Abstract
Background
To investigate the differential diagnosis of girls aged 6 to 8 years with idiopathic premature thelarche (IPT) and central precocious puberty (CPP) during the COVID-19 pandemic. We explored predicted adult height (PAH) discrepancy to guide appropriate diagnosis and treatment.
Methods
From January 2020 to December 2021, Chinese girls aged 6 to 8 years with precocious puberty were recruited. They were divided into IPT and CPP groups. Clinical characteristics, including height, weight, body mass index (BMI), basal luteinizing hormone (LH), oestradiol, uterine length and volume, follicle numbers (d > 4 mm) and bone age (BA) were recorded. We analysed differential diagnosis and PAH discrepancy in both groups. Binary logistic regression analysis was used to explore risk factors for CPP, and receiver operating characteristic (ROC) curves were generated to evaluate the diagnostic value of related indexes.
Results
Sixty patients, including 40 girls with IPT and 20 girls with CPP, were recruited. The prevalence of overweight and obesity in the entire cohort was 25% (15/60) and was significantly higher in IPT than CPP, 32.5% (13/40) vs. 10% (2/20), respectively (P=0.045). There were significant differences in LH, uterine volume, follicle numbers and BA (P<0.05). The impaired PAH of IPT and CPP was 0.01 ± 1.19 SD and 0.62 ± 0.94 SD with significant differences (P=0.047). Logistic regression analysis showed that LH and follicle numbers were independent risk factors for CPP. The ROC curve showed that the area under the curve (AUC) of LH and follicle numbers were 0.823 and 0.697. The sensitivity and specificity of LH with a cut off of 0.285 IU/L were 78.9% and 77.8%. The sensitivity and specificity of follicle numbers with a cut off of 3.5 were 89.5% and 52.8%.
Conclusion
The prevalence of overweight and obesity in 6- to 8-year-old girls with precocious puberty was high. Auxological data should not be used in the differential diagnosis of IPT and CPP. Basal LH above 0.285 IU/L and follicle numbers greater than 4 were important features suggestive of CPP. PAH was impaired in individuals with CPP, but it was not impaired in individuals with IPT.
Similar content being viewed by others
Introduction
Precocious puberty is a common endocrinological disease. According to its pathogenesis, precocious puberty is classified into peripheral precocious puberty (PPP), central precocious puberty (CPP) and benign variants, including isolated premature thelarche (IPT) [1, 2]. CPP in girls is commonly defined as the development of secondary sexual characteristics caused by premature activation of the hypothalamic-pituitary-gonadal (HPG) axis before 8 years of age [3,4,5]. IPT is defined as the appearance of isolated breast development before 8 years in girls without other signs of puberty [6, 7]. IPT is a benign disorder; however, in some patients, IPT can be induced by HPG axis activation, leading to CPP [6, 8]. CPP causes psychological disturbances and physical damage, such as short adult stature, early age at menarche, and risks of cardiovascular diseases [3, 9]. From the perspective of cartilage growth plate procedural ageing, early detection of CPP and prompt treatment are more effective in improving final adult height (FAH) [10]. Therefore, it is particularly crucial to differentiate IPT from CPP for prognosis and management.
Pubertal development varies notably at the individual level. A trend towards earlier pubertal onset in girls has been observed [11]. The initiation of puberty depends on genetic alterations and neurosecretory activity and is influenced by nutrition, exercise, and emotional and environmental factors [11, 12]. CPP is caused by early activation of the HPG axis with both FSH and LH pulsatile secretion, which cause ovarian oestrogen secretion and breast development [13]. The pathophysiology of IPT is complex due to partial HPG axis initiation mainly by follicle-stimulating hormone (FSH) secretion, lack of luteinizing hormone (LH) secretion, breast tissue sensitivity, or excessive oestrogens release from multiple origins [14]. LH is considered a powerful marker of gonadal axis activation; however, there was a lack of agreement on basal LH cut-offs for CPP diagnosis in different centres [3]. The gonadotropin-releasing hormone (GnRH) stimulation is the gold standard for CPP diagnosis [15]. In girls with breast development, excessive stimulation tests could be a waste of medical resources and result in psychological burdens on the patients [1]. Therefore, it is important to explore basal LH cut-offs to distinguish between IPT and CPP in our centre [3, 15].
