Abstract
Background
Neonatal sepsis is the major cause of neonatal mortality and morbidity, especially in low and middle-income countries. Continuous monitoring of pathogens and their antibiotic resistance pattern is crucial for managing neonatal sepsis. This study aimed to determine neonatal sepsis due to bacteria, antibiotic resistance patterns, associated risk factors and patient outcomes at St. Paul’s Hospital Millennium Medical College.
Method
An institutional-based cross-sectional study was conducted on 400 neonates suspected of sepsis at St. Paul’s Hospital Millennium Medical College from March 2020 to July 2020. A questionnaire was used to collect socio-demographic information, clinical parameters and potential risk factors from study participants. About 2ml of blood was drawn aseptically and inoculated into Tryptone Soya Broth at the patient’s bedside. Bacterial identification was performed by using standard microbiological techniques. The disk diffusion method was used to determine the antibiotic susceptibility patterns of each isolated bacteria. Data entry and analysis were done using Statistical Package for Social Sciences (SPSS) version 20 software. Bivariate and multivariable logistic regressions were used to assess associated risk factors of neonatal sepsis. A p-value less than 0.05 was considered statically significant with a 95% confidence interval.
Results
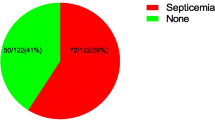
The overall prevalence of neonatal septicemia was 21% (84/400). Of these, 67 (79.8%) and 17 (20.2%) were gram-negative and gram-positive bacteria, respectively. Klebsiella spp, 37 (44%), E. coli 19 (21.6%) and Coagulase negative Staphylococci 13 (15.47%) were the leading cause of neonatal sepsis. Ciprofloxacin and amikacin were the most effective antibiotics for gram-negative and gram-positive bacteria. Multidrug resistance was observed in 84% of the bacterial isolates. Low birth weight and preterm were associated with neonatal septicemia (AOR = 49.90, 95% CI = 15.14-123.081, P = 0.002) and (AOR = 18.20, 95% CI = 6.835–27.541, P = 0.004) respectively.
Conclusion
Klebsiella spp and E. coli were frequently isolated bacteria in our study. The proportion of multidrug-resistance was significantly high. Most isolated bacteria were resistant to ampicillin, ceftazidime, cefotaxime and gentamycin, which indicates the necessity of continuous evaluation of antibiotic resistance rate.
Similar content being viewed by others
Introduction
Neonatal sepsis is a bloodstream infection that occurs in infants less than 28 days of life [1]. It is the primary cause of morbidity and mortality among neonates, especially in resource limited countries [2]. Several literatures define neonatal sepsis as either early-onset or late-onset, depending on the symptoms occur before or after 72 h of life [3,4,5]. Early onset sepsis (EOS) is defined as sepsis occurs less than or equal to three days of birth, and is caused by microorganisms occur in the maternal genital tract before or at the time of birth [6]. Premature and low birth weight, infection during pregnancy, vaginal colonization with Group B Streptococcus (GBS) and lack of appropriate antenatal care are frequently reported risk factors of early-onset sepsis [7]. Based on global report, the incidence rate of early-onset sepsis is almost three times that of late-onset sepsis, and 70% of the causative etiological agents are mainly GBs and E.coli [8]. However, intrapartum antibiotic prophylaxis can reduce significantly the incidence of GBS in newborns [9].
Late-onset sepsis (LOS) begins after three days of life, mainly due to bacteria carried from the hospital or the society at the time of delivery [10]. It is usually associated with hospital and community acquired bacterial infections including intravenous catheterization. Most of these risk factors are preventable through early diagnosis and timely appropriate clinical management [8]. The most common etiological agents are Staphylococcus aureus, Coagulase-negative Staphylococcus (CoNS), E. coli and Klebsiella pneumoniae [11,12,13]. However, the distribution of bacteria causing neonatal sepsis varies from country to country and even with the same geographical locations [14]. Globally, neonatal sepsis is the leading cause of morbidity and mortality in the first 4 weeks of life with an estimated annual death rate of 400,000 to 700,000, and accounts 15% of all neonatal deaths. Of these, around 42% of neonatal deaths occurred in the first week of life [8, 15, 16]. It is also the third most common cause of neonatal mortality [17]. In developing countries, nearly 50% of neonatal death is due to sepsis and the highest neonatal death rate has been reported from Sub-Saharan Africa with 27 deaths/1000 live births [18].
