Abstract
Background
The early diagnosis and treatment of bacterial meningitis (BM) in young infants was very critical. But, it was difficult to make a definite diagnosis in the early stage due to nonspecific clinical symptoms. Our objectives were to find the risk factors associated with BM and develop a prediction model of BM especially for young infants.
Methods
We retrospectively reviewed the clinical data of young infants with meningitis between January 2011 and December 2020 in Children’s Hospital of Soochow University. The independent risk factors of young infants with BM were screened using univariate and multivariate logistic regression analyses. The independent risk factors were used to construct a new scoring model and compared with Bacterial Meningitis Score (BMS) and Meningitis Score for Emergencies (MSE) models.
Results
Among the 102 young infants included, there were 44 cases of BM and 58 of aseptic meningitis. Group B Streptococcus (22, 50.0%) and Escherichia coli (14, 31.8%) were the main pathogens of BM in the young infants. Multivariate logistic regression analysis identified procalcitonin (PCT), cerebrospinal fluid (CSF) glucose, CSF protein as independent risk factors for young infants with BM. We assigned one point for CSF glucose ≤ 1.86 mmol/L, two points were assigned for PCT ≥ 3.80 ng/ml and CSF protein ≥ 1269 mg/L. Using the not low risk criterion (score ≥ 1) with our new prediction model, we identified the young infantile BM with 100% (95% CI 91.9%-100%) sensitivity and 60.3% (95% CI 46.4%-72.9%) specificity. Compared with BMS and MSE model, our prediction model had larger area under receiver operating characteristic curve and higher specificity, the differences were statistically significant.
Conclusion
Our new scoring model for young infants can facilitate early identification of BM and has a better performance than BMS and MSE models.
Similar content being viewed by others
Background
Bacterial meningitis (BM) is a life-threatening bacterial infection, with the highest incidence reported in young infants [1]. Due to the low resistance of young infants, and the blood–brain barrier has not been fully developed, the bacteria are easy to reach the meninges through the blood–brain barrier to cause infection of the central nervous system. But, diagnostic signs for BM in infants are nonspecific, they do not often exhibit the general symptoms and may only be fevered or look unwell [2, 3]. It is important for young infantile BM to early diagnose and properly manage to reduce the mortality and complication.
Physicians have been trained to administer antibiotics for infants suspected bacterial infection as soon as possible. If antibiotics need to be given before the lumbar puncture (LP) is performed, the antibiotics can sterilize the cerebrospinal fluid (CSF) making it less likely that bacteria will grow [4]. This can cause difficulty in confirming the diagnosis of BM, especially if there are other abnormalities in the CSF such as pleocytosis. Several models have been developed to predict BM [5,6,7,8,9], which can aid physician in their diagnostic approach. However, none of the existing models performed well enough to recommend as routine use in individual patient management which might be attributed to the wide range of applicable ages of the models. Nigrovic et al. identified a classic Bacterial Meningitis Score (BMS) model [5], but this model misclassified a few infants aged ≤ 60 days with BM as being at low risk for the disease [10], also it had low specificity and should not be applied clinical use to young infants [11]. Therefore, our objective was to generate a new scoring model for young infants (29–90 days) suspected BM who performed LP and had CSF pleocytosis. Moreover, we assessed the role of BMS model [5] and Meningitis Score for Emergencies (MSE) model [9] in our study population.
Materials and methods
Patients
We retrospectively reviewed the clinical records of young infants aged from 29 to 90 days with suspicions of meningitis (the ICD code of initial diagnosis was G04.913), and patients in whom a LP was accepted and with CSF pleocytosis (CSF leukocyte count > 10 × 106 /L). In our study, young infants suspected of meningitis and who have completed LP must have the following examinations: peripheral blood cell count, peripheral blood inflammatory markers (C-reactive protein CRP, procalcitonin PCT), CSF cell count, CSF glucose and protein, CSF Gram stain, CSF and peripheral blood culture, cranial magnetic resonance imaging or computerized tomography. It was also suggested to conduct CSF virus detection or CSF metagenomics next generation sequencing if conditions permit. We excluded those patients who were not previously healthy (with the history of severe neurological disease or ventricular drainage or primary immune-deficiencies), underwent a traumatic LP (> 1000 × 106 /L red blood cells in the CSF), diagnosed with a definite viral meningitis (such as enteroviral meningitis, herpes simplex viral meningitis), or treated with antibiotics within 72 h before the diagnostic LP. We also excluded the cases with incomplete clinical data. Then, we selected eligible patients who were finally diagnosed as BM (the ICD code was G00.900) or Aseptic meningitis (AM, the ICD code was G03.001). The eligible patients were from Children’s Hospital of Soochow University between January 2011 and December 2020. The study was approved by the Ethics Committee of Children’s Hospital of Soochow University.
