Abstract
Background
Oropharyngeal administration of colostrum (OAC) has an immune-stimulating effect on oropharyngeal-associated lymphoid tissue, and can promote the maturation of the gastrointestinal tract. However, how OAC promotes intestinal maturation in preterm infants by altering gut microbiota remains unclear. We aim to assess changes in gut microbiota and metabolites after OAC in very preterm infants.
Methods
A multicenter, double-blind, randomized controlled trial will be conducted in three large neonatal intensive care units in Shenzhen, China, with preterm infants with gestational age less than 32 weeks at birth and birth weight less than 1500 g. It is estimated that 320 preterm infants will be enrolled in this study within one year. The intervention group will receive oropharyngeal administration of 0.2 ml colostrum every 3 h, starting between the first 48 to 72 h and continued for 5 consecutive days. Following a similar administration scheme, the control group will receive oropharyngeal administration of sterile water. Stool samples will be collected at the first defecation, as well as on the 7th, 14th, 21st and 28th days after birth for analysis of effect of OAC on gut microbiota and metabolites through 16sRNA gene sequencing and liquid chromatography-mass spectrometry.
Discussion
This proposal advocates for the promotion of OAC as a safe and relatively beneficial protocol in neonatal intensive care units, which may contribute to the establishment of a dominant intestinal flora. Findings of this study may help improve the health outcomes of preterm infants by establishment of targeted gut microbiota in future studies.
Trial registration
NCT05481866 (registered July 30, 2022 on ClinicalTrials.gov).
Similar content being viewed by others
Background
Human milk can improve the health outcomes of preterm infants and reduce the risk of necrotizing enterocolitis (NEC) and late onset sepsis (LOS) in early life [1]. This is because of bioactive components tin beneficial breast milk [2], especially colostrum, which expresses essential biological factors such as fatty acids, hormones, immunoglobulin, lactoferrin and oligosaccharides [3,4,5]. These beneficial biological factors in colostrum also seem to have a unique preventive effect on allergic diseases and chronic diseases later life [6, 7].
Early colostrum feeding can have many benefits for preterm infants. However, some very low birth weight infants often exhibit unstable clinical symptoms such as unstable breathing, poor sucking and poor gastrointestinal function shortly after birth. Therefore, fasting or the slow progress of enteral feeding is a common clinical manifestation of preterm infants [8]. Thus, these preterm infants do not receive all protective biological factors provided by human milk in the early stage of life. In addition, even though some preterm infants can be fed with human milk at an early stage, they need nasal feeding instead of direct oral feeding because of uncoordinated sucking and swallowing function [9]. This prevents establishment of protective biological factors in the oropharynx of very preterm infants within the first few weeks of life, predisposing them to many diseases in early life [10].
Oropharyngeal administration of colostrum (OAC) can be used as a natural alternative administration technique. In OAC, a small amount of colostrum rich in biological factors is injected into the surface of oral mucosa to stimulate an immunostimulatory effect on the infant's oropharyngeal-associated lymphoid tissue [11, 12], including promoting the absorption of secretory immunoglobulin A and lactoferrin [13]. In addition, it can promote gastrointestinal immune function and systemic anti-infection ability, thereby promoting the maturation of the gastrointestinal tract [14, 15].
An optimized human gut microbiota composition is one of the signs of optimal maturation of the gastrointestinal tract and plays a key role in regulating the health and disease risk in preterm infants [16, 17]. With recent advances in high-throughput multiomics technology, including metagenomics and metabonomics, as well as the measurement of host diseases and microbiota, many bacteria and bacterial products causing human diseases (such as inflammatory bowel disease and type 2 diabetes) have been identified [18]. Many intestinal microorganisms and metabolites are involved in the pathogenesis of LOS and NEC in preterm infants [19, 20]. Although LOS pathogens are diverse (including Staphylococci and Enterobacteriaceae and obligate anaerobes), they are typically enriched a few days before the onset of the disease and produce unique metabolites (such as ethanol and formic acid) [21, 22].
Before diagnosis of NEC, notable differences in enrichment of gut microbiota occur. The gut becomes enriched with Proteobacteria dominated by Enterobacteriaceae, whereas Firmicutes, dominated by Staphylococcus, is relatively lacking. As a result, there is excessive growth of pathogenic bacteria under specific conditions and the reduction of biodiversity. Microbiome optimization may provide a new strategy for the prevention of NEC [23, 24]. In addition, studies have explored preclinical diagnostic value of fecal metabolites (such as short chain fatty acids, including acetate and butyrate) for NEC [25, 26].
Although evidence is inadequate and the mechanism is not clear, gut microbiota and metabolites are potentially useful as biomarkers for early diagnosis of NEC and LOS [27]. Therefore, it is beneficial to identify customized mode of intestinal microorganisms and the biological mechanism underlying metabolite changes before NEC and LOS onset [28, 29].
The microbiome-metabolome association is bidirectional [30]. Host and gut microbiota interact through either symbiosis or imbalance of small molecule metabolites. Metabonomics is undoubtedly a promising method to study the interaction between host and gut microbiota [31]. Metabolites of bacteria may also be novel therapeutic targets for human chronic diseases [32]. Several previous studies have investigated the effect of OAC on enteral feeding, NEC, LOS, mortality, etc. [33,34,35,36,37,38,39]. Because of limited sample size and inconsistent OAC measures, among other reasons, there is no sufficient evidence to prove that OAC can reduce the incidence of NEC, LOS and death [40]. But OAC can promote intestinal maturation and rapidly achieve total enteral feeding [41, 42]. However, the mechanism by which OAC promotes intestinal maturation by changing intestinal microbiota and metabolites remains elusive, which is the significant contribution of our study.
