Abstract
Background
Suboptimal vitamin D status is common in people with celiac disease (CeD), a disease that can be characterized by the presence of serum anti-tissue transglutaminase antibodies (TG2A) (i.e., TG2A positivity). To date, it remains unclear whether childhood TG2A positivity is associated with vitamin D status and how this potential association can be explained by other factors than malabsorption only, since vitamin D is mainly derived from exposure to sunlight. The aim of our study was therefore to assess whether childhood TG2A positivity is associated with vitamin D concentrations, and if so, to what extent this association can be explained by sociodemographic and lifestyle factors.
Methods
This cross-sectional study was embedded in the Generation R Study, a population-based prospective cohort. We measured serum anti-tissue transglutaminase antibodies (TG2A) concentrations and serum 25-hydroxyvitamin D (25(OH)D) concentrations of 3994 children (median age of 5.9 years). Children with serum TG2A concentrations ≥ 7 U/mL were considered TG2A positive. To examine associations between TG2A positivity and 25(OH)D concentrations, we performed multivariable linear regression, adjusted for sociodemographic and lifestyle factors.
Results
Vitamin D deficiency (serum 25(OH)D < 50 nmol/L) was found in 17 out of 54 TG2A positive children (31.5%), as compared to 1182 out of 3940 TG2A negative children (30.0%). Furthermore, TG2A positivity was not associated with 25(OH)D concentrations (β -2.20; 95% CI -9.72;5.33 for TG2A positive vs. TG2A negative children), and this did not change after adjustment for confounders (β -1.73, 95% CI -8.31;4.85).
Conclusions
Our findings suggest there is no association between TG2A positivity and suboptimal vitamin D status in the general pediatric population. However, the overall prevalence of vitamin D deficiency in both populations was high, suggesting that screening for vitamin D deficiency among children, regardless of TG2A positivity, would be beneficial to ensure early dietary intervention if needed.
Similar content being viewed by others
Background
Celiac disease (CeD) is a common chronic immune-mediated enteropathy triggered by the ingestion of gluten in genetically-predisposed individuals [1]. The multifactorial etiology of the disease possibly involves a complex interplay between genetics, gluten intake, intestinal microbiota, and both innate and adaptive immune responses [2]. Approximately 1–3% of the general population across various geographic areas are diagnosed with CeD [3]. It is assumed that the actual prevalence is even higher, because the disease often elapses clinically atypical and therefore often remains undiagnosed [4]. CeD incidence has risen in the past decades [5]. This rise cannot be explained by genetic factors only, and therefore other factors such as environmental and lifestyle factors are expected to play an important role [4].
In addition to the wide range of symptoms of CeD, such as abdominal bloating and pain, low serum concentrations of vitamin D are often found at the moment of CeD diagnosis [6]. Vitamin D is known for its key role in bone mineral health and calcium homeostasis, as well as for its immune regulatory effect by modulating production of inflammatory cytokines and the proliferation of proinflammatory cells [6]. On the other hand, chronic inflammation of the small bowel in CeD leads to villous atrophy and, consequently, malabsorption of vitamin D may occur [7]. However, vitamin D is mainly produced in the skin upon sunlight exposure and to a lesser extent obtained from dietary intake [8]. Thus, other factors than malabsorption leading to low vitamin D concentrations should be considered, such as an increased catabolism of vitamin D due to the chronic inflammation, or insufficient sunlight exposure due to chronic illness [9]. However, as current recommendations indicate to limit sun exposure because of its association with skin cancer, dietary intake remains an important source of vitamin D [10].
Despite the unresolved issue of vitamin D deficiency being a risk factor or consequence of pediatric CeD [7, 11], Snyder et al. (2016) recently recommended to screen for vitamin D status at the time a diagnosis of CeD is considered [12]. Notwithstanding this, a concomitant vitamin D deficiency could further affect health in a child with CeD. Current guidelines indicate that CeD diagnosis requires serological testing and histological confirmation from small-bowel biopsies, a very invasive assessment. Fortunately, CeD can also be characterized by the presence of serum anti-tissue transglutaminase antibodies (TG2A) (i.e., TG2A positivity). A recent systematic review and meta-analysis indicated that TG2A has a sensitivity and specificity of over 90% for detection of small intestinal enteropathy [13]. Therefore, in the current study we used TG2A positivity as a reflection of potential CeD.