In Ethiopia, neonatal sepsis is the major newborn health problem which accounts 33% of all neonatal deaths [14]. According to 2011 and 2016 Ethiopian Demographic and Health Survey (EDHS) report, the neonatal mortality rate was significantly decreased from 37 to 29 deaths/1000 live births [19]. However, increasing death rate was reported in 2019 EDHS, 30 deaths /1000 lives [20]. According to a systematic review in Ethiopia, the neonatal sepsis was estimated at 45% with a range of 17–78% of neonatal sepsis [21]. These data indicate that the prevalence of neonatal death due to sepsis or other causes varies time to time, hence continuous assessment is essential to prevent and control morbidity. Another big challenge in neonatal life is the emergence of multidrug resistant bacteria which complicates the sepsis management, and around 30% of neonatal death is linked to antimicrobial resistance [22]. This is mainly due to frequent use of antibiotics without bacteriological and susceptibility evidence [23].
Therefore, reliable diagnosis including identification and antibiotic susceptibility is crucial to prevent and control neonatal mortality due to bacterial infection. Moreover, neonatal sepsis still continues to be the primary cause of neonatal morbidity and mortality globally as well as in Ethiopia. Therefore, the current study aimed to determine the bacterial etiology of sepsis and their antibiotic susceptibility pattern among neonates at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia.
Materials and methods
Study setting
This study was conducted at St. Paul’s Specialized Hospital Millennium Medical College (SPHMMC). It is located in Addis Ababa, the capital city of Ethiopia, and one of the largest teaching referral hospitals in Ethiopia. It was established in 1968 by Emperor Haile Selassie, and the medical school opened in 2007 under the name of Millennium Medical College. The hospital has around 800 beds from these 26 beds for pediatrics medical ward,16 beds for pediatrics surgical ward and 4 beds for pediatric intensive care unit (ICU) and 4 neonatal intensive care unit (NICU). It gives diagnostic and treatment services for 370,000- 500,000 patients per year, from these around 3600 were neonates.
Study design and study period
An institutional-based cross-sectional study was conducted from March 2020 to July 2020.
Source population
All neonates who were admitted to NICU of SPHMMC were the source population.
Study population
All neonates with the age of 0–28 days who were admitted in the neonatal ward presenting with clinical features suggestive of sepsis such as difficulty in breathing, refusal to feed, fever, lethargy and respiratory distress were recruited for the study.
Inclusion
All neonates born in or outside of SPHMMC during arrival or during stay int the hospital showing signs and symptoms of sepsis were included.
Exclusion
Neonates who took antibiotics for the last two weeks during data collection were excluded in this study. This was to minimize false negative blood cultures because antibiotics may not kill all bacteria but it can decrease their number (unable to detect with blood culture). Neonates whose parents/ legal guardians were not available for interview were also excluded.
Study variables
Dependent variables
Prevalence of bacterial isolates.
Independent variables
Age, sex, weight at birth, gestational week, medical devices, recovery or death of neonates, maternal malnutrition and chronic diseases.
Sample size determination and Sampling technique
The sample size was determined using a single population proportion formula. Accordingly, the minimum sample size was calculated by taking the prevalence (p) of neonatal sepsis (46.6%) reported from Gondar specialized hospital, Ethiopia [24], the margin of error (5%), and 95% Confidence level (z = 1.96).
n = (Z α/2)2 * P (1- P)/ d2.
n= (1.96)2 *0.466(1-0.466)/0.052 = 382.
The minimum sample size was 382, and non-probability sampling technique was employed using inclusion criteria till sample size fulfilled. Nevertheless, we have collected 400 samples.
Data collection
Structured questionnaire was used to collect socio-demographic data, clinical characteristics and associated risk factors of neonatal sepsis. In addition, maternal information such as chronic disease, indwelling medical device, birth weight, nutritional status and HIV status were collected by reviewing medical records. Outcome of neonates assessed using checklists.
Blood specimen collection
After obtaining written informed consent from parents or legal guardians, 2ml of blood samples were collected from peripheral vein by aseptic technique. The vein puncture site was cleansed with 70% isopropyl alcohol and 2% tincture iodine before collecting. The collected blood samples were inoculated into Tryptone soya broth and transported to the microbiology laboratory within 5–10 min at room temperature. The blood samples were collected prior to antibiotic treatment.
Laboratory methods
Blood culture and bacterial identification
The blood culture bottles were incubated at 35–37 °C under aerobic conditions for 24 h and inspected daily for 7 days until growth detected. Bottles were observed macroscopically daily for visible evidence of bacterial growth such as hemolysis, turbidity, and gas production. Sub-culture was done on MacConkey agar and incubated aerobically at 35–37 °C for 24 h. Whereas, sub-culture was done on sheep blood agar and chocolate agar and incubated at 5% CO2 for 48–72 h. The same procedure was repeated until the seventh day before the blood cultures considered to be negative.
Bacterial identification was done through colony morphology, Gram stain and biochemical tests. Biochemical tests were performed on sub cultured media by isolating the pure colonies. For gram-positive bacteria, catalase, coagulase, novobiocin and mannitol fermentation were performed. For gram-negative bacteria, indole, citrate utilization, triple sugar iron, urea, mannitol, oxidase, nitrate reduction, lysine decarboxylase, lysine deaminase and motility test were carried out based on Clinical and Laboratory Standard Institute (CLSI) guideline 2020 [25].
