the use of unclean fuel by families increased the odds of ARIs among children by 2.41 times (OR = 2.41, 95% CI: 1.56, 3.73; p = < 0.001) compared to those using clean fuels. Additionally, a significant proportion of family members in both case and control categories reported in-house smoking habits (40.23% in cases and 29.89% in controls). In households with in-house smokers, the risk of childhood ARI significantly increased by 58% when compared to households with nonsmokers or those without in-house smoking (OR = 1.58, 95% CI: 1.02, 2.47; p = 0.04). Approximately 45% of families relied on tube-well or surface water for drinking, which was associated with 2.45 times higher odds of childhood ARI (OR = 2.45, 95% CI: 1.59, 3.79; p = < 0.001) compared to those consuming tap or piped water. Encouragingly, approximately three-fourths (75.86%) of families used improved sanitation facilities, signifying a positive trend. However, the utilization of unimproved toilet facilities significantly increased the risk of childhood ARI by 5.29 times (OR = 5.29, 95% CI = 3.03, 9.66; p = < 0.001). Interestingly, the sharing of toilet facilities did not yield a significant increase in the risk of childhood ARI [Table 2].
Association of maternal and child characteristics with childhood ARI
We then assessed the effect of various maternal and child characteristics on childhood ARI in the bivariate logistic regression model [Table 3]. The data indicated that most children were firstborn in the family (33.91% among cases and 48.28% among controls). An intriguing trend became apparent – the odds of ARI increased with ascending birth order. The first- and second-born children had an effective defence against ARI related to third- or more-ordered children (OR = 0.31, 95% CI: 0.19, 0.58; p = < 0.001 and OR = 0.38, 95% CI: 0.22, 0.69; p = 0.002, respectively). Most children were born after 37 weeks of gestation (54% in the case group and 68.39% in the control group). Remarkably, premature birth amplified the odds of ARI by a substantial 84% (OR = 1.84, 95% CI: 1.19, 2.86; p = 0.006) compared to full-term (reference category). Only 56.32% of all children received exclusive breastfeeding. However, children who breastfed exclusively were 50% (OR = 0.50, 95% CI: 0.32, 0.77; p = 0.002) less likely to develop ARI than those not. Most children completed vaccination for age (79.31% for the group of cases and 92.53% for the group of controls). The odds ratio indicated that children who did not receive immunization by the recommended age were 3.5 times (OR = 3.50, 95% CI: 1.80, 7.26; p < 0.001) more inclined to develop ARI in comparison to their counterparts who received completely (the reference category). The majority of all children (47.13%) were delivered through the typical vaginal route (52.30%). In a bivariate model, the nutritional status and mode of delivery among under-five children were not significantly correlated with ARI [Table 3].
Conditional logistic regression: an adjusted model
We finally conducted a multivariable conditional logistic regression analysis to estimate the adjusted odds ratios (AORs) for identifying potential in-house environmental factors. After adjusting the effect of household, maternal and child characteristics, we found that several factors remained significantly associated with childhood ARIs [Table 4].
Unimproved toilets were significantly associated with increased odds of childhood ARI by 4.35 times (AOR = 4.35, 95% CI = 2.14, 9.26; p < 0.001)). Crowded households with more than three people per bedroom also showed a significantly elevated risk of childhood ARI by 2.66 times while all other variables were kept constant (AOR = 2.66, 95% CI = 1.52, 4.71; p < 0.001) while adjusting the other covariates. Likewise, households with family members who smoked indoors also demonstrated increased odds of childhood ARIs by 1.74 times (AOR = 1.74, 95% CI: 1.01, 3.05, p = 0.04).
The adjusted odds of childhood ARI were also found to be significant among maternal and child characteristics in the conditional logistic regression model; in terms of preterm birth by 2.44 times (AOR = 2.44, 95% CI: 1.43, 4.23; p < 0.001) (AOR = 2.44, 95% CI: 1.43, 4.23; p < 0.001) and having three- or more-birth ordered children by 4.26 times (AOR = 4.26, 95% CI = 2.07, 9.03; p < 0.001), and in-house smoking (AOR = 1.74, 95% CI = 1.01, 3.05; p = 0.04).
In addition, exclusive breastfeeding alone significantly minimizes ARI in under-five children (AOR = 0.48, 95% CI = 0.28, 0.82; p = 0.01) [Table 4].