During the novel coronavirus COVID-19 pandemic, the prevalence of precocious puberty in girls has increased across all social classes [14, 16, 17]. Few studies have been performed on the clinical features of girls with precocious puberty during this unique period [1, 16]. This study focused on the clinical characteristics of Chinese girls aged 6 to 8 years with breast development during the COVID-19 pandemic [17]. We investigated growth and development data, LH, oestradiol (E2), pelvic ultrasound, bone age (BA) and predicted adult height (PAH) damage in girls with precocious puberty, providing a basis of differential diagnosis in IPT and CPP.
Materials and methods
Participants
This prospective observational study was performed in the paediatric department of Beijing Jishuitan Hospital during the COVID-19 pandemic from January 2020 to December 2021. The project was approved by the Ethics Committee of Beijing Jishuitan Hospital. Clinical data from 60 Chinese girls aged 6 to 8 years with precocious puberty were collected. Data from their physical records included chronological age (CA) at visit, onset of breast budding, height, weight, body mass index (BMI), breast stage, LH, E2, uterine length, uterine volume, number of follicles with diameter greater than 4 mm (d > 4 mm), and BA. Uterine volume = length*width*thickness*0.5233. PAH was calculated according to BA assessed by a radiologist and a paediatrician using the Greulich and Pyle (G-P) atlas and growth curves of Chinese girls [18]. Mid-parental height (MPH) = (paternal height + maternal height-13)/2. PAH discrepancy = MPH - PAH.
The diagnostic criteria for CPP in girls were as follows [19]: (1) Breast development before 8 years; (2) Linear growth acceleration; (3) Advanced bone age by more than one year; (4) Uterine and ovary enlargement and multiple follicles with diameters greater than 4 mm; and (5) Hypothalamic-pituitary-gonadal axis activation. The exclusion criteria were as follows (6): (1) exogenous oestrogen intake; (2) gonadal tumour, adrenal disease or other organic diseases; and (3) chromosomal abnormalities and genetic diseases.
GnRH stimulation test
The GnRH stimulation test was performed using subcutaneous administration of the GnRH analogue triptorelin (Ferring AG, Saint-Prex, Switzerland). The dosage was 2.5 µg/kg with a maximum dose of 100 µg. Then, blood samples were drawn at the 0′, 30′, 60′, and 90′ time points to examine LH and follicle-stimulating hormone (FSH) concentrations. Serum LH and FSH were measured by immunochemiluminescent assay (ICMA) using a Beckman UniCel DxI800 automatic chemiluminescence analyser. The GnRH stimulation test was performed in patients with breast development and LH > 0.1–0.2 IU/L. CPP was diagnosed when the peak value of LH was greater than 5.0 U/L, and the LH/FSH ratio was greater than 0.6.
Statistical analysis
Statistical analyses were performed using the Statistical Packages for the Social Sciences (SPSS) version 24.0 software (Chicago, USA). To compare the differences between IPT and CPP, independent-samples t tests were used to analyze the significant differences in normally distributed measurement data, which was expressed as M ± SDS. The Mann‒Whitney U test was used for nonnormally distributed data, which are expressed as [M (QR)]. Binary logistic regression using the forward likelihood ratio (LR) method was applied for CPP risk factor analysis. Receiver operating characteristic (ROC) curves were constructed to evaluate the sensitivity and specificity of the risk factors. Youden’s J index (sensitivity + specificity-1) was used to determine the optimal diagnostic cut-off points based on the ROC curves. The prevalence of overweight and obesity in both groups was analysed using a chi-square (χ2) test. Data from these patients were further categorized and analysed according to BMI. The cut-off for statistical significance was accepted as P < 0.05.
Results
The cohort included 40 individuals with IPT (66.7%, 40/60) and 20 individuals with CPP (33.3%, 20/60). Clinical features are shown in Table 1. In the IPT group, 17 patients completed GnRHa stimulation tests, and 12 patients had isolated breast development with LH < 0.1–0.2 IU/L. Eleven patients completed follow-up of more than 3 to 6 months, and symptoms of breast budding were found to have receded. In the CPP group, 13 patients completed GnRHa stimulation tests, and 4 patients were diagnosed with CPP based on elevated LH and oestradiol (E2), uterine enlargement, and multiple follicles. The other 3 cases were followed up for more than 3 to 6 months, and their secondary sexual characteristics developed progressively. The final diagnosis was based on clinical manifestations, GnRHa stimulation tests and follow-up.