Antimicrobial susceptibility testing
Antimicrobial susceptibility test was performed for each bacterial isolate based on Clinical and Laboratory Standard Institute guideline by using disc diffusion method on Muller Hinton agar [25]. Muller Hinton agar with 5% sheep blood was used for fastidious organisms. Approximately, three to five pure colony of the test organism was taken by using a sterile wire loop and emulsify in 2 ml of normal saline. McFarland (0.5) was used as a standard to check the 5% turbidity of bacterial suspensions. Then a sterile cotton swab was dipped in to the suspension and squeeze free from excess fluid against the inside wall of the test tube. The test organisms were uniformly seed on the surface of Muller-Hinton agar for non-fastidious group and Muller Hinton agar with 5% sheep blood for fastidious group and expose to a concentration gradient of antibiotic diffusing from antibiotic impregnated paper disc into the agar medium and the medium was incubated at 37 °C for 24 h.
Antimicrobial agents tested include erythromycin (15 µg), clindamycin (2 µg), chloramphenicol (30 µg), vancomycin (30 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), ampicillin (10 µg), cloxacillin (30 µg), gentamicin (10 µg), amikacin (30 µg), ciprofloxacin (5 µg), ceftazidime (30 µg), ceftriaxone (30 µg) and cefotaxime (30 µg). Grades of susceptibility pattern were interpreted by comparison of the zone of inhibition according to CLSI guideline 2020 [25].
Quality control
Quality control of the culture media was performed for new batch of media. Visual inspection was done to check cracks in media, hemolysis, evidence of freezing and presence of air bubbles. The sterility of culture media was ensured by incubating 5% of each batch of the prepared media at 37 °C for 24 h. All prepared culture media were also checked by inoculating known strains such as E. coli (ATCC 25922) gram-negative bacteria, S. aureus (ATCC 25923) for gram-positive bacteria and N. gonorrhoeae (ATCC49226) for fastidious bacteria. To standardize the inoculums density of bacterial suspension for the susceptibility test, 0.5 McFarland standards was used.
Statistical analysis
All data were encoded in Microsoft Excel 2016 and exported to Statistical Package for Social Sciences (SPSS) version 20 software. We used descriptive statistics, such as frequencies and percentages to describe the proportion of bacterial isolates and associated risk factors of neonatal sepsis. Binary logistic regression model was performed to determine the association between neonatal sepsis and associated factors. Moreover, all variables with a p-value < 0.25 in the bivariable analysis were included in multivariable analysis to account for possible confounding variables. P-value less than 0.05 considered as statistically significant. The strength of the association was interpreted using an odds ratio in a 95% confidence interval. Finally, the results presented on words, graphs and tables.
Operational definition
MDR: MDR was defined as non-susceptibility to at least one agent in three or more antimicrobial categories.
Early onset sepsis: The onset of signs and symptoms of sepsis in neonates less than or equal to 3 days of life.
Late onset sepsis: The onset of signs and symptoms of sepsis after 3 days and up to 28 days of life.
Term: Babies born between 37 and 41 weeks of pregnancy.
Preterm: Babies born before 37 completed weeks of pregnancy.
Low birth weight: Newborn weight at birth < 2.5 kg.
Normal birth weight: Newborn weight at birth ≥ 2.5 kg.
Results
Socio-demographic characteristics of the study participants
Out of 400 sepsis suspected neonates, 246 (61.5%) were males. About 166 (41.5%) of the neonates had low birth weight (< 2.5 kg), and 118 (29.5%) were preterm. The majority of neonates, 364 (91%) were in the first three days of life (Table 1).
Proportion of bacterial isolates
Of all bacterial isolates, 67/84 (79.8%) were gram-negative bacteria, while the remaining 17 (20.23%) were gram-positive bacteria. The majority of culture confirmed cases of sepsis were in the first three days of life 65 (77.4%). About 59 (90.76%) of gram-negative bacteria were detected from the EOS, whereas 11 (57.87%) of gram-positive bacteria were detected from the LOS. The leading causes of neonatal sepsis in this study were Klebsiella spp 37 (44%) and E. coli 19 (22.6%) (Fig. 1).
Antibiotic resistance pattern of bacterial species
Coagulase negative staphylococcus (CoNS) was found to be highly resistant to ampicillin 11 (85%), trimethoprim-sulfamethoxazole 10 (77%) and cloxacillin 13 (100%) (Table 2). The overall level of antibiotic resistance in gram-negative bacteria ranged from 13 (20%) to 54 (84%). Regarding specific bacterial species, Klebsiella spp were extremely resistant to gentamicin 35 (95%) and cefotaxime 35 (95%). Similarly, Acinetobacter spp showed the highest resistance to gentamycin 8 (100%) and trimethoprim-sulfamethoxazole 8 (100%). E. coli was resistant to ceftazidime and cefotaxime at the rate of 19 (100%) and 13 (68%) respectively. On the other hand, almost all identified gram-negative bacteria showed least resistance rate to ciprofloxacin and amikacin (Table 2).