We hypothesized that OAC could promote relative enrichment of intestinal Firmicutes but reduce relative enrichment of Proteobacteria. In addition, the production of short chain fatty acids and other metabolites would change accordingly to resemble the gut microbiota profile in normal full-term breast fed infants, improving the health outcomes of preterm infants. The purpose of this multicenter, double-blind, randomized controlled trial is to evaluate whether OAC can modulate gut microbiota diversity and metabolites (such as short chain fatty acids) compared with oral care with sterile water.
Methods
This study is based on the standard protocol items: recommendations for interactive trials (SPIRIT) [43].
Study design
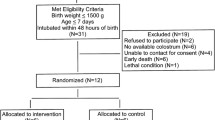
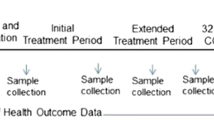
This multicenter, double-blind, randomized controlled trial will be conducted in three large neonatal intensive care units (NICUs) in Shenzhen, Guangdong Province, China, from October 1, 2022 to September 31, 2023. The purpose of this study is to evaluate the effect of OAC on gut microbiota and metabolites. Preterm infants with gestational age < 32 weeks and weight < 1500 g will be evaluated. Infants who meet these inclusion criteria will be randomly divided into two groups in a ratio of 1:1. The intervention group and control group will receive 0.2 ml oropharyngeal colostrum and 0.2 ml sterile water, respectively, every three hours for five days. Stool samples will be collected at the first defecation, as well as on the 7th, 14th, 21st and 28th days to detect the gut microbiota and metabolites. A flow diagram of the research process including the planned study phases is shown in Fig. 1.
Subjects will be recruited from three level III NICUs in Shenzhen, Guangdong Province, China: 1) Shenzhen People's Hospital (about 6000 births in 2021 and a preterm birth rate of about 11%), 2) Longgang Maternal and Child Health Hospital (about 13,000 births in 2021 and a preterm birth rate of about 6.7%), and 3) Bao’an Maternal and Child Health Hospital (about 18,000 births in 2021 and preterm birth rate of about 7%). Shenzhen People’s Hospital designed and initiated the study. It is the coordinating center and the affiliation of the sponsor (ZY). These three NICUs are all located in economically developed areas in China, provide prenatal care, delivery, postnatal care, neonatal treatment and other services.
Inclusion/exclusion criteria
Inclusion criteria: 1. gestational age less than 32 weeks and birth weight less than 1500 g; 2. admission to NICU ≤ 24 h; and 3. be able to start the agreement within 72 h of birth.
Exclusion criteria: 1. birth asphyxia (defined as umbilical artery / first hour arterial PH < 7.0 or cardiopulmonary resuscitation in the delivery room); 2. birth complicated by severe gastrointestinal malformations (such as intestinal atresia, esophago-tracheal fistula, intestinal rotation abnormalities, congenital megacolon); 3. prenatal diagnosis of congenital chromosomal abnormalities or suspected congenital genetic metabolic diseases; and 4. maternal drug abuse or contraindications to breastfeeding (HIV and cytomegalovirus infection).
Collection of milk and oropharyngeal administration procedure
Obstetric nurses will encourage the mother of each newborn to start lactation within 24 h of delivery and educate them on breast massage and electric breast suction every 3 h. Mothers of preterm infants will be encouraged to milk frequently with hygienic hands to ensure a steady supply of breast milk. To ensure the feasibility of oropharyngeal administration of mother’s milk to preterm infants in the treatment group, the mother’s milk will be collected in a pre-labeled sterile milk collection bag. A minimum colostrum volume of 1.6 ml will be collected and stored in the breast milk refrigerator of NICU. After ascertaining that baby's information is consistent with details in the label on the breast milk storage bag, an on-site investigator (nurses preparing syringes) will select the storage bag with the latest lactation time and use sterile gloves and syringes to suck 0.1 ml of colostrum or sterile water for oropharyngeal administration, and cover them with opaque tape and needle cap. Each syringe will be labeled with serial number, hospitalization number, name and date. Then, the bag will be stored at 0 ~ 4℃ for 24 h for subsequent intervention. In this study, all other storage bags will be stored in a freezer at -18 °C until the infant begins enteral feeding.
In the first 48–72 h the treatment group will receive the treatment measures: two 1 mL sterile syringes each aspirated with 0.1 mL of colostrum, placed at room temperature for 5 min. The colostrum will be maintained at room temperature for OAC use. After removing the tip and cap of the first syringe, the colostrum will be slowly injected along the right oral mucosa for at least 20 s. Then, the right buccal mucosa will be wiped with a sterile cotton swab for at least 10 s. The second syringe will be used in the same way on the left oral mucosa. The procedure will be performed every three hours for five days. The control group will be treated with sterile water following similar operation steps. Colostrum for the control group will be frozen in the NICU breast milk refrigerator for enteral feeding. Vital signs will be monitored throughout the process, including heart rate, respiration, body temperature and pulse oxygen saturation. During treatment, newborns will be regularly monitored and observed. If their clinical symptoms are unstable (heart rate is greater than 200 or less than 100, breathing is greater than 80, or the oxygen saturation is maintained above 85% only when the oxygen concentration increases by 10%) [44], the intervention will be interrupted. Once the clinical situation stabilizes, the treatment will be resumed immediately, and the final intervention dose and adverse reactions recorded.
Outcomes
Primary outcome measures: 1a) a between-group difference in gut microbial alpha diversity will be measured using Shannon diversity index at the 7th day and 1b) a between-group difference in the concentration of fecal metabolites (short chain fatty acids) will be quantitatively measured using non-targeted liquid chromatography-mass spectrometry (LC–MS) at the 7th day.