The aim of our study was to assess whether childhood TG2A positivity is associated with vitamin D concentrations, and if so, to what extent this association can be explained by sociodemographic and lifestyle factors.
Methods
Study design and population
This cross-sectional study was part of the Generation R Study, a multiethnic, population-based prospective cohort study performed in Rotterdam, the Netherlands. Mothers were invited to enroll with an expected delivery between 2002 and 2006, and their children have been followed from fetal life onwards (n = 9749). Detailed and extensive data were collected from the participants, including biological samples such as blood samples. All data collection and measurements were carried out in accordance with applicable guidelines and regulations and approved by the Medical Ethical Committee of Erasmus MC, University Medical Center Rotterdam. Further details on the design of the Generation R Study are described extensively elsewhere [14]. Written informed consent for participation was obtained from the parents of the participating children. The study was approved by the Medical Ethics Committee of the Erasmus Medical Center [15].
At a median age of 5.9 years (IQR; 5.8-6.0), 6690 children visited the research center. Serum samples were collected from 4593 children. Subsequently, children were excluded from our analysis if: (1) insufficient amount of serum was available; (2) their IgA concentrations were below the detection limit (possibly IgA deficient); (3) they were twins (to avoid clustering of similar environmental and genetic risk of celiac disease); (4) they had an established CeD diagnosis; and/or (5) were on a gluten-free diet (Fig. 1). This resulted in a total study population of 3994 children, of whom we measured the serum anti-tissue transglutaminase antibodies (TG2A) concentrations and serum 25-hydroxyvitamin D (25(OH)D) concentrations.
Anti-tissue transglutaminase antibodies (TG2A) positivity
Serum TG2A concentrations were determined using a fluorescence enzyme immunoassay (ELiA Celikey IgA, PhadiaImmunocap 250; EliA IgA, Phadia AB, Uppsala Sweden) at the Department of Immunology of the Erasmus MC [16]. Intra-essay and inter-assay coefficients of variability were < 10% and < 15%, respectively. Children with serum TG2A concentrations of < 7 U/ml were considered TG2A negative and children with TG2A concentrations of ≥ 7 U/ml were considered TG2A positive, as per the manufacturer’s instructions. TG2A positive children were offered further clinical follow-up at the Department of Pediatric Gastroenterology, Erasmus MC, Rotterdam.
To identify the genetic predisposition to celiac disease, an approximation of HLA-DQ2 or HLA-DQ8 carriership was determined using a tag single nucleotide polymorphism approach as described in detail earlier [16, 17]. Children were considered HLA-DQ2/DQ8 positive when at least 1 of the haplotypes was present.
Vitamin D status assessment
Measurements of 25(OH)D concentrations were performed at the Endocrine Laboratory of the VU University Medical Center, Amsterdam. Isotope dilution on-line solid phase extraction liquid chromatography-tandem mass spectrometry (ID-XLC-MS/MS) was used, as described in detail earlier [18]. The limit of quantitation was 4.0 nmol/L, intra-assay and inter-assay coefficients of variation were < 6% and < 8%, respectively. On the basis of recommendations and previous studies in the pediatric population we defined 3 cut-offs for vitamin D status: deficient (< 50 nmol/L), sufficient (50 to < 75 nmol/L) and optimal (≥ 75 nmol/L) [19,20,21,22,23,24,25].