Infantile BM was diagnosed according to either one of the following two criteria: (1) the CSF culture was positive for a bacterial pathogen (Streptococcus pneumonia, group B Streptococcus (GBS), Escherichia coli, Staphylococcus aureus, Neisseria meningitidis, Haemophilus influenza, etc.); or (2) the presence of CSF pleocytosis and with a positive blood culture. Organisms (such as coagulase-negative staphylococci, Propionobacterium acnes, Streptococcus viridans, Corynebacterium spp, and other diphtheroids) cultured in previously healthy patients were considered to be contaminants. AM was defined as CSF pleocytosis with negative bacterial cultures of the CSF and blood. In our study, AM also include the presence of negative viral tests if performed. Complications of acute meningitis included seizure, subdural effusion, hydrocephalus, brain abscess, suspected ventriculitis and cerebral infarcts.
Data collection
We collected information about demographic, clinical, laboratory characteristics, and LP results. Demographic and clinical characteristics included age, gender, occurrence of seizures, anterior fontanel pressure (AFP). The presence of seizures and increased AFP were determined by two treating physicians. The laboratory values were obtained closest before the time to the LP, the data included peripheral white blood cell (WBC) count, peripheral absolute neutrophil count (ANC), CRP, PCT measurements, blood culture. LP results included CSF WBC count and CSF ANC, CSF glucose, CSF protein, CSF Gram stain, CSF culture.
Statistical analyses
Statistical analyses were performed using SPSS 27.0. Our analysis showed that the measurement data in this study were not normally distributed. Therefore, the measurement data were expressed as medians (quartiles), and the count data were expressed as frequencies (percentages). Mann–Whitney test for measurement date and χ2 test for count date were used to compare variables between groups. We conducted a receiver operating characteristic (ROC) curve analysis including significant continuous variables selected by univariate analysis. Continuous variables were converted to dichotomous variables according to the optimal cutoff points used by the Youden index. Risk factor analysis was performed using univariate and multivariate logistic regression analyses. In multivariate logistic regression analysis, the forward stepwise method was used to select independent risk factors for young infants with BM. Hosmer–Lemeshow test was used to identify the fitness of the regression model. The score point of each predictor was determined by the value of logistic coefficient. Area under the ROC curve was calculated to evaluate capacity of the models. The sensitivity and specificity for each scoring models were calculated. Two-tailed analysis with P < 0.05 indicated that the difference was statistically significant.
Results
Main characteristics of patients
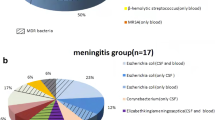
There were 133 infants aged from 29 to 90 days who had CSF pleocytosis and were initially diagnosed with meningitis, 27 cases with incomplete data were excluded. We also excluded 4 infants who were finally diagnosed with enteroviral meningitis. Eventually, the remaining 102 young infants including 44 (43.1%) with BM and 58 (56.9%) with AM were enrolled in our study. In the AM group, 32.8% (19/58) of the cases received Enterovirus testing, 77.6% (45/58) received Herpes simplex virus testing, and none of them had positive results. These children without positive pathogens were finally diagnosed as AM. Cases of BM were caused by the following pathogens: GBS (22, 50.0%), Escherichia coli (14, 31.8%), Streptococcus pneumonia (2, 4.5%), Klebsiella pneumonia (1, 2.3%), Enterococcus species (4, 9.1%), and Staphylococcus aureus (1, 2.3%). In the BM group, there were 6 infants with positive CSF Gram stain, and all of them were eventually cultured pathogenic bacteria. The bacterial pathogen was identified in both CSF and blood culture in 18 patients (40.9%), CSF culture alone in 16 patients (36.4%), and blood culture alone in 10 patients (22.7%). All the complications identified (37.9% of the patients) were seizures in AM group. Infants with BM could be combined with multiple complications rather than a single complication, the incidence of one or more complications was 59.1% in BM group. The main characteristics of the patients with BM and AM are shown in Table 1.