Secondary outcome measures: 2a) between-group differences in other diversity indicators of gut microbiota, including Simpson index (an indicator of species richness and evenness less affected by rare species than Shannon index) and Chao1 index (estimated total number of species) at the 7th day; 2b) between-group differences in alpha diversity of gut microbiota measured using Shannon index, Simpson index and Chao1 index on the 14th, 21st and 28th days of life; 2c) Between-group differences in the concentration of fecal metabolites quantitatively measured by non-targeted LC–MS at the 14th, 21st and 28th days of life; 2d) proportion of gut microbiota at phylum and genus level; 2e) gut microbial beta diversity explaining between-sample dissimilarity calculated using the Vegan package; and 2f) correlation between the comparative dominant flora and metabolites (Table 1).
Subgroup analysis will be performed on preterm infants with gestational age ≤ 28 weeks (Table 1).
Statistical analysis
Intention to treat (ITT) method will be used for statistical analysis. Baseline maternal and infant demographic and clinical characteristics of OAC group and placebo group will be compared using chi-square or Fisher’s exact test (such as infant sex, mode of delivery and presence or absence of chorioamnionitis) and t-test or Wilcoxon test for continuous variables. After removing low-quality sequences, original data will be analyzed following the steps of 16S rRNA discovery, clustering and identification. The number of operational taxonomic units (OTUs) will be calculated for each sample at a 97% sequence similarity level. A specific taxonomic unit will represent a specific species. Subsequently, a sparse curve will be drawn based on the results of OTUs generated using sample sequencing. Wilcoxon test will be used to analyze the diversity and richness of microbiota within individuals. A t-test or Wilcoxon test will be used to compare alpha diversity (Shannon index, Simpson index, and Chao1 index) between the two groups of infants with and without OAC intervention within four weeks of birth. Repeated measures analysis of variance (ANOVA) (for normally distributed data) or Kruskal Wallis test (for non-normal data) will be used to evaluate the microbial alpha diversity at different time points. The proportion difference of gut microbiota at the phylum and genus levels will be calculated using chi-square or Fisher's exact test. Values with p < 0.05 will be considered statistically significant. Beta diversity will be calculated using the Vegan package, and a principal co-ordinates analysis (PCOA) based on Bray–Curtis distances will be plotted. Adonis permutational multivariate analysis of variance of Bray–Curtis distances with 9999 permutations will be used to compare microbial community structure between each of the two groups.
Redundancy analysis (RDA) will be performed using the Vegan package to define the factors that could significantly affect gut microbial composition in each group. We will use Linear discriminant analysis (LDA) of effect size (LEfSe) to determine the most discriminant taxa between the two groups. If the effect of OAC intervention on gut microbiota is less than that of other confounding factors (delivery mode, antibiotic use, feeding type, etc.), we will adjust it with RDA.
Gut microbiota can regulate the signal pathways regulating intestinal mucosal homeostasis through the production of metabolites. Non-targeted LC–MS will be used to evaluate short chain fatty acids and other organic acids and alcohols. The relative and absolute concentrations of metabolites will be calculated using fold change (FC) value, and the difference in metabolite expression between the two groups will be explored. Principal component analysis or partial least squares discriminant analysis will be used to compare different metabolites among the sample types. A time dynamic curve will be used to further analyze metabolites of interest. The correlation between the relative abundance of dominant bacterial taxa based on 16S rRNA gene sequencing and the intensity of metabolites of interest will be determined using sparse partial least squares regression [45].
Details of all methods of analysis are provided in Table 1.
Sample size
The purpose of this study is to detect the alpha diversity of gut microbiota in preterm infants (Shannon index is a better indicator of species richness and evenness) and group differences in short chain fatty acids of interest. At present, no study has reported the effect of OAC on the difference in alpha diversity of gut microbiota and metabolites between preterm infants receiving oropharyngeal administration of colostrum and those receiving sterile water. Therefore, it is difficult to estimate the expected change. However, clinically relevant differences in the Shannon index have been determined based on previous studies on the effect of different feeding types on the Shannon index of preterm infants at the 7th day of age [46,47,48,49]. According to these studies, the mean between-group difference in Shannon index ranged from 0.2 to 0.6 with a standard deviation of 0.2 to 0.8 and a mode of 0.6 [46,47,48,49]. In a study of the effect of OAC on oral microbiota, the mean between-group difference in Shannon index ranged from 0.2 to 0.5 [50]. Combined with these basic data, the mean difference in Shannon index is assumed to be 0.2, with a standard deviation of 0.6. We conducted sample size estimation using PASS 15.0 (NCSS, Kaysville, Utah, United States). To achieve a statistical power of 80% (2-sided type 1 error of 0.05), the calculated sample size of 143 patients for each group (286 in total) with a ratio of 1:1 will be used. In addition, the relevant difference in short chain fatty acids is based on the previous difference of acetic acid, the main metabolite of preterm infants at the 7th day of life with different feeding types. The mean value of the difference between groups of acetic acid is about 0.025 µmol/g, with a standard deviation of 0.02–0.1 for each group [51, 52]. Therefore, a sample of 92 infants in each group (184 in total) will provide 80% power to detect a difference in short chain fatty acids of at least 0.025, assuming a standard deviation of 0.06 and a 2-sided type I error of 0.05. In combination with these two sample sizes, we will use a relatively larger sample (143 cases). Assuming a loss of 10–12% to follow-up, we will need to include 160 infants in each group, resulting in a total sample size of 320. The sample size will be adjusted based on the results of interim analysis.