The prevalence of overweight and obesity in the entire cohort was 25% (15/60) and was significantly higher in the IPT group than in the CPP group: 32.5% (13/40) vs. 10% (2/20), respectively (χ2 = 4.030, P = 0.045). There were no significant differences in CA at visit, MPH, birth weight, age at maternal menarche, height, weight, BMI, uterine volume or BA advancement (BA-CA) between the groups (P≥0.05). There were significant differences in age at onset, LH, uterine volume, number of follicles (d > 4 mm), BA, PAH and PAH discrepancy between the groups (P < 0.05).
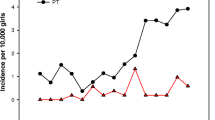
Binary logistic regression analysis showed that basal LH levels (OR = 13.958, CI: 1.917-101.621) and numbers of follicles (d > 4 mm) (OR = 1.486, CI: 1.081–2.041) were two independent risk factors for CPP. The area under the curve (AUC) of LH levels was 0.823 (95% CI: 0.706–0.940, P = 0.000). The AUC for follicle number was 0.697 (95% CI: 0.557–0.836, P = 0.017). The maximum Youden’s J index was found for a cut-off point of 0.285 IU/L at the LH level and follicle number of 3.5. The sensitivity and specificity of basal LH with a cut off of 0.285 IU/L were 78.9% and 77.8%, respectively. The sensitivity and specificity of follicle numbers with a cut off of 3.5 were 89.5% and 52.8%, respectively. These results are shown in Fig. 1.
There were 27 IPT cases and 18 CPP cases with normal BMI. There were no significant differences in CA, MPH, birth weight, age at maternal menarche, age at onset, height, weight, BMI, uterine length, BA advancement, or PAH discrepancy between the two groups (P≥0.05). There were significant differences in LH, uterine volume, follicle numbers, BA, and PAH between the groups (P < 0.05). These results are shown in Table 2.
Among girls with IPT, 27 had a normal BMI and 13 had overweight or obesity. There were no significant differences in CA, MPH, age at maternal menarche, age at onset, height, LH, E2, uterine length and volume, follicle numbers (d > 4 mm), BA, BA advancement, PAH or PAH discrepancy (P ≥ 0.05). There were significant differences in birth weight (P = 0.018) and height Z score minus MPH Z score (P = 0.018). These results are shown in Table 2.
Discussion
We investigated the differential diagnosis of 6- to 8-year-old girls with IPT and CPP and their influence on PAH in the unique period [13]. There were no significant differences in MPH, birth weight or age at maternal menarche between the two groups, which implied similar genetic backgrounds. There were no significant differences in height, weight, or BMI. Therefore, anthropometric parameters should not be used to distinguish CPP from IPT [7]. Our study demonstrated that LH, uterine volume, follicle numbers and BA exhibited important differences between IPT and CPP. Binary regression analysis showed that basic LH levels and follicle numbers were two independent risk factors for CPP. Patients with LH exceeding 0.285 IU/L and more than 4 follicles were more likely to have CPP. Our study proposes that girls with LH above 0.285 IU/L should further undergo the GnRH stimulation test to confirm CPP diagnosis, greatly reducing stimulation numbers and alleviating medical burdens. These results were consistent with those of previous studies, in which basal LH levels above 0.255 IU/L or 0.3 IU/L (ICMA) were considered indicative of puberty [1, 5, 6]. Girls with breast development and LH levels below 0.285 IU/L should be followed up and monitored to rule out CPP.
Recent studies have shown that the increased prevalence of precocious puberty in girls was associated with a high incidence of overweight and obesity [6]. In this study, the prevalence of overweight and obesity in individuals with precocious puberty was high [1, 20]. During the COVID-19 pandemic, increased energy intake, reduced physical activity, excessive sedentary behaviour, and exposure to various stressors have contributed to an increase in childhood overweight and obesity [20, 21]. Childhood adiposity increases risks of cardiovascular disease and endocrine disturbances, especially early pubertal development in girls [22]. Most of the girls with breast development were diagnosed with IPT. It was demonstrated that IPT was more common than CPP in girls aged 6 to 8 years with breast development and overweight and obesity. Our results partially indicated that earlier breast development was associated with higher BMI [12]. The potential mechanisms of correlation between nutritional excess and pubertal advance in girls may include leptin-mediated stimulation, aromatase overactivation or IGF-1 increasement [11, 23].