Covariates
We identified potential covariates in the association between CeD and vitamin D status based on both existing literature [7, 9, 26,27,28,29] and previously reported predictors in our own study population [16, 18, 30]. Information on demographics, ethnicity and lifestyle characteristics were assessed using questionnaires, detailed physical and serological examinations, and medical records. Child’s age, sex and birth weight were obtained from obstetric records. From a subgroup, cord blood samples were collected at birth and a genome-wide association analysis (GWA) was done using Illumina Infinium II HumanHap610 Quad Arrays, following manufacturer’s protocols and imputed to the combined HapMap Phase II CEU, CHB/JPT, YRI panel [31]. To assess the risk for vitamin D deficiency, four SNPs involving vitamin D synthesis and metabolism were obtained from the GWA dataset: rs10741657 (near CYP2R1), rs12785878 (near DHCR7), rs2282679 (near GC) and rs6013897 (near CYP24A1) [28]. We determined child’s ethnicity by country of birth of the parents [32,33,34], and classified ethnicity as Western or non-Western, based on the geographical distribution of CeD and according to the criteria we used in a previous study [30]. Data on breastfeeding exclusiveness and timing of gluten introduction were obtained from delivery reports and postnatal questionnaires at 2, 6 and 12 months. Dietary intake was assessed with a semi-quantitative food frequency questionnaire (FFQ) at around the age of 1 year. To assess the quality of the children’s diets, we applied a previously developed diet quality score reflecting adherence to dietary guidelines for young children [35]. Diet quality scores were categorized as high and low diet quality scores, based on the population mean score. Data on the use of vitamin D supplementation at the age of 1 year were obtained from the same FFQ. Information on gastroenteritis was assessed by questionnaires and based on the report of fever combined with diarrhoea during the year before the 6-year visit to the research center. At the age of 6 years, anthropometric data were collected during the children’s visit to the research center. The season of blood draw was based on the date of the visit and categorized into winter/spring and summer/autumn. Information on the amount of playing outside during daytime was obtained from a questionnaire filled out by the parents.
Maternal educational level was obtained at enrollment and categorized into low (≤ secondary education) and high (≥ higher education). Smoking during pregnancy (yes or no) was assessed by prenatal questionnaires. During the 2nd trimester of pregnancy, serum samples were collected and 25(OH)D concentrations were obtained from 3039 mothers.
Statistical analysis
Chi-square tests (for categorical variables), Mann-Whitney U tests (for continuous non-normally distributed variables), and Student’s T tests (for continuous normal distributed variables) were performed to compare the characteristics of TG2A negative (i.e., serum TG2A concentrations of < 7 U/ml) and TG2A positive (i.e., serum TG2A concentrations of ≥ 7 U/ml) children. Fisher’s exact test was obtained in case the expected count was less than five. Associations between TG2A positivity and vitamin D concentrations were examined using linear regression analysis, with serum 25(OH)D concentrations as continuous dependent variable and TG2A positivity (TG2A negative and TG2A positive) as binary independent variable. Covariates with p < 0.10 or with a change in effect estimate ≥ 10% in univariable regression analysis, were considered as potential confounding variables [36]. We performed three final regression models: a crude model, a multivariable model adjusted for season of blood draw and ethnicity (model 1) and a multivariable model adjusted for season of blood draw, ethnicity, serum 25(OH)D concentrations of the mother during pregnancy, child’s birth weight, gender and breastfeeding exclusiveness (model 2). In addition, analyses were performed to test whether the association between TG2A positivity and 25(OH)D concentrations was different for HLA DQ2/DQ8 positive versus HLA DQ2/DQ8 negative children.
Sensitivity analyses were performed to test whether the association between TG2A positivity and 25(OH)D concentrations was different for children with high versus low diet scores (based on the mean diet score). Furthermore, we investigated possible effect modification by the four different vitamin D deficiency risk alleles.
To reduce attrition bias, covariates were imputed (n = 5 imputations), using the Fully Conditional Specification method. Pooled results of the imputed data were presented. Regression coefficients were pooled by taking the average of the coefficients of the five datasets. The pooled results of the regression analysis were presented as beta’s and 95% confidence intervals (CIs). All statistical analyses were performed using the software package IBM SPSS Statistics, version 22.0, for Windows (IBM Corp, Armonk, NY).
Results
Subject characteristics
Out of 3994 children tested, 54 (1.4%) wereTG2A positive (Fig. 1). Of them, 51 children agreed on further clinical follow-up at the Department of Pediatric Gastroenterology. Thirty-one children (60.8%) had CeD, 10 (19.6%) did not have CeD, and 10 (19.6%) were considered potential CeD cases because of inconclusive serologies [37]. TG2A positive children more often were girls (64.8% vs. 48.2%), had on average a lower BMI (15.6 kg/m2 vs. 16.2 kg/m2) (Table 1), and were more often HLA DQ2 and/or HLA DQ8 positive (89.7% vs. 41.1%) (Supplementary Table 1). Of the n = 1105 HLA DQ2 and/or HLA DQ8 positive children, 1070 children (96.8%) were TG2A negative, and 35 children (3.2%) were TG2A positive (data not shown).