Multidrug resistance pattern of bacterial isolates
About 84% of bacterial isolates were resistant to three or more classes of antimicrobial agents. Around 59 (70%) of bacterial isolates were resistant to three different categories of antimicrobial agents, while 10 (11.9%) were resistant to four different classes of antimicrobial agents. MDR isolates were found in 8 (100%) of Acinetobacter spp and 35 (95%) of Klebsiella spp (Table 3).
Factors associated with neonatal sepsis
Bivariable and multivariable regressions were used to assess the association between associated factors and neonatal sepsis. Low birth weight was found to be associated with neonatal septicemia (AOR = 45.90, 95% CI = 15.14-123.081, P = 0.002). In addition, gestational age less than 37 weeks and neonates whose mothers infected with HIV were showed a higher odd to develop sepsis (AOR = 18.2, 95% CI = 6.835–27.541, P = 0.004 and AOR = 5.30, 95% CI = 2.864–13.583, P = 0.003) respectively (Table 4).
Discussion
In Ethiopia, one-third of the neonatal deaths are caused by sepsis [26]. The spectrum of bacteria that cause neonatal sepsis changes over time, which necessitates ongoing assessment and monitoring [8]. In this study, the prevalence of confirmed blood culture sepsis was 21%. Similar results were reported from Dire Dawa, Ethiopia 23% [27], Nigeria, 22.4% [28] and Ghana 21% [29]. However, it was lower than studies in Ethiopia (Addis Ababa 27% [30], Tigray 36.6% [31], Gondar 46.6% [32],) and other countries such as Zambia 33% [33], Egypt 40.7% [34] and India 26.2% [35]. The lower prevalence of sepsis in our finding might be due to similar signs and symptoms of septicemia with non-bacterial infections such as viruses and fungi that can cause a negative blood culture result [36]. Another reason could be due to differences in diagnostic method, sample size, study settings and study period.
Klebsiella spp 37 (44%) and E. coli 19 (22.6%) were the most prevalent bacteria in our finding. On the other hand, previous studies reported that S. aureus and CoNS were the primary organisms causing newborn sepsis [32, 37, 38]. The higher prevalence of Klebsiella spp in this study could be explained by the fact that Klebsiella spp are prevalent in the hospital environment and can cause infection at delivery or during their hospital stay [39, 40]. It can also be due to poor personal hygiene of caregivers or families, as well as contaminated medical devices [41].
The current study has shown that EOS was more prevalent than LOS, with a proportion of 77.8% and 22.61%, respectively. The results were comparable to previous studies in Wolega, Ethiopia 75.5% [42], Myanmar 77.8% [43], and Nepal 78.3% [44]. This higher prevalence of EOS could be attributed to prematurity, low birth weight, and poor hygienic conditions during delivery [45]. In our finding, Klebsiella spp and E. coli were the primary cause of EOS while CoNS and S. aureus were the predominant cause of LOS. The findings were consistent with a study in Nepal [46] and South Africa [47]. However, African reports indicated that S. aureus and CoNS were the predominant causal agents of EOS [48]. These inconsistent results might be due to the etiological agents of EOS and LOS change with time and geographical location [11]. Another reason could be due to early horizontal transmission in the delivery room or vertical transmission from maternal genital tract colonized with these pathogen [41, 49, 50].
The trend of antibiotic resistance change over time and may vary among countries [51]. Many literatures recommend ampicillin and gentamicin as a first-line treatment for neonatal sepsis [51,52,53]. Conversely, our finding showed that the majority of bacterial isolates were resistant to ampicillin and gentamicin, ranging from 68 to 85% and 75–100% respectively. This was consistent with previous studies in Ethiopia that showed high resistance rates of bacteria against ampicillin and gentamicin (90–100%) [31, 54]. Similarly, almost all bacterial isolates were 100% resistant to ampicillin in Egypt [55] and India [38]. The reason for this high resistance may be related with frequent use of these antibiotics.
In our study, the number of S. aureus and Enterobacter species isolates were very low to discuss about their susceptibility pattern, so we have discussed about bacterial species that have sufficient number. Accordingly, CoNS were the only gram-positive bacteria that have adequate number and showed least sensitivity to gentamycin (69%), ampicillin (85%) and cloxacillin (94%) respectively. This finding agreed with previous studies from Tanzania [56] and Nepal [57] that reported the resistance rate of CoNS to gentamicin (75%) and ampicillin (100%). The reason for this high resistance may be related with frequent use of these antibiotics which create selective pressure of antimicrobial agents.