Different from previous studies, BMI Z scores and height Z scores in individuals with IPT were both higher than those in individuals with CPP, although with no significant differences [14]. The height Z scores were synchronized with BA advancement, so PAH was not affected in IPT patients. Because of accelerated skeletal maturation, PAH in CPP patients was significantly decreased [24]. Nevertheless, in girls with normal BMI and precocious puberty, the height Z scores of CPP patients were higher than those of IPT patients due to accelerated linear growth [2]. However, PAH in the CPP group was also significantly lower than that in the IPT group. Therefore, IPT, with or without overweight/obesity, had no obvious impact on PAH, but girls with CPP had impaired PAH [2]. GnRHa treatment should be tailored to prevent pubertal progression and bone age acceleration to improve final adult height (FAH) in CPP patients [25].
Compared with girls with IPT and a normal BMI, the height Z score minus the MPH Z score was significantly higher in girls with IPT and overweight/obesity, in whom BA advancement was dominant. These results also showed that overweight and obesity can cause growth acceleration and BA advancement [20, 26]. There was no difference in PAH between individuals with normal BMI and those with overweight/obesity. The PAH in girls with IPT and overweight or obesity was not higher than their target height. In addition, their birth weights were significantly higher than those of girls with a normal BMI. It was suggested that fetal weight could affect childhood BMI and that high birth weight was significantly associated with childhood obesity [20, 27, 28]. Management of the fetal weight gain was considered to be beneficial to prevent childhood obesity and early puberty [27].
As a prospective observational study, we need to further expand the sample size to confirm the findings of these study. In addition, PAH based on BA has bias, and follow-up of FAH is necessary [2].
Conclusions
In summary, the prevalence of overweight and obesity in girls with precocious puberty during the COVID-19 pandemic was high. Auxological data should not be used in the differential diagnosis between IPT and CPP in 6- to 8-year-old girls. Basal serum LH levels and follicle numbers are important indexes for the differential diagnosis of CPP. LH above 0.285 IU/L and more than 4 follicles were important features suggestive of CPP. PAH was impaired in CPP patients, but it was not impaired in IPT patients.
Data Availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Abbreviations
- PPP:
-
Peripheral precocious puberty
- CPP:
-
Central precocious puberty
- IPT:
-
Isolated premature thelarche
- HPG:
-
Hypothalamic-pituitary-gonadal
- FAH:
-
Final adult height
- FSH:
-
Follicle-stimulating hormone
- LH:
-
Luteinizing hormone
- GnRH:
-
Gonadotropin-releasing hormone
- E2:
-
Oestradiol
- BA:
-
Bone age
- PAH:
-
Predicted adult height
- CA:
-
Chronological age
- BMI:
-
Body mass index
- MPH:
-
Mid-parental height
- ICMA:
-
Immunochemiluminescent assay
- SPSS:
-
Statistical Packages for the Social Sciences
- LR:
-
Likelihood ratio
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- FAH:
-
Final adult height
References
Cao R, Liu J, Fu P, Zhou Y, Li Z, Liu P. The diagnostic utility of the basal luteinizing hormone level and single 60-minute post GnRH agonist stimulation test for idiopathic central precocious puberty in girls. Front Endocrinol (Lausanne). 2021;12:713880.
Cantas-Orsdemir S, Eugster EA. Update on central precocious puberty: from etiologies to outcomes. Expert Rev Endocrinol Metab. 2019;14(2):123–30.
Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265–74.
Leger J, Carel JC. Central precocious puberty - management and long-term outcomes. Eur Endocrinol. 2015;11(1):45–6.
Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358(22):2366–77.
Cheuiche AV, da Silveira LG, de Paula LCP, Lucena IRS, Silveiro SP. Diagnosis and management of precocious sexual maturation: an updated review. Eur J Pediatr. 2021;180(10):3073–87.
Dura-Trave T, Gallinas-Victoriano F, Malumbres-Chacon M, Ahmed-Mohamed L, Guindulain MJC, Berrade-Zubiri S. Clinical data and basal gonadotropins in the diagnosis of central precocious puberty in girls. Endocr Connect. 2021;10(2):164–70.
Neely EK, Crossen SS. Precocious puberty. Curr Opin Obstet Gynecol. 2014;26(5):332–8.
Luo X, Liang Y, Hou L, Wu W, Ying Y, Ye F. Long-term efficacy and safety of gonadotropin-releasing hormone analog treatment in children with idiopathic central precocious puberty: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2021;94(5):786–96.
Ab Rahim SN, Omar J, Ismail TST. Gonadotropin-releasing hormone stimulation test and diagnostic cutoff in precocious puberty: a mini review. Ann Pediatr Endocrinol Metab. 2020;25(3):152–5.
Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol (Oxf). 2021;95(4):542–55.
Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Brauner EV, Juul A. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatr. 2020;174(4):e195881.
Kaplowitz PB. Update on precocious puberty: who should be treated? Adv Pediatr. 2020;67:93–104.
Khokhar A, Mojica A. Premature thelarche. Pediatr Ann. 2018;47(1):e12–15.
Yeh SN, Ting WH, Huang CY, Huang SK, Lee YC, Chua WK, Lin CH, Cheng BW, Lee YJ. Diagnostic evaluation of central precocious puberty in girls. Pediatr Neonatol. 2021;62(2):187–94.
Chen Y, Chen J, Tang Y, Zhang Q, Wang Y, Li Q, Li X, Weng Z, Huang J, Wang X, et al. Difference of precocious puberty between before and during the COVID-19 pandemic: a cross-sectional study among Shanghai school-aged girls. Front Endocrinol (Lausanne). 2022;13:839895.
Stagi S, De Masi S, Bencini E, Losi S, Paci S, Parpagnoli M, Ricci F, Ciofi D, Azzari C. Increased incidence of precocious and accelerated puberty in females during and after the italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr. 2020;46(1):165.
Greulich WW, Pyle SI. Radiographic Atlas of skeletal development of the Hand and wrist: based on the Brush Foundation Study of Human Growth and Development initiated by T. Wingate Todd, MB, Ch.B., FRCS. Stanford: Stanford University Press; 1950.
Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases. The Society of Pediatrics, Chinese Medical Association, Editorial Board, Chinese Journal of Pediatrics: Consensus statement for the diagnosis and treatment of central precocious puberty (2015). Zhonghua Er Ke Za Zhi. 2015;53(6):412–8.
Gungor NK. Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol. 2014;6(3):129–43.
Weihrauch-Bluher S, Kromeyer-Hauschild K, Graf C, Widhalm K, Korsten-Reck U, Jodicke B, Markert J, Muller MJ, Moss A, Wabitsch M, et al. Current guidelines for obesity prevention in childhood and adolescence. Obes Facts. 2018;11(3):263–76.
Papadimitriou A, Nicolaidou P, Fretzayas A, Chrousos GP. Clinical review: constitutional advancement of growth, a.k.a. early growth acceleration, predicts early puberty and childhood obesity. J Clin Endocrinol Metab. 2010;95(10):4535–41.
Papadimitriou A, Marakaki C, Papadimitriou DT. Growth variations with opposite clinical outcomes and the emerging role of IGF-1. Trends Endocrinol Metab. 2022;33(5):359–70.
Hur JH, Park S, Jung MK, Kang SJ, Kwon A, Chae HW, Kim HS, Kim DH. Insulin resistance and bone age advancement in girls with central precocious puberty. Ann Pediatr Endocrinol Metab. 2017;22(3):176–82.
Vuralli D, Gonc NE, Ozon ZA, Kandemir N, Alikasifoglu A. Which parameters predict the beneficial effect of GnRHa treatment on height in girls with central precocious puberty? Clin Endocrinol (Oxf). 2021;94(5):804–10.
Su H, Su Z, Pan L, Wang L, Xu Z, Peng G, Li X. Factors affecting bone maturation in chinese girls aged 4–8 years with isolated premature thelarche. BMC Pediatr. 2020;20(1):356.
Brown CL, Halvorson EE, Cohen GM, Lazorick S, Skelton JA. Addressing childhood obesity: opportunities for prevention. Pediatr Clin North Am. 2015;62(5):1241–61.
Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97(12):1019–26.
Acknowledgements
We thank all members of the family participating in this study.
Funding
This study was supported by the Beijing Municipal Administration of Hospitals Incubating Program (PZ2018002 and PZ2019004).
Author information
Authors and Affiliations
Contributions
HS and LL conceived and designed the research. YQ analyzed the data. HS and NW wrote the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Beijing Jishuitan Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardians. The study was conducted according to the World Medical Association Declaration of Helsinki.
Consent for publication
Written informed consent to publish the clinical details was obtained from the participants and parents of the patients in the study.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, H., Qian, Y., Wan, N. et al. Differential diagnosis of precocious puberty in girls during the COVID-19 pandemic: a pilot study. BMC Pediatr 23, 185 (2023). https://doi.org/10.1186/s12887-023-04009-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-023-04009-x