TG2A positivity and vitamin D status
Child and maternal characteristics did not differ between TG2A negative and TG2A positive children, except for gender (TG2A positive children more often were female) and BMI at 6 years of age (TG2A positive children had a slightly lower BMI at 6 years of age) (Table 1).
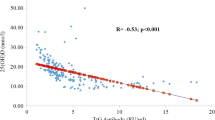
At the age of 6 years, median 25(OH)D serum concentrations in the TG2A negative group was 63.9 (IQR: 45.1–81.0; rage: 4.1 to 211) nmol/L, as compared to 62.0 (IQR: 44.9–82.7; range: 15 to 131) nmol/L in the TG2A positive group (p = 0.8) (Supplementary Table 1).
Vitamin D status did not differ between TG2A negative and TG2A positive children. Of TG2A negative children, 30% had deficient vitamin D concentrations, 36.8% had sufficient vitamin D concentrations and 33.2% had optimal vitamin D concentrations. In the TG2A positive group, 31.5% of the children was vitamin D deficient, 27.8% had sufficient vitamin D concentrations and 40.7% had optimal vitamin D concentrations, respectively. (Supplementary Table 1).
In univariable regression analysis with 25(OH)D concentration as outcome, no association was found with TG2A positivity at 6 years of age (β= -2.20 nmol/L, 95% CI -9.72; 5.33). Results did not change after adjustment for season of blood draw and ethnicity (multivariable model 1: β= -2.20 nmol/L, 95% CI -8.73; 4.33), nor after additional adjustment for maternal 25(OH)D concentrations during pregnancy, birth weight, gender, and breastfeeding exclusiveness (multivariable model 2: β= -1.73 nmol/L, 95% CI -8.31; 4.85) (Table 2). Similar results were found for the associations between TG2A positivity and 25(OH)D concentrations in crude data (Supplementary Table 2). Also no associations were found between TG2A positivity and 25(OH)D concentrations separately adjusted for season of blood draw and ethnicity, maternal 25(OH)D concentrations during pregnancy, birth weight, gender, or breastfeeding exclusiveness in crude and imputed data (Supplementary Tables 3 and 4). Analyses stratified on HLA DQ2/DQ8 haplotype positivity showed similar results to those for the total study population (Table 2 and Supplementary Table 3).
In sensitivity analyses, no associations were found between TG2A positivity and 25(OH)D concentrations stratified on mean diet score (i.e., diet quality scores < 4.17 and ≥ 4.17), although a trend was observed towards lower 25(OH)D concentrations among those with TG2A positivity and lower diet quality scores (Supplementary Table 5a and 5b). Additional adjustments for body mass index (BMI; kg/m2) did not change our results (data not shown). No interactions were found for allele frequencies associated with vitamin D deficiency (Supplementary Table 6).
Discussion
This study does not confirm the hypothesis that TG2A positivity is associated with suboptimal vitamin D status in the general pediatric population. Although we observed no association between vitamin D concentrations and TG2A positivity in children, we did find a high overall prevalence of vitamin D deficiency in both children with and without TG2A positivity.
Our results showed a high overall prevalence of vitamin D deficiency in children, regardless of TG2A positivity. This finding is in accordance with the current reports on vitamin D deficiency as a worldwide phenomenon [11, 18, 38, 39]. However, our results suggested no association between childhood TG2A positivity and vitamin D status. This is in line with the results of Villanueva et al., who found no difference in mean vitamin D concentrations between children with CeD and their controls [7]. This might be explained by our focus on the pediatric population, as previous research by Lerner et al. (2011) studying childhood and adulthood CeD in two countries with relatively high amounts of sunshine indicates a negative correlation between age and vitamin D status, unrelated to CeD and intestinal injury or inflammation [11]. They found that vitamin D concentrations were not different between the individuals with or without CeD, making it unlikely that the intestinal inflammation and malabsorption in CeD affected serum vitamin D concentrations as all participants were on a gluten containing diet [11].
It is important to note that sunlight is the main source of vitamin D (i.e., rather than dietary intake and dietary supplements), as vitamin D is made in the skin from exposure to sunlight [9]. Indeed, sun exposure is one of the main conditions affecting vitamin D status [11]. This might help explain the negative correlation between age and vitamin D status, as children are often more exposed to sunlight because they are unaware of the harmful effects of sun exposure [11].