Amikacin and ciprofloxacin were effective against E. coli and Klebsiella spp, which was comparable to previous study [54]. This can indicate that these two antibiotics could be a potential choice for empiric treatment of neonatal sepsis in the future.
The prevalence of MDR was 84% in our study, which was slightly lower than a previous study in the same setting, where the rate was 88% [58]. However, it was higher than other studies [24, 38, 44, 59]. The proportion of MDR among gram-negative bacteria was 87%, which was consistent with previous studies in Bahir Dar, Ethiopia with 88.2% [60], Ghna 71.7% [61] and Nepal with 81% [44]. The high percentage of MDR in gram-negative bacteria may be due to their ability to transfer genes, have intrinsic antibiotic resistance and produce beta-lactamase.
We also assessed associated factors of neonatal sepsis. Septicemia neonates with low birth weight were 45.90 times more likely to develop sepsis than neonates with normal birth (AOR = 45.9, 95% CI = 15.14-123.08, P = 0.002). This finding was consistent with a study in Gondar and Bahir Dar, Ethiopia [32, 60]. The significant association might be due to low birth weight newborns are mostly premature, underdeveloped immune system, unable to feed, and easily lose their heat which may increase the likelihood of neonatal infections. Preterm neonates with septicemia were 18.2 times more likely to develop sepsis than the term neonates (AOR = 18.2,95% CI = 6.83–27.54, P = 0.004), this was similar to previous studies [32, 62]. This association could be due to weaker immune system of the preterm infants that can easily develop systemic infection [63]. This can be better explained by the fact that neonates have low neutrophil storage pools which increases the occurrence of reduced number of neutrophils with serious infection [64]. Neonates whose mothers were seropositive were five times more likely to develop sepsis (AOR = 5.30, 95% CI = 2.86–13.58, P = 0.003). This might be due to the fact that mothers who are infected with HIV have low immune status, which causes infants to be susceptible to infection.
Age, sex, and maternal nutrition status were not significantly associated with neonatal sepsis. In this study, the death rate due to sepsis was7% and was not significantly related to blood culture results. This result was much smaller than study which reported a 20.9% death rate [65], but higher than studies in Bahir Dar and Dessie, Ethiopia that reported 4.0% and 4.8% death rate [66, 67].
Limitations
This study has several limitations: The first limitation was unable to perform drug resistance enzymes, cefoxitin and genes because of budget and facility limitations. Laboratory analysis was the second limitation which fail to detect some pathogenic bacteria such as Pneumococcus and Group B streptococcus. Another limitation was data collection tool (questionnaire) for maternal related factors which suffers from recall bias. Therefore, care must be taken in interpreting and drawing conclusions based on such information, as there is a tendency for respondents to provide what they believe. The result from this study could not infer to the general population because of the nature of the cross-sectional study at a single center.
Conclusions
In conclusion, EOS was caused primarily by Klebsiella spp and E. coli, while LOS was caused by CoNS. Gram-negative bacteria were highly resistant to gentamycin, ceftazidime and cefotaxime. Similarly, CoNS was also resistant against gentamycin, ceftazidime and cloxacillin. In addition, low birth weight, gestational age less than or equal to 37 weeks and maternal HIV status were associated with neonatal sepsis. Therefore, periodic review of antibiotic resistance profile is recommended. Further, large scale study is important to characterize each bacterial strain and to study more risk factors.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CoNS :
-
Coagulase negative Staphylococcus
- EDHS:
-
Ethiopian demography and health survey
- EOS:
-
Early onset sepsis
- GBs:
-
Group B streptococcus
- LOS:
-
Late onset sepsis
- MDR:
-
Multidrug resistance
- NICU:
-
Neonatal intensive care unit
References
Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106(8):745–52.
Seale AC, Blencowe H, Manu AA, Nair H, Bahl R, Qazi SA, et al. Estimates of possible severe bacterial Infection in neonates in sub-saharan Africa, south Asia, and Latin America for 2012: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):731–41.
Ekman B, Paudel P, Basnet O, Kc A, Wrammert J. Adherence to World Health Organisation guidelines for treatment of early onset neonatal sepsis in low-income settings; a cohort study in Nepal. BMC Infect Dis. 2020;20(1):1–8.
Kamalakannan SK. Neonatal sepsis past to present. Biomed J Sci & Tech Res. 2018;3(3):3309–14.
Nyenga A, Mukuku O, Wembonyama S. Neonatal sepsis: a review of the literature. Theory and Clinical Practice in Pediatrics. 2021;3:94–101.
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK Jr, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev. 2012;88:69–S74.
Hammad E, Zainab M. Meta-analysis on factors influencing early onset neonatal sepsis. Sch J Appl Sci Res. 2018;1(8):20–2.
World Health Organization. Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. 2020. World Health Organization. https://apps.who.int/iris/handle/10665/334216.