Participant recruitment and informed consent
Data collection will begin in October 2022 and is expected to end in September 2023. Shenzhen People’s Hospital is the pilot research unit, and the pilot research will be carried out from August to September 2022. The annual census of very preterm infants in this facility is relatively high and the breastfeeding rate is very high (above 80%), hence ideal for the recruitment of subjects. Mothers who meet the inclusion criteria (fetal weight < 1500 g estimated by ultrasound) will be invited to participate. To achieve full registration of participants and target sample size, breast-feeding education will be provided in obstetrics to improve lactation awareness. Professional staff will guide pregnant women and provide them with breast care (such as breast massage). The principal investigator (PI) will explain the study in detail and respond to any questions raised. Mothers will be informed of their right to withdraw from the intervention at any time. They will also be informed that if they decline to participate in the study, their care and that of their infants will not be affected.
Written informed consent will be obtained from the patient's guardian. This study complies with the Declaration of Helsinki.
Randomization
A 1:1 block randomization scheme will be adopted. Each group will be stratified by birth gestational age (< 28 weeks, 28 weeks, and 32 weeks). A random serial number will be randomly generated for each layer using SAS (v9.4 SAS Inst. Inc., Cary, NC) before the start of the study. The block length will be 6. Statisticians will prepare sealed, numbered, opaque envelopes for each site and hand them over to the study coordinator, who will ensure that the envelopes are used in a numerical order. After receiving the notice, the study coordinator will open the envelope, take down the group assignment form, copy the form and send it to the statistician. The manuscript will be left in the case file of the preterm infant. To achieve blinding, the study coordinator preparing the syringe (colostrum or placebo) will be different from the nurse conducting the intervention. The subjects, intervention nurses, the principal investigator and the data verifier will be blinded. During the pilot test, non-fixed codes will be used for blinding to represent each allocation scheme to prevent unblinding by inadvertently losing blindness to all trial participants.
Data collection
Descriptive and clinical data be collected from both groups. Birth status characteristics, such as delivery mode, gestational age, weight and length, Apgar score will be collected in addition to the mothers’ occupation, education level, disease, use of drugs, supplements and antibiotics. During hospitalization, clinical data will be collected on enteral feeding (start time, duration, starting dose, feeding type and total enteral feeding time); parenteral nutrition (start time and duration); antibiotics use in infants (type, start time, duration and dose); NEC, LOS and mortality; and respiratory support.
We hypothesize that OAC would regulate gut microbiota and its metabolites in very preterm infants, resulting in an increase in microbial diversity, with increasing dominance of Lactobacillus/Bifidobacterium predominating and a relative decrease in Proteus species. Stool samples will be collected from selected subjects at the first defecation, as well as on the 7th, 14th, 21st and 28th days and immediately frozen at − 80 °C. Fecal samples refrigerated with dry ice will be transported to the Microbiology Research Institute of Shenzhen People’s hospital. After extracting DNA from frozen fecal samples, 16S rRNA gene will be amplified by polymerase chain reaction (PCR) (v3-v4 hypervariable region) and sequenced using Illumina miseq platform. All data collection procedures will be performed following the local coronavirus disease 2019 (COVID-19) epidemic prevention and control policy.
Missing data
Retention of participants will be ensured during the monitoring period through the support of mothers, lactation encouragement and provision of psychological services. The following events are considered as follow-up losses: the study is not continued due to neonatal death, unstable clinical symptoms and other reasons; the intervention dose does not reach the expected 70%; and parents of preterm infants withdraws from the trial at any time. Eliminated or corrected observations and related reasons will be recorded for future reference.
Most types of missing data in the study will come from random missing. All missing data will be processed with SAS software using the double-robust inverse probability weighting method [53]. Its underlying principle is to estimate the weighted average of the outcome indicators of the complete data sample. Among them, the weight of each individual is the reciprocal of the probability that the outcome index of the individual is not missing, yielding an effect estimation closer to the real value.
Data management
The purpose of data management is to ensure the reliability, integrity and accuracy of data. All study participants will be given a study identification number. Data from each research center will be imported to the collected electronic data capture (EDC) system and protected by password. These data will be verified, corrected, blind audited and locked. The backup is scheduled at midnight every day. Shenzhen People’s Hospital, as the supervision unit, will provide a detailed definition of each variable in the EDC system and ensure consistent data entry. The research team's access to participant information will be strictly limited to the purpose of running the study. In future studies, the storage and use of these data will require additional consent of both parents. For this analysis, the researchers will have access to the finalized test data set.
Quality control
In clinical research, quality control should be carried out in the whole process of trial design, implementation and completion. In the design stage, to improve the quality of test data, a data monitoring committee (DMC) will be established. DMC will composed of the data verifiers (ZY and NW) and the principal investigators at each center (XY, RL and HP). The research team at each center will be composed of the principal investigator (XY, RL and HP), data quality controller (CC, LZ and ZY), syringe preparing nurse and the intervener. In the design phase, calculation of sample size, training of researchers, formulation of data management plan, and reasonable selection of efficacy observation indicators will be considered. The training will cover standardized operation process, including recruitment, randomization and web-based data collection. In the implementation phase, a standardized data collection process will be adopted, and the DMC will conduct regular verification of the filling of case report form (CRF) and research records at each center. Data verification will mainly focus on the following aspects: 1) the timeliness and completeness of data filling in CRFs, and the education on mother lactation to minimize data loss; 2) normalization of data filling; 3) records of adverse events, including the number and duration of adverse events during OAC, and records of colostrum intervention; and 4) monitor the progress of the study and verify the implementation of the study protocol, blind method performance and adverse events. After the study, the data will be collated, verified and statistically analyzed (including missing values, inclusion and exclusion criteria, and adverse events). The quality control process of the protocol is shown in Fig. 2.