Although several other studies showed that CeD is frequently associated with vitamin D deficiency [6, 40, 41], the potential role of vitamin D in the pathogenesis of CeD remains unclear. Furthermore, if an association is found, it remains of debate whether CeD is the consequence or the cause in relation to vitamin D deficiency. Recently, Wessels et al. reported that vitamin D status in relation to CeD might depend on more than a gluten free diet and vitamin D supplementation, since two-thirds of their patients remained vitamin D deficient after five-year follow up despite a prescription for vitamin D supplementation [42]. Interestingly, a study by Aronsson et al. (2021) showed a U-shaped relation between vitamin D concentrations and risk of CeD, whereas our results showed no indication for non-linearity [43]. Specifically, they found that besides low vitamin D concentrations also vitamin D concentrations above 75 nmol/L, most likely due to frequent vitamin D supplementation, were associated with an increased risk of developing CeD, which might be explained by upregulation of Th2 cell cytokines associated with immune reaction to external stimuli by high doses of vitamin D [43]. Our results do not support this hypothesis as we found no indication for non-linearity. To date, no clear consensus is reached regarding the optimal vitamin D concentration in childhood [43].
Children with CeD generally have suboptimal bone health, and clinical predictors for low bone mineral density in this population are currently lacking [44, 45]. Vitamin D is mainly known for its essential role in bone mineral health, by maintaining the homeostasis of calcium and phosphate [9, 46, 47]. It is thought that intestinal malabsorption due to chronic inflammation and villous atrophy is an important causative factor for vitamin D deficiency in CeD, and consequently might be one of the main causes of low bone mineral density (BMD) among children with CeD [6, 26, 40, 45, 46, 48]. Although calcitriol is the biologically active form of vitamin D, the total serum concentration of 25(OH)D is usually measured to determine vitamin D status and also considered a better measure, because of its higher concentrations and longer half-life [9, 40, 49]. However, concerning vitamin D status in CeD, it might be of importance to measure serum calcitriol concentrations as well, because low 25(OH)D does not necessarily reflect a deficiency of calcitriol and thus calcium imbalance [49, 50]. Furthermore, the question remains whether a strict association exists between intestinal vitamin D absorption and serum vitamin D concentrations. Vitamin D deficiency has been reported in CeD patients with normal intestinal vitamin D absorption, whereas patients with severely impaired intestinal vitamin D absorption showed normal serum vitamin D concentrations [9]. Schøsler et al. found decreased BMD in children with CeD, whereas not all children were vitamin D deficient [26]. An explanation for these findings might be that calcium malabsorption in CeD (and consequently impaired bone mineral health) may not be the result of vitamin D deficiency, but rather of a reduced level of calcium binding proteins due to intestinal villous atrophy [49]. Our results also do not support the hypothesis that decreased BMD in children with CeD is associated with vitamin D status, as our results showed no association between TG2A positivity and suboptimal vitamin D status.
Methodological considerations
A major strength of our study is the large population-based prospective design. We measured both TG2A and 25(OH)D concentrations in a large group of children and we had information available on a wide range of child and maternal characteristics, allowing us to correct our analyses for multiple potential confounding factors. Another strength of our study is that children were unaware of the TG2A and 25(OH)D status at the time of visit to the research center, which minimises the risk of selection bias. Furthermore, we compared TG2A positive children with TG2A negative children. Several studies on vitamin D deficiency in relation to CeD are conducted without a comparison group [26, 41], which may lead to an overestimation of vitamin D deficiency because of intestinal malabsorption because vitamin D deficiency is already highly prevalent in the general population.
However, some limitations should be taken into account. Firstly, TG2A positivity ideally would have been complemented by additional measures (i.e., characteristic histological changes in the duodenal biopsy) to diagnose CeD. However, we were able to include HLA-DQ2/DQ8 positivity (reflecting genetic predisposition to celiac disease) and the diagnostic accuracy of serum TG2A concentration measurements is high. Therefore, for the aim of our study, TG2A positivity provided a suitable proxy for potential CeD [51]. Unfortunately, our study lacks information on serum parathyroid hormone (PTH) concentrations and thus we cannot say whether low vitamin D concentrations in TG2A positive children are likely to be associated with impaired bone mineral health. Furthermore, vitamin D serum concentration at 6 years of age was measured only once, whereas serum concentrations fluctuate over time. However, largest within-person fluctuations would be expected due to seasonality, which we adjusted for in our analyses. Further, vitamin D serum concentrations are influenced by dietary or supplemental vitamin D intake, of which we had no specific information available. We did however have information on overall dietary quality, which reflects adherence to dietary guidelines and therefore is a proxy for nutrient (and vitamin D) adequacy. Furthermore, a previous study by Voortman et al. (2015) has shown that diet quality in early childhood indeed was associated with vitamin D status at the age of 6 years [18].