Warrier LM, Joy S, Bashir RA. Group B Streptococcal colonization among pregnant women and neonates in a tertiary care hospital in South India. Indian J Pediatr. 2022;89(12):1187–94.
Weiss SL, Peters MJ, Alhazzani W, Agus MS, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020;46(1):10–67.
Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27(1):21–47.
Sanderson E, Yeo K, Wang A, Callander I, Bajuk B, Bolisetty S, et al. Dwell time and risk of central-line-associated bloodstream Infection in neonates. J Hosp Infect. 2017;97(3):267–74.
Berardi A, Sforza F, Baroni L, Spada C, Ambretti S, Biasucci G, et al. Epidemiology and Complications of late-onset sepsis: an Italian area-based study. PLoS ONE. 2019;14(11):e0225407.
Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC Public Health. 2013;13(1):1–14.
Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med. 2017;377(5):414–7.
UNICEF. Levels & trends in child mortality: Reports in 2019.
Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35.
Masaba BB, Mmusi-Phetoe RM. Neonatal survival in Sub-sahara: a review of Kenya and South Africa. J Multidiscip Healthc. 2020;13:709.
ICF, CSACEa. Ethiopia Demographic and Health Survey 2016. Maryland, USA: Addis Ababa, Ethiopia, and Rockville; 2016.
Ministry of Health in Ethiopia. Ethiopian Mini Demographic Health Survey.2019. https://www.moh.gov.et/ejcc/sites/default/files/2019-08. access date, 20/7/2023.
Assemie MA, Alene M, Yismaw L, Ketema DB, Lamore Y, Petrucka P et al. Prevalence of neonatal sepsis in Ethiopia: a systematic review and meta-analysis. Int J Pediatr. 2020;2020.
Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen J-A, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–75.
Yusef D, Shalakhti T, Awad S, Algharaibeh Ha, Khasawneh W. Clinical characteristics and epidemiology of sepsis in the neonatal intensive care unit in the era of multi-drug resistant organisms: a retrospective review. Pediatr Neonatol. 2018;59(1):35–41.
G/eyesus T, Moges F, Eshetie S, Yeshitela B, Abate E. Bacterial etiologic agents causing neonatal sepsis and associated risk factors in Gondar, Northwest Ethiopia. BMC Pediatr. 2017;17(1):137.
CLSI. Performance standards for antimicrobial susceptibility testing. 29th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
Wardlaw T, You D, Hug L, Amouzou A, Newby H. UNICEF Report: enormous progress in child survival but greater focus on newborns urgently needed. Reproductive Health. 2014;11(1):1–4.
Serbesa ML, Oumer A, Iffa M. Assessment of reason for admission and Factors Associated with their treatment outcome of neonates in Dilchora Referral Hospital, Eastern, Ethiopia. J Neonatal Stud. 2018;1:103.
Dedeke I, Arowosegbe A, Shittu O, Ojo D, Akingbade O. Neonatal sepsis in a Nigerian Tertiary Hospital: clinical features, clinical outcome, aetiology and antibiotic susceptibility pattern. South Afr J Infect Dis. 2017;32(4):127–31.
Acheampong EN, Tsiase JA, Afriyie DK, Amponsah SK. Neonatal Sepsis in a Resource-Limited Setting: Causative Microorganisms and Antimicrobial Susceptibility Profile. Interdiscip Perspect Infect Dis 2022;2022.
Negussie A, Mulugeta G, Bedru A, Ali I, Shimeles D, Lema T, et al. Bacteriological profile and antimicrobial susceptibility pattern of blood culture isolates among Septicemia suspected children in selected hospitals Addis Ababa, Ethiopia. Int J Biol Med Res. 2015;6(1):4709–17.
Weldu Y, Naizgi M, Hadgu A, Desta AA, Kahsay A, Negash L, et al. Neonatal Septicemia at intensive care unit, Ayder Comprehensive Specialized Hospital, Tigray, North Ethiopia: bacteriological profile, drug susceptibility pattern, and associated factors. PLoS ONE. 2020;15(6):e0235391.
Moges F, Eshetie S, Yeshitela B, Abate E. Bacterial etiologic agents causing neonatal sepsis and associated risk factors in Gondar, Northwest Ethiopia. BMC Pediatr. 2017;17(1):1–10.
Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in Zambia. Pediatr Infect Dis J. 2016;35(7):e191–e8.
Shehab El-Din EMR, El-Sokkary MMA, Bassiouny MR, Hassan R. Epidemiology of neonatal sepsis and implicated pathogens: a study from Egypt. Biomed Res Int 2015;2015.
Kumar DVP, Mohan J, Rakesh P, Prasad J, Joseph L. Bacteriological profile of neonatal sepsis in a secondary care hospital in rural Tamil Nadu, Southern India. J Family Med. 2017;6(4):735.