Quality control process of the protocol. DMC, data monitoring committee (composed of data verifiers at the coordination center and the principal investigators at each center); CRF, case report form. * The research team of each center is composed of the principal investigator, data quality controller, syringe preparing nurse and the intervener. ** The trial steering committee (composed of the directors of neonatal pediatrics at each center and the heads of the medical safety review team)
The whole research team will hold online meetings every month through multimedia video conferencing software to discuss the implementation of the scheme as needed and quickly solve any problems. When 50% of randomly assigned patients completed five stool samples collection, the primary endpoint was analyzed in the interim. An interim-analysis will be conducted by independent statisticians without knowledge of treatment allocation. Statisticians will report to the DMC. The DMC will conduct non-blind access to all data and discuss the results of the interim analysis, adverse events and compliance with the trial steering committee (composed of the directors of neonatal pediatrics at each center and the heads of the medical safety review team) at the multicenter meeting. Adverse events are defined as aspiration to the lungs, resuscitation due to severe bradycardia or apnea after intervention, and postnatal infection with cytomegalovirus thought to be associated with fresh breast milk. If cases related to these adverse events occur, we will intervene immediately. To ensure the safety of the included infants, vital signs will be carefully monitored during oropharyngeal administration. If the vital signs are unstable, the intervention will be stopped in time. The trial steering committee will decide to continue the trial and report to the ethics committee, which is independent of the sponsor and investigators (Fig. 2).
Ethical and dissemination
Our protocol has been approved by the medical ethics committee of each hospital participating in the recruitment. Ethical approval will be provided before each center starts recruiting. Informed consent, participant education, recruitment materials and subsequent modifications will also be reviewed and approved by the ethics committee. Researchers trained in this method will explain the research objectives, risks and postnatal benefits to the mothers. The guardians of all subjects will be provided with educational materials and will sign the informed consent form. The researcher will submit the safety and progress report to the ethics committee at least every year and three months after the termination or completion of the research, as well as data on the safety and effectiveness review by the data monitoring committee. The results of this study will be published in academic conferences and peer-reviewed open access journals.
Discussion
This multicenter, double-blind RCT study will be performed based on 16sRNA gene sequencing and non-targeted metabolic mass spectrometry. The purpose of the trial is to evaluate the effect of OAC on the gut microbiota and metabolites of preterm infants.
OAC often occurs before or in the early stage of enteral feeding of preterm infants. Previous studies have confirmed that OAC is safe and effective [54]. It increases IgA, IgM, and lactoferrin, to regulate the immune function [55]. The role of human microbiota and metabolic activities in health and disease have been revealed through the application of new technologies. However, the effect of OAC on gut microbiota and metabolites is not clear. Previous studies have suggested that microbiota regulate the development of the immune system and brain in newborns [56]. Therefore, we hypothesized that OAC can increase intestinal bacterial diversity, promote the abundance of Bifidobacteria and other dominant microbiota, as well as the production of short chain fatty acids, thereby reduce vascular damage caused by oxidative stress [57]. It can also protect the intestinal barrier integrity and prevent the occurrence of preterm infant-related diseases by modulating the brain-gut axis.
It should be noted that the gut microbiota are affected by many factors. Previous studies have shown that infants receiving antibiotic treatment have increased relative abundance of Proteobacteria, and decreased the relative abundance of Actinobacteria, Firmicutes, and Bacteroidetes decreases [23]. The relative abundance of Bacteroides decreased after cesarean section and was higher during vaginal delivery [58]. Formula-fed infants showed higher relative abundance of Firmicutes, whereas breast-fed infants showed increased abundance of the phyla Actinobacteria and the genus Bifidobacterium [59]. Therefore, we plan to conduct a multicenter, double-blind RCT study to evaluate the effect of OAC on the gut microbiota and metabolites of preterm infants. The design of the proposed study considers group differences in mode of delivery, antibiotic exposure, feeding type, among other factors to evaluate the effectiveness of OAC comprehensively. The relationship between bacterial species composition and environmental variables is determined by controlling some environmental factors which affect gut microbiota imbalance using statistical methods such as RDA.
Lactational stages are categorized as colostrum (≤ 5 days postpartum), transitional milk (6–15 days postpartum), and mature milk (≥ 16 days postpartum) [3, 60, 61]. In addition, the peak of IgA in human milk has been reported to range between the fourth and fifth days after delivery [62]. Therefore, the duration of OAC is set to 5 days in the proposed study. However, the OAC program is not unified in previous studies. Therefore, whether a longer duration results in optimal intestinal microbiota regulation deserves further discussion. Another limitation is that we intend to use fecal samples for non-targeted LC–MS analysis. As fecal substances are such a complex matrix, the discovery of biomarkers is more susceptible to inter individual variation (metabolites and confounding factors) and differences in analytical methods [63]. Although metabolome analysis of blood, urine, saliva, and other tissues combined with nuclear magnetic resonance (NMR), gas chromatography-mass spectrometry, and other analytical techniques will yield valuable results [31], these techniques are expensive and complex.
The study population will be recruited from three large NICU. This study advocates administration of early colostrum to preterm infants and encourages breast-feeding to improve the health outcomes of preterm infants because early colostrum enriches gut microflora.
Trial status
This is a current, ongoing trial which is actively recruiting study participants. We expect to finish patient recruitment in September 2023 and will present the final results during 2024.
Availability of data and materials
Not applicable.