Conclusion
We found no association between TG2A positivity and suboptimal vitamin D status in the general pediatric population. We did, however, find a high overall prevalence of vitamin D deficiency in both populations, suggesting that screening for vitamin D deficiency among children, regardless of TG2A positivity, would be beneficial to ensure early dietary intervention if needed.
Data Availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CeD:
-
celiac disease
- CI:
-
confidence interval
- TG2A:
-
anti-tissue transglutaminase antibodies
- 25(OH)D:
-
25-hydroxyvitamin D
- β:
-
beta
- IQR:
-
inter quartile range
- IgA:
-
immunoglobulin A
- HLA:
-
human leukocyte antigen
- GWA:
-
genome-wide association
- FFQ:
-
food frequency questionnaire
- BMI:
-
body mass index
- PTH:
-
parathyroid hormone
References
Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43–52.
Lebwohl B, Ludvigsson JF, Green PH. Celiac disease and non-celiac gluten sensitivity. BMJ. 2015;351:h4347.
Szajewska H, Shamir R, Mearin L, Ribes-Koninckx C, Catassi C, Domellof M, et al. Gluten introduction and the risk of Coeliac Disease: A position paper by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2016;62(3):507–13.
Lionetti E, Gatti S, Pulvirenti A, Catassi C. Celiac disease from a global perspective. Best Pract Res Clin Gastroenterol. 2015;29(3):365–79.
King JA, Jeong J, Underwood FE, Quan J, Panaccione N, Windsor JW et al. Incidence of Celiac Disease Is Increasing Over Time: A Systematic Review and Meta-analysis. Official J Am Coll Gastroenterol | ACG. 2020;115(4).
Ahlawat R, Weinstein T, Pettei MJ. Vitamin D in pediatric gastrointestinal disease. Curr Opin Pediatr. 2017;29(1):122–7.
Villanueva J, Maranda L, Nwosu BU. Is vitamin D deficiency a feature of pediatric celiac disease? J Pediatr Endocrinol Metab. 2012;25(5–6):607–10.
Touvier M, Deschasaux M, Montourcy M, Sutton A, Charnaux N, Kesse-Guyot E, et al. Determinants of vitamin D status in caucasian adults: influence of Sun exposure, Dietary Intake, Sociodemographic, Lifestyle, Anthropometric, and genetic factors. J Invest Dermatology. 2015;135(2):378–88.
Margulies SL, Kurian D, Elliott MS, Han Z. Vitamin D deficiency in patients with intestinal malabsorption syndromes–think in and outside the gut. J Dig Dis. 2015;16(11):617–33.
Reichrath J. Skin cancer prevention and UV-protection: how to avoid vitamin D-deficiency? Br J Dermatol. 2009;161(s3):54–60.
Lerner A, Shapira Y, Agmon-Levin N, Pacht A, Ben-Ami Shor D, Lopez HM, et al. The clinical significance of 25OH-Vitamin D status in celiac disease. Clin Rev Allergy Immunol. 2012;42(3):322–30.
Snyder J, Butzner JD, DeFelice AR, Fasano A, Guandalini S, Liu E et al. Evidence-Informed Expert Recommendations for the Management of Celiac Disease in Children. Pediatrics. 2016;138(3).
Giersiepen K, Lelgemann M, Stuhldreher N, Ronfani L, Husby S, Koletzko S, et al. Accuracy of diagnostic antibody tests for coeliac disease in children: summary of an evidence report. J Pediatr Gastroenterol Nutr. 2012;54(2):229–41.
Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, et al. The Generation R Study: Biobank update 2015. Eur J Epidemiol. 2014;29(12):911–27.
Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, van Franco OH. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31(12):1243–64.