Dolin HH, Papadimos TJ, Chen X, Pan ZK. Characterization of pathogenic sepsis etiologies and patient profiles: a novel approach to triage and treatment. Microbiol Insights. 2019;12:1178636118825081.
Yadav NS, Sharma S, Chaudhary DK, Panthi P, Pokhrel P, Shrestha A, et al. Bacteriological profile of neonatal sepsis and antibiotic susceptibility pattern of isolates admitted at Kanti Children’s Hospital, Kathmandu, Nepal. BMC Res Notes. 2018;11(1):1–6.
Mohakud NK, Mishra JP, Nayak MK, Mishra J, Pradhan L, Panda SS, et al. Bacteriological Profile and Outcome of Culture-positive neonatal Sepsis in a special Newborn Care Unit setting, Odisha. Cureus. 2022;14(5):1–7.
Le T, Wang L, Zeng C, Fu L, Liu Z, Hu J. Clinical and microbiological characteristics of nosocomial, healthcare-associated, and community-acquired Klebsiella pneumoniae Infections in Guangzhou, China. Antimicrob Resist Infect Control. 2021;10(1):1–11.
Bhatta DR, Hosuru Subramanya S, Hamal D, Shrestha R, Gauchan E, Basnet S, et al. Bacterial contamination of neonatal intensive care units: how safe are the neonates? Antimicrob Resist Infect Control. 2021;10(1):1–6.
Bitew K, Gidebo DD, Ali MM. Bacterial contamination rates and drug susceptibility patterns of bacteria recovered from medical equipment, inanimate surfaces, and indoor air of a neonatal intensive care unit and pediatric ward at Hawassa University Comprehensive Specialized Hospital, Ethiopia. IJID. 2021;1:27–33.
Fekadu G, Abera T, Tekle T. Clinical treatment outcomes of neonatal sepsis in neonatal intensive care unit of Wollega University Teaching and Referral Hospital, Nekemte Town, Western Ethiopia. Pediatr Ther. 2019;9(353):2161–06651000353.
Oo NAT, Edwards JK, Pyakurel P, Thekkur P, Maung TM, Aye NSS, et al. Neonatal sepsis, antibiotic susceptibility pattern, and treatment outcomes among neonates treated in two tertiary care hospitals of Yangon, Myanmar from 2017 to 2019. Trop Med Infect Disease. 2021;6(2):62.
Pokhrel B, Koirala T, Shah G, Joshi S, Baral P. Bacteriological profile and antibiotic susceptibility of neonatal sepsis in neonatal intensive care unit of a tertiary hospital in Nepal. BMC Pediatr. 2018;18(1):1–8.
Ogundare E, Akintayo A, Aladekomo T, Adeyemi L, Ogunlesi T, Oyelami O. Presentation and outcomes of early and late onset neonatal sepsis in a Nigerian hospital. Afr Health Sci. 2019;19(3):2390–9.
Ansari S, Nepal HP, Gautam R, Shrestha S, Neopane P, Chapagain ML. Neonatal septicemia in Nepal: early-onset versus late-onset. International journal of pediatrics. 2015;2015.
Van Staaden H, Hendricks C, Spicer K. Bacteraemia and antibiotic sensitivity in a tertiary neonatal intensive care unit. South Afr J Infect Dis. 2021;36(1):195.
Hamer D, Darmstadt G, Carlin J, Zaidi A, Yeboah-Antwi K, Saha S, et al. Young Infants Clinical Signs Study Group: etiology of bacteremia in young infants in six countries. Pediatr Infect Dis J. 2015;34:e1–e8.
Sands K, Spiller OB, Thomson K, Portal EA, Iregbu KC, Walsh TR. Early-onset neonatal sepsis in low-and middle-income countries: current challenges and future opportunities. Infect Drug Resist. 2022:933–46.
Bulabula A, Dramowski A, Mehtar S. Transmission of multidrug-resistant Gram-negative bacteria from colonized mothers to their infants: a systematic review and meta-analysis. J Hosp Infect. 2020;104(1):57–67.
Korang SK, Safi S, Gluud C, Lausten-Thomsen U, Jakobsen JC. Antibiotic regimens for neonatal sepsis-a protocol for a systematic review with meta-analysis. Syst Reviews. 2019;8(1):1–13.
Manan MM, Ibrahim NA, Aziz NA, Zulkifly HH, Al-Worafi YMA, Long CM. Empirical use of antibiotic therapy in the prevention of early onset sepsis in neonates: a pilot study. Archives of Medical Science. 2016;12(3):603–13.
Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, et al. Early and late Infections in newborns: where do we stand? A review. Pediatr Neonatology. 2016;57(4):265–73.