Discussion
This case-control study, individually matched by age and gender- was conducted at a tertiary children’s hospital in Bangladesh and aimed to investigate the association of in-house environmental factors and acute respiratory infection among children aged 6–59 months in the country’s population where we identified that several household environmental factors had a critical role to developing childhood acute respiratory infection (ARI). The study encompassed 348 participants and performed both bivariate and multivariate conditional logistic regression models to explore the associations.
Our study observed a substantial increase in ARI among children living in - crowded households with more than three persons per bedroom. Specifically, our findings indicated a 2.12-fold increase in the odds of ARI in such conditions (OR = 2.12, 95% CI: 1.38, 3.28). Even after adjusting all other variables, it remained unchanged. This aligned with previous research in Canada, which demonstrated a similar association where indoor CO2 where indoor CO2 levels increased with the number of indoor occupants, significantly elevating the risk of respiratory infections among young children [27]. Congested living conditions made spreading infections easier, increasing the risk of cross-infection when family members sneezed, coughed, or talked. Such houses were mostly ill-ventilated [28]. Studies revealed that poor ventilation in the house increases the odds of ARIs in young children [29, 30]. However, indoor humidity was also influenced by reduced out-to-indoor airflow rates, which augmented the growth of microorganisms in the house, increasing the risk of ARI in the susceptible group [31]. The result of the study was consistent with other countries, such as India and Nigeria, where the variable overcrowding in the house was considered [19, 32].
The present study also shed light on a significant association between unimproved toilet facilities and the increased odds of childhood ARI by 4.35 times (AOR = 4.35, 95% CI: 2.14, 9.26) after adjusting for the effect of other covariates. Our findings aligned with studies conducted in similar settings, including Bangladesh, India and Pakistan, which consistently reported similar results [17, 19, 33]. Unimproved toilets were primarily prevalent in rural areas where access to modern sanitation facilities was often limited or unavailable. These unimproved facilities were typically utilized by people with lower levels of education and financial means [34, 35]. The lack of access to improved sanitation not only increases the risk of ARIs but also contributes to a broader public health challenge by facilitating the transmission of infectious diseases.
In Bangladesh, young children generally accompany their mothers while cooking. In our study, dirty cooking fuel was found to be significantly associated with ARIs. Such fuels were mostly used in rural areas. The analysis revealed higher odds of ARIs among children of rural dwellings than among children of urban dwellings and the findings were compatible with several studies [19, 36]. Studies have also shown that irritant air pollutants, such as particulate matter, carbon monoxide, nitrogen dioxide, sulfur dioxide, and formaldehyde, are produced more in solid biomass fuel combustion than in clean fossil fuels. These toxic air pollutants generated within the house become trapped and have the potential to increase microbial infection susceptibility [37]. A meta-analysis conducted with the studies in rural households showed that exposure to solid biomass fuel significantly increased the risk of ARI among children by 3.53 times [38].
The current study also brought to light the adverse impact of exposure to second hand smoke in children, increasing their susceptibility to acute respiratory infections (ARIs). This association was consistent with findings from various regions, Nepal, Bangladesh, and Cameroon [10, 33, 36]. Second hand smoke, often found in households where family members smoke indoors, can impair the natural protective mechanisms against ARI in children [39]. However, it is noteworthy that not all studies reported such a link. A notable exception was found in the study conducted in Ethiopia [37], which did not find a significant association. Contextual factors, such as smoking prevalence and cultural practices, may influence these variations in findings.
Furthermore, our findings aligned with a recent meta-analysis that revealed a concerning association. Pregnant women exposed to passive smoking at home amplify the risk of delivering preterm babies [40]. Preterm children were particularly vulnerable to infections [41]. The present study observed a 2.44-field elevated odds of ARI among the children with preterm birth (AOR = 2.44, 95% CI: 1.43, 4.23. Additionally, our findings underlined the heightened susceptibility of premature babies to infections, particularly during their first year of life [42]. Preterm birth, a complex and multifaceted issue, is associated with an increased risk of various health challenges, including a higher likelihood of developing acute respiratory infections (ARIs). Our study indeed aligned with these trends as we observed significantly higher odds of ARI among preterm children under the age of five in a study conducted in India [11].
Our study findings emphasized the vital role of parental education in reducing the occurrence of childhood ARIs. The health and well-being of children are intricately linked to their caregivers’ knowledge, attitudes, and practices regarding water, sanitation and hygiene (WASH) [43]. Studies from various regions have consistently demonstrated the protective association between parental education and a reduced risk of ARI. For instance, research conducted in Nigeria found a protective correlation between childhood ARI and parental education [32]. Similarly, a study encompassing several developing nations indicated that maternal education played a pivotal role in reducing the risk of childhood ARI [44].