Kiefte-de Jong JC, Jaddoe VW, Uitterlinden AG, Steegers EA, Willemsen SP, Hofman A, et al. Levels of antibodies against tissue transglutaminase during pregnancy are associated with reduced fetal weight and birth weight. Gastroenterology. 2013;144(4):726–35. e2.
Monsuur AJ, de Bakker PI, Zhernakova A, Pinto D, Verduijn W, Romanos J, et al. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLoS ONE. 2008;3(5):e2270.
Voortman T, van den Hooven EH, Heijboer AC, Hofman A, Jaddoe VW, Franco OH. Vitamin D deficiency in school-age children is associated with sociodemographic and lifestyle factors. J Nutr. 2015;145(4):791–8.
Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy european paediatric population. J Pediatr Gastroenterol Nutr. 2013;56(6):692–701.
Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124(3):e362–70.
Carpenter TO, Herreros F, Zhang JH, Ellis BK, Simpson C, Torrealba-Fox E, et al. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr. 2012;95(1):137–46.
Sioen I, Mouratidou T, Kaufman JM, Bammann K, Michels N, Pigeot I, et al. Determinants of vitamin D status in young children: results from the belgian arm of the IDEFICS (identification and Prevention of Dietary- and Lifestyle-Induced Health Effects in Children and Infants) study. Public Health Nutr. 2012;15(6):1093–9.
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–8.
Wagner CL, Greer FR, American Academy of Pediatrics Section on B, American Academy of Pediatrics Committee on N. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52.
Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–91.
Schosler L, Christensen LA, Hvas CL. Symptoms and findings in adult-onset celiac disease in a historical danish patient cohort. Scand J Gastroenterol. 2016;51(3):288–94.
Yang J, Tamura RN, Aronsson CA, Uusitalo UM, Lernmark A, Rewers M, et al. Maternal use of dietary supplements during pregnancy is not associated with coeliac disease in the offspring: the environmental determinants of diabetes in the Young (TEDDY) study. Br J Nutr. 2017;117(3):466–72.
Marild K, Tapia G, Haugen M, Dahl SR, Cohen AS, Lundqvist M, et al. Maternal and neonatal vitamin D status, genotype and childhood celiac disease. PLoS ONE. 2017;12(7):e0179080.
Wierdsma NJ, van Bokhorst-de MA, Berkenpas M, Mulder CJ, van Bodegraven AA. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients. 2013;5(10):3975–92.
Jansen MAE, Beth SA, van den Heuvel D, Kiefte-de Jong JC, Raat H, Jaddoe VWV, et al. Ethnic differences in coeliac disease autoimmunity in childhood: the Generation R Study. Arch Dis Child. 2017;102(6):529–34.
Medina-Gomez C, Felix JF, Estrada K, Peters MJ, Herrera L, Kruithof CJ, et al. Challenges in conducting genome-wide association studies in highly admixed multi-ethnic populations: the Generation R Study. Eur J Epidemiol. 2015;30(4):317–30.
Swertz O, Duimelaar P, Thijssen J. Migrants in the Netherlands 2004, https://www.cbs.nl/nr/rdonlyres/3c60b3e9-09e0-491f-87f2-99b8e54936a1/0/2004a3pub.pdf. Statistics Netherlands. 2016.
Year reportI. 2012, https://www.cbs.nl/nr/rdonlyres/cf430ff1-4623-4d60-b34b-cbe33749006a/0/2012b72pub.pdf. Statistics Netherlands. 2016.
https://www.volksgezondheidenzorg.info/onderwerp/bevolking/regionaal-internationaal/etniciteit. 2016.
Voortman T, Kiefte-de Jong JC, Geelen A, Villamor E, Moll HA, de Jongste JC, et al. The development of a diet quality score for preschool children and its validation and determinants in the Generation R Study. J Nutr. 2015;145(2):306–14.
Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129(1):125–37.
Jansen M, van Zelm M, Groeneweg M, Jaddoe V, Dik W, Schreurs M, et al. The identification of celiac disease in asymptomatic children: the Generation R Study. J Gastroenterol. 2018;53(3):377–86.
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035.
Van Belle TL, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab. 2011;25(4):617–32.
Caruso R, Pallone F, Stasi E, Romeo S, Monteleone G. Appropriate nutrient supplementation in celiac disease. Ann Med. 2013;45(8):522–31.