Abbreviations
- OAC:
-
Oropharyngeal administration of colostrum
- NICU:
-
Neonatal intensive care unit
- LC–MS:
-
Liquid chromatography-mass spectrometry
- NEC:
-
Necrotizing enterocolitis
- LOS:
-
Late onset sepsis
- ITT:
-
Intention to treat
- OTU:
-
Operational taxonomic unit
- ANOVA:
-
Analysis of variance
- PCOA:
-
Principal co-ordinates analysis
- LDA:
-
Linear discriminant analysis
- RDA:
-
Redundancy analysis
- FC:
-
Fold change
- PCA:
-
Principal component analysis
- PLS-DA:
-
Sparse partial least squares
- SPLs:
-
Partial least squares-discriminant analysis
- PI:
-
Principal investigator
- PCR:
-
Polymerase chain reaction
- COVID-19:
-
Coronavirus disease 2019
- EDC:
-
Electronic data capture system
- CRF:
-
Case report form
- DMC:
-
Data monitoring committee
- RCT:
-
Randomized controlled trial
- NMR:
-
Nuclear magnetic resonance
References
Miller J, Tonkin E, Damarell RA, McPhee AJ, Suganuma M, Suganuma H, Middleton PF, Makrides M, Collins CT. A systematic review and meta-analysis of human milk feeding and morbidity in very low birth weight infants. Nutrients. 2018;10(6):707.
Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2020;3(3):Cd007137.
Ballard O, Morrow A. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60(1):49–74.
Li M, Chen J, Shen X, Abdlla R, Liu L, Yue X, Li Q. Metabolomics-based comparative study of breast colostrum and mature breast milk. Food Chem. 2022;384:132491.
Bardanzellu F, Fanos V, Reali A. “Omics” in human colostrum and mature milk: looking to old data with new eyes. Nutrients. 2017;9(8):843.
Fujimori M, França E, Morais T, Fiorin V, de Abreu L, Honório-França A. Cytokine and adipokine are biofactors can act in blood and colostrum of obese mothers. BioFactors. 2017;43(2):243–50.
Reichardt P, Müller D, Posselt U, Vorberg B, Diez U, Schlink U, Reuter W, Borte M. Fatty acids in colostrum from mothers of children at high risk of atopy in relation to clinical and laboratory signs of allergy in the first year of life. Allergy. 2004;59(4):394–400.
Moore TA, Wilson ME. Feeding intolerance: a concept analysis. Adv Neonatal Care. 2011;11(3):149–54.
Brooks C, Vickers AM, Aryal S. Comparison of lipid and calorie loss from donor human milk among 3 methods of simulated gavage feeding: one-hour, 2-hour, and intermittent gravity feedings. Adv Neonatal Care. 2013;13(2):131–8.
Rodriguez NA, Vento M, Claud EC, Wang CE, Caplan MS. Oropharyngeal administration of mother’s colostrum, health outcomes of premature infants: study protocol for a randomized controlled trial. Trials. 2015;16:453.
Gephart SM, Weller M. Colostrum as oral immune therapy to promote neonatal health. Adv Neonatal Care. 2014;14(1):44–51.
Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: theoretical perspectives. J Perinatol. 2009;29(1):1–7.
Maffei D, Brewer M, Codipilly C, Weinberger B, Schanler RJ. Early oral colostrum administration in preterm infants. J Perinatol. 2020;40(2):284–7.
Chen XC, Tong YF, Han ZM, Lin ZL. The Effects of Early Oropharyngeal Administration of Microdosed Colostrum on Feeding Status in Ventilated Extremely Low-Birth-Weight Infants. Breastfeed Med. 2021;16(8):648–53.
Lee J, Kim HS, Jung YH, Choi KY, Shin SH, Kim EK, Choi JH. Oropharyngeal colostrum administration in extremely premature infants: an RCT. Pediatrics. 2015;135(2):e357-366.
Cuna A, Morowitz MJ, Ahmed I, Umar S, Sampath V. Dynamics of the preterm gut microbiome in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021;320(4):G411–9.
Mastromarino P, Capobianco D, Campagna G, Laforgia N, Drimaco P, Dileone A, Baldassarre M. Correlation between lactoferrin and beneficial microbiota in breast milk and infant’s feces. Biometals. 2014;27(5):1077–86.
Metwaly A, Reitmeier S, Haller D. Microbiome risk profiles as biomarkers for inflammatory and metabolic disorders. Nat Rev Gastroenterol Hepatol. 2022;19(6):383–97.
Stewart CJ, Embleton ND, Marrs ECL, Smith DP, Fofanova T, Nelson A, Skeath T, Perry JD, Petrosino JF, Berrington JE, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome. 2017;5(1):75.
Hong L, Zhang L, Zhou Q, Li S, Han J, Jiang S, Han X, Yang Y, Hong S, Cao Y. Impacts of enriched human milk cells on fecal metabolome and gut microbiome of premature infants with stage i necrotizing enterocolitis: a pilot study. Mol Nutr Food Res. 2022;66(1):e2100342.
Shaw AG, Sim K, Randell P, Cox MJ, McClure ZE, Li MS, Donaldson H, Langford PR, Cookson WO, Moffatt MF, et al. Late-Onset Bloodstream Infection and Perturbed Maturation of the Gastrointestinal Microbiota in Premature Infants. PLoS One. 2015;10(7):e0132923.
Graspeuntner S, Waschina S, Künzel S, Twisselmann N, Rausch TK, Cloppenborg-Schmidt K, Zimmermann J, Viemann D, Herting E, Göpel W, et al. Gut dysbiosis with bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin Infect Dis. 2019;69(2):268–77.
Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, Gregory KE, Kroll JS, McMurtry V, Ferris MJ, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31.
Lindberg TP, Caimano MJ, Hagadorn JI, Bennett EM, Maas K, Brownell EA, Matson AP. Preterm infant gut microbial patterns related to the development of necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2020;33(3):349–58.