Our study showed that a family with a relatively high income (exceeding 25,000 BDT/month) had significant protection against ARI among children under five in conditional logistic regression analysis. However, in-house environmental factors were regarded as an indication of social and economic disadvantage [45]. Individuals with economic constraints were usually forced to live in a house with insufficient household environmental facilities for their necessities. Previous studies from Bangladesh also revealed such results [14,15,16,17].
In our study, we found that those who were exclusively breastfed, properly immunized for age, and had fewer than two siblings (in cases of multiple births) had significantly lower odds of ARI in childhood. Studies from different countries have provided similar results [10, 32, 36]. Having more siblings reduces the mothers’ attention on individual children, thus increasing the chance of different diseases. Immunoglobulins in breast milk help significantly reduce the risk of ARI in children. The healthy children had fewer odds of ARI, but the association was not significant, which contrasts with the findings of other countries [36, 46].
Improving home ventilation is a low-cost strategy. It involves simply opening windows and doors to disperse excess CO2 and create an environment less conducive to the growth of harmful microorganisms. Additionally, discouraging indoor smoking is another low-cost measure that can reduce ARIs in children and help mitigate the risk of premature birth, which is also independently associated with ARIs. Supporting this, continuation of breastfeeding for at least six months and offering guidance to mothers with larger families is essential. Furthermore, the population-based approach prioritizes health education, especially in rural areas, to reduce reliance on unclean cooking fuels and promote improved sanitation facilities. Complementing these efforts, broader initiatives aimed at poverty reduction, social equity and establishing a supportive social welfare system can ensure that residents are not compelled to live in overcrowded, inadequate spaces due to sudden financial shocks.
Our analysis had some limitations that need to be acknowledged. It was tertiary hospital-based research and, therefore, did not highlight the hidden aspect of the iceberg epidemic, including mother-child pairs who could not use hospital facilities or even primary health care. Recruiting controls at the hospital may have introduced a self-selection bias. Future research should use a longitudinal sample to confirm the causal relations between public and private hospitals nationwide. Further, research could comprehensively explore the causal relationships between various factors and ARI incidence.
Conclusion
The study hypotheses were adequately tested to confirm that in-house environmental factors in Bangladesh have an apparent, significant influence on childhood ARI, especially unimproved household sanitation facilities, in-house crowding (children living in a house where more than three people live in a bedroom), and in-house smoking, which were found to be important influencers in developing acute respiratory infection among under-five children. In addition, higher birth order and preterm birth were also found to play an important role in the development of ARI. Conversely, exclusive breastfeeding might significantly reduce ARIs in children under five in Bangladesh.
Data availability
The data underlying the results presented in this study will be provided upon reasonable request to Dr Delwer H. Hawlader. Email: mohammad.hawlader@northsouth.edu.
Abbreviations
- ARI:
-
Acute Respiratory Infections
- WHO:
-
World Health Organization
- UNICEF:
-
United Nations Children’s Fund
- MDG:
-
Millennium Development Goal
- GAPPD:
-
Global Action Plan for the Prevention and Control of Pneumonia and Diarrhea
- OR:
-
Odds Ratio
- AOR:
-
Adjusted Odds Ratio
- CI:
-
Confidence Interval
- MUAC:
-
Mid-Upper Arm Circumference
- EPI:
-
Expanded Program on Immunization
- VIF:
-
Variance Inflation Factor
- LPG:
-
Liquefied Petroleum Gas
- BDT:
-
Bangladeshi Taka
- C-section:
-
Cesarean Section
References
WHO. Child mortality (under 5 years). Fact Sheets. 2022;37:20–3.
UNICEF. A child dies of pneumonia every 43 seconds. 2023.
World Health Organization. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025 The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). 2013;:1–61.
WHO;UNICEF. WHO/Unicef Joint Statement Management of Pneumonia In Community Settings. 2004.
Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax. 2000;55:518–32.
Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:1405–16.
Indwar P, Debnath F, Sinha A. Reporting measles case fatality due to complications from a tertiary care hospital of Kolkata, West Bengal 2011–2013. J Fam Med Prim Care. 2016;5:777.
National Institute of Population Research and Training (NIPORT)., Mitra and Associates, and ICF International. Bangladesh Demographic and Health Survey 2014: key indicators. Dhaka, Bangladesh,and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and.