Sorsa A, Früh J, Stötter L, Abdissa S. Blood culture result profile and antimicrobial resistance pattern: a report from neonatal intensive care unit (NICU), Asella teaching and referral hospital, Asella, South East Ethiopia. Antimicrob Resist Infect Control. 2019;8(1):1–6.
Almohammady MN, Eltahlawy EM, Reda NM. Pattern of bacterial profile and antibiotic susceptibility among neonatal sepsis cases at Cairo University Children Hospital. J Taibah Univ Med Sci. 2020;15(1):39–47.
Godfrey E, Majaliwa E, Assenga EN. Aetiology, antimicrobial susceptibility and outcome of children with sepsis, admitted at Muhimbili National Hospital, Dar Es Salaam. Pan Afr Med J. 2022;42.
Manandhar S, Amatya P, Ansari I, Joshi N, Maharjan N, Dongol S, et al. Risk factors for the development of neonatal sepsis in a neonatal intensive care unit of a tertiary care hospital of Nepal. BMC Ifect Dis. 2021;21(1):1–11.
Solomon S, Akeju O, Odumade OA, Ambachew R, Gebreyohannes Z, Van Wickle K, et al. Prevalence and risk factors for antimicrobial resistance among newborns with gram-negative sepsis. PLoS ONE. 2021;16(8):e0255410.
Dhir SK, Sundaram V, Gautam V, Munda VS, Tiewsoh JBA, Angurana SK, et al. Microorganisms profile and antimicrobial resistance pattern in outborn neonates in Northern India: a hospital-based observational study. J Trop Pediatr. 2021;67(3):1–9.
Zenebe Y, Molla T, Beza L, Mekonnen D. Bacterial profile and antimicrobial susceptibility pattern of neonatal sepsis in Felege-Hiwot Referral Hospital, Bahir Dar, northwest Ethiopia: a cross-sectional study design. Ethiop J Health Dev. 2021;35(1):1–28.
Labi A-K, Obeng-Nkrumah N, Bjerrum S, Enweronu-Laryea C, Newman MJ. Neonatal bloodstream Infections in a Ghanaian Tertiary Hospital: are the current antibiotic recommendations adequate? BMC Infect Dis. 2016;16(1):1–12.
Hayun M, Alasiry E, Daud D, Febriani DB, Madjid D. The risk factors of early onset neonatal sepsis. Am J Clin Experimental Med. 2015;3(3):78–82.
Collado MC, Cernada M, Neu J, Pérez-Martínez G, Gormaz M, Vento M. Factors influencing gastrointestinal tract and microbiota immune interaction in preterm infants. Pediatr Res. 2015;77(6):726–31.
Collins A, Weitkamp J-H, Wynn JL. Why are preterm newborns at increased risk of Infection? Archives of Disease in childhood-fetal and neonatal Edition. 2018;103(4):F391–F4.
Ali A-KM, Abdul-Kareem L, Rashed EJ. Identification of bacterial agentsand antimicrobial susceptibility of neonatal sepsis with patient, s outcome. Al-Qadisiyah Med J. 2014;10(17):148–61.
Fenta GM, Woldemariam HK, Metaferia Y, Seid A, Gebretsadik D. Admission outcome and Antimicrobial Resistance Pattern of Bacterial isolates among neonates with suspected Sepsis in neonatal intensive care unit at Dessie Comprehensive Specialized Hospital, Dessie, Northeastern Ethiopia. Interdisciplinary Perspect Infect Dis. 2022;2022:1–13.
Tewabe T, Mohammed S, Tilahun Y, Melaku B, Fenta M, Dagnaw T, et al. Clinical outcome and risk factors of neonatal sepsis among neonates in Felege Hiwot referral hospital, Bahir Dar, Amhara Regional State, North West Ethiopia 2016: a retrospective chart review. BMC Res Notes. 2017;10(1):1–7.
Acknowledgements
We gratefully thank parents or guardians and data collectors for their willing to participate in this study.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
MS conceived and designed the study and participated in field data collection, data analysis and prepared the preliminary results. DA participated in data analysis, interpretation and prepared the manuscript. KD participated in the study design and the critical review of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
Ethical approval was obtained from Department of Research and Ethical Review Committee (DRERC) of Medical laboratory Science, College of Health Science, Addis Ababa University with a protocol number of DRERC/493/20/MLS. Permission letter was secured from St. Paul Hospital Millennium Medical College. Informed consent was obtained from all parents or legal guardians of the neonates. Parents or legal guardians were informed about the right to refuse to take part in the study as well as to withdraw at any time during the study period. Blood culture results were communicated to the responsible physician for immediate and appropriate treatment. This study was carried out based on the declaration of Helsinki. All the results were kept confidentially.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sherif, M., Abera, D. & Desta, K. Prevalence and antibiotic resistance pattern of bacteria from sepsis suspected neonates at St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. BMC Pediatr 23, 575 (2023). https://doi.org/10.1186/s12887-023-04399-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04399-y