Probert C, Greenwood R, Mayor A, Hughes D, Aggio R, Jackson RE, Simcox L, Barrow H, García-Finana M, Ewer AK. Faecal volatile organic compounds in preterm babies at risk of necrotising enterocolitis: the DOVE study. Arch Dis Child Fetal Neonatal Ed. 2020;105(5):474–9.
Neu J, Pammi M. Necrotizing enterocolitis: The intestinal microbiome, metabolome and inflammatory mediators. Semin Fetal Neonatal Med. 2018;23(6):400–5.
Berkhout DJC, Niemarkt HJ, de Boer NKH, Benninga MA, de Meij TGJ. The potential of gut microbiota and fecal volatile organic compounds analysis as early diagnostic biomarker for necrotizing enterocolitis and sepsis in preterm infants. Expert Rev Gastroenterol Hepatol. 2018;12(5):457–70.
de Meij TG, van der Schee MP, Berkhout DJ, van de Velde ME, Jansen AE, Kramer BW, van Weissenbruch MM, van Kaam AH, Andriessen P, van Goudoever JB, et al. Early detection of necrotizing enterocolitis by fecal volatile organic compounds analysis. J Pediatr. 2015;167(3):562-567.e1.
Garner CE, Ewer AK, Elasouad K, Power F, Greenwood R, Ratcliffe NM, Costello Bde L, Probert CS. Analysis of faecal volatile organic compounds in preterm infants who develop necrotising enterocolitis: a pilot study. J Pediatr Gastroenterol Nutr. 2009;49(5):559–65.
Nguyen Q, Karagas M, Madan J, Dade E, Palys T, Morrison H, Pathmasiri W, McRitche S, Sumner S, Frost H, et al. Associations between the gut microbiome and metabolome in early life. BMC microbiol. 2021;21(1):238.
Chen MX, Wang SY, Kuo CH, Tsai IL. Metabolome analysis for investigating host-gut microbiota interactions. J Formos Med Assoc. 2019;118(Suppl 1):10–22.
Li D, Lu Y, Yuan S, Cai X, He Y, Chen J, Wu Q, He D, Fang A, Bo Y, et al. Gut microbiota-derived metabolite Trimethylamine-N-oxide (TMAO) and multiple health outcomes: an umbrella review and updated meta-analysis. Am J Clin Nutr. 2022;116(1):230–43.
Aggarwal R, Plakkal N, Bhat V. Does oropharyngeal administration of colostrum reduce morbidity and mortality in very preterm infants? A randomised parallel-group controlled trial. J Paediatr Child Health. 2021;57(9):1467–72.
OuYang X, Yang CY, Xiu WL, Hu YH, Mei SS, Lin Q. Oropharyngeal administration of colostrum for preventing necrotizing enterocolitis and late-onset sepsis in preterm infants with gestational age ≤ 32 weeks: a pilot single-center randomized controlled trial. Int Breastfeed J. 2021;16(1):59.
Abd-Elgawad M, Eldegla H, Khashaba M, Nasef N. Oropharyngeal administration of mother’s milk prior to gavage feeding in preterm infants: a pilot randomized control trial. JPEN J Parenter Enteral Nutr. 2020;44(1):92–104.
Ferreira D, Oliveira A, de Leves D, de Bem É, Fatureto G, Navarro N, Afonso N, Santiago F, Mineo J, Sopelete M, et al. Randomized controlled trial of oropharyngeal colostrum administration in very-low-birth-weight preterm infants. J Pediatr Gastroenterol Nutr. 2019;69(1):126–30.
Sharma D, Kaur A, Farahbakhsh N, Agarwal S. Role of oropharyngeal administration of colostrum in very low birth weight infants for reducing necrotizing enterocolitis: a randomized controlled trial. Am J Perinatol. 2020;37(7):716–21.
Romano-Keeler J, Azcarate-Peril M, Weitkamp J, Slaughter J, McDonald W, Meng S, Latuga M, Wynn J. Oral colostrum priming shortens hospitalization without changing the immunomicrobial milieu. J Perinatol. 2017;37(1):36–41.
Sohn K, Kalanetra K, Mills D, Underwood M. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. 2016;36(2):106–11.
Tao J, Mao J, Yang J, Su Y. Effects of oropharyngeal administration of colostrum on the incidence of necrotizing enterocolitis, late-onset sepsis, and death in preterm infants: a meta-analysis of RCTs. Eur J Clin Nutr. 2020;74(8):1122–31.
Xavier Ramos MS, Martins CDC, Souza ES, Vieira GO, Gomes-Filho IS, Figueiredo A, Pereira MG, Cruz SSD. Oropharyngeal colostrum immunotherapy and nutrition in preterm newborns: meta-analysis. Rev Saude Publica. 2021;55:59.
Silva AP, Machado RCM, Nascimento BF, da Cunha LVS, Padilha PC. Analysis of clinical outcomes of oropharyngeal colostrum administration in very low-birth-weight preterm newborns. Nutrition. 2021;90:111292.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin JA, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Chen LL, Liu J, Mu XH, Zhang XY, Yang CZ, Xiong XY, Wang MQ. Oropharyngeal administration of mother’s own milk influences levels of salivary sIgA in preterm infants fed by gastric tube. Sci Rep. 2022;12(1):2233.
Lê Cao KA, González I, Déjean S. integrOmics: an R package to unravel relationships between two omics datasets. Bioinformatics. 2009;25(21):2855–6.
Chi C, Fan Y, Li C, Li Y, Guo S, Li T, Buys N, Clifton VL, Colditz PB, Yin C, et al. Early Gut Microbiota Colonisation of Premature Infants Fed with Breastmilk or Formula with or without Probiotics: A Cohort Study. Nutrients. 2021;13(11):4068.