Bangladesh Bureau of Statistics [BBS]. Report on Bangladesh sample vital statistics 2018. Dhaka: Statistics and Informatics Division (SID), Ministry of Planning, 2019.
Tazinya AA, Halle-Ekane GE, Mbuagbaw LT, Abanda M, Atashili J, Obama MT. Risk factors for acute respiratory infections in children under five years attending the Bamenda Regional Hospital in Cameroon. BMC Pulm Med. 2018;18.
Taksande AM, Yeole M. Risk factors of Acute respiratory infection (ARI) in under-fives in a rural hospital of Central India. J Pediatr Neonatal Individ Med. 2015;5:e050105.
Lutpiatina L, Sulistyorini L, Notobroto HB, Raya RP, Utama RD, Thuraidah A. Multilevel analysis of Lifestyle and Household Environment for toddlers with symptoms of Acute respiratory infection (ARI) in Indonesia in 2007, 2012, and 2017. Glob Pediatr Heal. 2022;9:2333794X221078700.
Islam M, Sultana ZZ, Iqbal A, Ali M, Hossain A. Effect of in-house crowding on childhood hospital admissions for acute respiratory infection: a matched case–control study in Bangladesh. Int J Infect Dis. 2021;105:639–45.
Md Abul Kalam Azad K. Risk factors for Acute Respiratory infections (ARI) among children under five years in Bangladesh. J Sci Res. 2009;1:72–81.
Sultana M, Sarker AR, Sheikh N, Akram R, Ali N, Mahumud RA et al. Prevalence, determinants and health care-seeking behavior of childhood acute respiratory tract infections in Bangladesh. PLoS ONE. 2019;14.
Imran MIK, Inshafi MUA, Sheikh R, Chowdhury MAB, Uddin MJ. Risk factors for acute respiratory infection in children younger than five years in Bangladesh. Public Health. 2019;173:112–9.
Yaya S, Bishwajit G. Burden of acute respiratory infections among under-five children in relation to household wealth and socioeconomic status in Bangladesh. Trop Med Infect Dis. 2019;4.
World Health Organization (WHO). IMCI Integrated Management of Childhood Illness. 2005.
Kumar Sg, Majumdar A, Kumar V, Naik B, Selvaraj K, Balajee K. Prevalence of acute respiratory infection among under-five children in urban and rural areas of puducherry, India. J Nat Sci Biol Med. 2015;6:3.
Government of Bangladesh. National Micronutrient Survey 2011-12. 2014.
Hossain I, Hossain G, Ismail Hossain M, Mamun MA, Nurul Islam AS, Ripter Hossain M. M, et al. Assessment of Nutritional Status by using Mid Upper Arm Circumference of School going children (6–10 years) in Rajshahi District. Bangladesh: A Statistical Analysis; 2020.
World Health Organization(WHO). Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. WHO Guidel. 2014;:1–156.
Gray A, Ashby T, Worthington P, Rochford M, Le Harivel J, Jensen J et al. Definitions of crowding and the effects of crowding on Health. msd.govt.nz.
World Health Organization. Defining clean fuels and technologies. World Heal Organ. 2022;:28–30.
World Health Organization. Improved sanitation facilities and drinking-water sources. Health Topics. 2023;:2. https://www.who.int/data/nutrition/nlis/info/improved-sanitation-facilities-and-drinking watersources#:~:text = Improved drinking-water sources are,protect.
World Health Organization. Preterm birth Preterm birth. World Health Organization 2018; February 2018:1–5. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
Kovesi T, Gilbert NL, Stocco C, Fugler D, Dales RE, Guay M et al. Indoor air quality and the risk of lower respiratory tract infections in young Canadian Inuit children. Can Med Assoc J. 2007;177:155 LP – 160.
Organization WH. Regional Office for Europe and R. P. Ranson, Guidelines for healthy housing. Copenhagen: WHO Regional Office for Europe, 1988.
Von Schirnding Y, Bruce N, Smith K, Ballard-Tremeer G, Ezzati M, Lvovsky K. Addressing the impact of household energy and indoor air pollution on the health of poor: implications for policy action and intervention measures. World Health Organization Geneva; 2002.
Admasie A, Kumie A, Worku A. Children under five from Houses of unclean fuel sources and poorly ventilated Houses have higher odds of suffering from Acute respiratory infection in Wolaita-Sodo, Southern Ethiopia: a case-control study. J Environ Public Health. 2018;2018:9320603.