Erdem T, Ferat C, Nurdan YA, Halime E, Muhammed Selcuk S, Hamza K, et al. Vitamin and mineral deficiency in children newly diagnosed with celiac disease. Turk J Med Sci. 2015;45(4):833–6.
Wessels MM, van Vriezinga VII, Putter SL, Rings H, Mearin EH. Complementary serologic investigations in children with Celiac Disease is unnecessary during Follow-Up. J Pediatr. 2016;169:55–60.
Andrén Aronsson C, Liu X, Norris JM, Uusitalo U, Butterworth MD, Koletzko S, et al. 25(OH)D levels in infancy is Associated with Celiac Disease Autoimmunity in At-Risk children: a case-control study. Front Nutr. 2021;8:720041.
Fedewa MV, Bentley JL, Higgins S, Kindler JM, Esco MR, MacDonald HV. Celiac Disease and Bone Health in Children and Adolescents: a systematic review and Meta-analysis. J Clin Densitometry. 2020;23(2):200–11.
Trovato CM, Albanese CV, Leoni S, Celletti I, Valitutti F, Cavallini C, et al. Lack of clinical predictors for low mineral density in children with celiac disease. J Pediatr Gastroenterol Nutr. 2014;59(6):799–802.
Grace-Farfaglia P. Bones of contention: bone mineral density recovery in celiac disease–a systematic review. Nutrients. 2015;7(5):3347–69.
Palermo NE, Holick MF, Vitamin D. Bone health, and other health benefits in pediatric patients. J Pediatr Rehabil Med. 2014;7(2):179–92.
Zanchi C, Di Leo G, Ronfani L, Martelossi S, Not T, Ventura A. Bone metabolism in celiac disease. J Pediatr. 2008;153(2):262–5.
Zingone F, Ciacci C. The value and significance of 25(OH) and 1,25(OH) vitamin D serum levels in adult coeliac patients: a review of the literature. Dig Liver Dis. 2018;50(8):757–60.
Bianchi ML, Bardella MT. Bone in celiac disease. Osteoporos Int. 2008;19(12):1705–16.
Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136–60.
Acknowledgements
We gratefully acknowledge all participants and professionals involved in the study.
Funding
This phase of the Generation R Study was supported by the Erasmus MC, Erasmus University Rotterdam and by NutsOhra. The sponsors had no role in the design of the study; the data collection and analyses; the interpretation of data; the preparation and review of the manuscript; and the decision to submit the manuscript.
Author information
Authors and Affiliations
Contributions
SAB contributed to the design of the study, the background literature research, data collection, analysis, interpretation of the data, writing of the manuscript, and collated comments from other authors. LAV contributed to the interpretation of the data, writing and revising the manuscript, and collated comments from other authors. TV contributed to the design of the study, and writing of the manuscript. JCK contributed to the design of the study, analysis, interpretation of the data, writing of the manuscript, and supervised the project. MCZ contributed to the design of the study, and writing of the manuscript. HAM obtained funds, designed the study, supervised the project, contributed to data interpretation and writing of the manuscript. All authors have access to all of the data, take responsibility for the integrity of the data, the accuracy of the data analysis and approved the final version of the manuscript. SAB and JCK are guarantors of the study and accept full responsibility for the work and the conduct of the study. All authors confirm that neither this manuscript nor any part of it has been published or is being considered for publication elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent for participation was obtained from the parents of the participating children in this study. The general design, all research aims and the specific measurements in the Generation R Study have been approved by the Medical Ethical Committee of the Erasmus Medical Center, Rotterdam. New measurements will only be embedded in the study after approval of the Medical Ethical Committee. Participants are asked for their written informed consent for the four consecutive phases of the study: prenatally (MEC 198.782/2001/31), birth to 4 years (MEC 217.595 /2002/202, MEC-2007-4), 4–16 years (MEC-2012-165), and from 16 years onwards (not relevant for this study). At the start of each phase, mothers and their partners received written and oral information about the study. Even with consent of the parents, when the child is not willing to participate actively, no measurements are performed. We confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van der Velde, L.A., Beth, S.A., Voortman, T. et al. Anti-tissue transglutaminase antibodies (TG2A) positivity and the risk of vitamin D deficiency among children - a cross-sectional study in the generation R cohort. BMC Pediatr 23, 286 (2023). https://doi.org/10.1186/s12887-023-04041-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04041-x