Gregory KE, Samuel BS, Houghteling P, Shan G, Ausubel FM, Sadreyev RI, Walker WA. Influence of maternal breast milk ingestion on acquisition of the intestinal microbiome in preterm infants. Microbiome. 2016;4(1):68.
Piñeiro-Ramos JD, Parra-Llorca A, Ten-Doménech I, Gormaz M, Ramón-Beltrán A, Cernada M, Quintás G, Collado MC, Kuligowski J, Vento M. Effect of donor human milk on host-gut microbiota and metabolic interactions in preterm infants. Clin Nutr. 2021;40(3):1296–309.
Li N, Yan F, Wang N, Song Y, Yue Y, Guan J, Li B, Huo G. Distinct Gut Microbiota and Metabolite Profiles Induced by Different Feeding Methods in Healthy Chinese Infants. Front Microbiol. 2020;11:714.
Cortez RV, Fernandes A, Sparvoli LG, Padilha M, Feferbaum R, Neto CM, Taddei CR. Impact of Oropharyngeal Administration of Colostrum in Preterm Newborns’ Oral Microbiome. Nutrients. 2021;13(12):4224.
Chen C, Yin Q, Wu H, Cheng L, Kwon JI, Jin J, Han T, Che H. Different effects of premature infant formula and breast milk on intestinal microecological development in premature infants. Front Microbiol. 2019;10:3020.
Pourcyrous M, Nolan VG, Goodwin A, Davis SL, Buddington RK. Fecal short-chain fatty acids of very-low-birth-weight preterm infants fed expressed breast milk or formula. J Pediatr Gastroenterol Nutr. 2014;59(6):725–31.
Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75(1):45–9.
Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study to determine the safety and feasibility of oropharyngeal administration of own mother’s colostrum to extremely low-birth-weight infants. Adv Neonatal Care. 2010;10(4):206–12.
Moreno-Fernandez J, Sánchez-Martínez B, Serrano-López L, Martín-Álvarez E, Diaz-Castro J, Peña-Caballero M, Martín-Peregrina F, Alonso-Moya M, Maldonado-Lozano J, Ochoa JJ, et al. Enhancement of immune response mediated by oropharyngeal colostrum administration in preterm neonates. Pediatr Allergy Immunol. 2019;30(2):234–41.
Niemarkt HJ, De Meij TG, van Ganzewinkel CJ, de Boer NKH, Andriessen P, Hütten MC, Kramer BW. Necrotizing enterocolitis, gut microbiota, and brain development: role of the brain-gut axis. Neonatology. 2019;115(4):423–31.
Yang J, Hou L, Wang J, Xiao L, Zhang J, Yin N, Yao S, Cheng K, Zhang W, Shi Z, et al. Unfavourable intrauterine environment contributes to abnormal gut microbiome and metabolome in twins. Gut. 2022;71(12):2451–62.
Aguilar-Lopez M, Dinsmoor AM, Ho TTB, Donovan SM. A systematic review of the factors influencing microbial colonization of the preterm infant gut. Gut Microbes. 2021;13(1):1–33.
Aguilar-Lopez M, Wetzel C, MacDonald A, Ho TTB, Donovan SM. Metagenomic profile of the fecal microbiome of preterm infants consuming mother's own milk with bovine milk-based fortifier or infant formula: a cross-sectional study. Am J Clin Nutr. 2022;116(2):435–45.
Christian P, Smith ER, Lee SE, Vargas AJ, Bremer AA, Raiten DJ. The need to study human milk as a biological system. Am J Clin Nutr. 2021;113(5):1063–72.
Khanna D, Yalawar M, Verma G, Gupta S. Century wide changes in macronutrient levels in indian mothers’ milk a systematic review. Nutrients. 2022;14(7):1395.
Araújo ED, Gonçalves AK, Cornetta Mda C, Cunha H, Cardoso ML, Morais SS, Giraldo PC. Evaluation of the secretory immunoglobulin A levels in the colostrum and milk of mothers of term and pre-term newborns. Braz J Infect Dis. 2005;9(5):357–62.
Karu N, Deng L, Slae M, Guo AC, Sajed T, Huynh H, Wine E, Wishart DS. A review on human fecal metabolomics: Methods, applications and the human fecal metabolome database. Anal Chim Acta. 2018;1030:1–24.
Acknowledgements
We would like to thank the Guangdong Provincial Government for launching the “Ascension Project”. This construction fund is managed and used by the hospital through its own budget, focusing on the construction of scientific research platforms and talent teams.
Funding
The study is funded by Ascension Project (1717), Guangdong, China. The funding body does not have any role in the design of the study and collection, analysis, and interpretation of data (which will be collected) and in writing the manuscript.
Contact for public and scientific queries: Zhangbin Yu, Department of Neonatology, Shenzhen People’s Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology), Shenzhen, Guangdong, China, 518,020, Telephone: 13,913,994,139, Email: yuzhangbin@126.com.
Author information
Authors and Affiliations
Contributions
NW and ZY designed the study; NW, HP, XY, and RL contributed to the drafting of the manuscript; CC and LZ critically reviewed the version to be published; ZY, JZ and NW provided the statistical analyses. All authors contributed to the revision of the manuscript and read and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study described in this protocol paper was approved by the Medical Ethics Committee of the three participating hospitals. Written material will also be provided. If they agree to participate, the guardians will sign informed consent forms.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, N., Zhang, J., Yu, Z. et al. Oropharyngeal administration of colostrum targeting gut microbiota and metabolites in very preterm infants: protocol for a multicenter randomized controlled trial. BMC Pediatr 23, 508 (2023). https://doi.org/10.1186/s12887-023-04346-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04346-x