WHO (World Health Organization). WHO Guidelines for indoor air quality: dampness and Mould. Copenhagen:World Health Organization; 2009.
Ujunwa F, Ezeonu C. Risk factors for acute respiratory tract infections in under-five children in Enugu Southeast Nigeria. Ann Med Health Sci Res. 2014;4:95.
Ram PK, Dutt D, Silk BJ, Doshi S, Rudra CB, Abedin J, et al. Household air quality risk factors associated with childhood pneumonia in urban Dhaka, Bangladesh. Am J Trop Med Hyg. 2014;90:968–75.
[BBS]. BB of S. Report on Bangladesh Sample Vital Statistics. Bangladesh Bureau of Statistics and Informatics Division (SID). Ministry of planning; government of the people’s Republic of Bangladesh. Bangladesh: Dhaka; 2018.
Akpakli DE, Manyeh AK, Akpakli JK, Kukula V, Gyapong M. Determinants of access to improved sanitation facilities in rural districts of southern Ghana: evidence from Dodowa Health and demographic surveillance site. BMC Res Notes. 2018;11.
Yadav S, Khinchi Y, Pan A, Gupta SK, Shah GS, Baral DD, et al. Risk factors for acute respiratory infections in hospitalized under five children in central Nepal. J Nepal Paediatr Soc. 2013;33:39–44.
Admasie A, Kumie A, Worku A. Children under Five from Houses of Unclean Fuel Sources and Poorly Ventilated Houses Have Higher Odds of Suffering from Acute Respiratory Infection in Wolaita-Sodo, Southern Ethiopia: A Case-Control Study. J Environ Public Health. 2018;2018.
Po JYT, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232 LP – 239.
Valencia-Gattas M, Conner GE, Fregien NL. Gefitinib, an EGFR tyrosine kinase inhibitor, prevents smoke-mediated ciliated airway epithelial cell loss and promotes their recovery. PLoS ONE. 2016;11.
C H, G TT, L CX. W. associations between passive maternal smoking during pregnancy and preterm birth: evidence from a meta-analysis of observational studies. PLoS ONE. 2016;11:e0147848.
Diggikar S, Paul A, Razak A, Chandrasekaran M, Swamy RS. Respiratory infections in children born preterm in low and middle-income countries: a systematic review. Pediatr Pulmonol. 2022;57:2903–14.
Collins A, Weitkamp JH, Wynn JL. Why are preterm newborns at increased risk of infection? Archives of Disease in Childhood: Fetal and Neonatal Edition. 2018;103:F391–4.
Mshida HA, Kassim N, Kimanya ME, Mpolya E. Influence of Water, Sanitation, and Hygiene Practices on Common Infections among Under-Five Children in Longido and Monduli Districts of Arusha, Tanzania. J Environ Public Health. 2017;2017.
Pinzón-Rondón AM, Aguilera-Otalvaro P, Zárate-Ardila C, Hoyos-Martínez A. Acute respiratory infection in children from developing nations: a multi-level study. Toxicol Lett. 2016;259:27.
Krieger J, Higgins DL. Housing and health: Time Again for Public Health Action. Am J Public Health. 2002;92:758–68.
Cunha A. Relationship between acute respiratory infection and malnutrition in children under 5 years of age. Acta Paediatr Int J Paediatr. 2000;89:608–9.
Acknowledgements
We would like to express our sincere gratitude to the children, families, dedicated physicians, and staff at Dhaka Shishu Hospital for their valuable cooperation in this study.
Funding
Open access funding provided by Mid Sweden University. This study did not receive any funds from the public or any donor agency.
Open access funding provided by Mid Sweden University.
Author information
Authors and Affiliations
Contributions
The manuscript was reviewed, accepted and approved by all contributors. Conceptualization and design: MDHH, KD. Data collection: MI, KI. Data curation: MDHH, MI, KI. Data analysis: MI, KI. Draft manuscript preparation: MI, KI, KD, MDHH. Review and editing: MDHH, KD. Supervision: MDHH. Critical review: KD.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the North South University Institutional Review Board (2019/OR-NSU/IRB-No.0701). All methods were performed following the relevant guidelines and regulations. Written informed consent was obtained from each child’s mother or father.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Islam, M., Islam, K., Dalal, K. et al. In-house environmental factors and childhood acute respiratory infections in under-five children: a hospital-based matched case-control study in Bangladesh. BMC Pediatr 24, 38 (2024). https://doi.org/10.1186/s12887-024-04525-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-024-04525-4