Prediction model for young infants with BM
Meningitis associated clinical characteristics and laboratory parameters were compared by using univariate analysis, significant differences(P < 0.05) were demonstrated in peripheral WBC count, CRP, PCT, CSF WBC count, CSF ANC, CSF glucose, CSF protein and positive Gram stain between the BM and AM (Table 1). Optimum cutoff values for above significant continuous variables were determined by analyzing the ROC curve and Youden index. Then the following dichotomous variables were selected in a forward stepwise multivariable logistic regression analysis: male, history of seizure, increased AFP, peripheral WBC count ≤ 7.43 × 109 /L, CRP ≥ 58.3 mg/L, PCT ≥ 3.80 ng/ml, CSF ANC ≥ 58.5 × 106 /L, CSF glucose ≤ 1.86 mmol/L, CSF protein ≥ 1269 mg/L. PCT ≥ 3.80 ng/ml, CSF glucose ≤ 1.86 mmol/L and CSF protein ≥ 1269 mg/L were independent predictors of young infants with BM (Table 2). Hosmer–Lemeshow test was 0.42, which indicated a lack of deviation between the model and observed event rate, and the prediction model worked well. The area under the ROC curve of this regression model was 0.93 (95% confidence interval (CI) 0.88–0.98).
We developed a new scoring model for young infants with BM based on the logistic coefficient of significant predictors, two points were assigned to PCT ≥ 3.80 ng/ml and CSF protein ≥ 1269 mg/L, one point to CSF glucose ≤ 1.86 mmol/L. The range of the resulting the new BM scoring model was thus 0 to 5 points. Distributions of the young infants in our study with bacterial and aseptic meningitis related to the value of our new scoring model are shown in Fig. 1. Infants with none of the above risk predictors were classified as being at very low risk for BM, whereas with any of the above risk predictors were classified as not being at low risk for BM. Using the not low risk criterion (score ≥ 1) with the new prediction model, we identified the young infantile BM with 100% (95% CI 91.9%-100%) sensitivity and 60.3% (95% CI 46.4%-72.9%) specificity (Table 3).
We also tested BMS model and MSE model in our study, the area under ROC curve was 0.64 (95% CI 0.53–0.75) for BMS model and 0.82 (95% CI 0.74–0.90) for MSE model. Compared with BMS and MSE model, our prediction model had a larger area under ROC curve, and the differences were statistically significant (our model versus BMS, Z = 5.41 P < 0.005; our model versus MSE, Z = 3.72 P < 0.005). We evaluated the performance of the three models in predicting young infants at not low risk of BM in terms of specificity and sensitivity. Thus, we got 100% (95% CI 91.9%-100%) sensitivity and 60.3% (95% CI 46.6%-72.9%) specificity in our new model, 90.9% (95% CI 78.3%-97.4%) and 10.3% (95% CI 3.9%-21.2%) in the BMS model, 100% (95% CI 91.9%-100%) and 19.0% (95% CI 9.9%-31.4%) in the MSE model, respectively. Our prediction model had a higher specificity than the other two models in our study patients (our model versus BMS, χ2 = 547.31 P < 0.005; our model versus MSE, χ2 = 356.41 P < 0.005). The results are shown in Table 4 and Fig. 2.
Finally, we calculated sensitivity and specificity of our new scoring model to identify BM complication. The incidence rate of complication of the BM was 59.1%, which was significantly higher than that of the AM patients (37.9%, χ2 = 4.50, p = 0.034). Using the not low risk criterion (score ≥ 1) with our new prediction model, we identified complication with 77.1% (95% CI 62.7%-88.0%) sensitivity and 38.9% (95% CI 25.9%-53.1%) specificity.
Discussion
GBS accounted for a half of cases of BM in young infants in our study, and Escherichia coli accounted for about one-third. GBS was predominant in young infants aged 1-3 months in other studies, accounting for 50% of cases from Japan [12], 38% of cases from United Kingdom and Ireland [13], and 32% of cases from Canada [14]. GBS prophylaxis strategies would impact only early-onset (0–6 days of age) GBS meningitis, but not prevent late-onset disease (7–89 days of age) [15, 16]. So there is a need for additional strategies such as GBS vaccines for prevention of late-onset GBS meningitis. In a French survey of Escherichia coli meningitis, neonatal cases accounted for 71%, with the 14 days old of the median age at diagnosis [17]. In Japan, there was more non-neonatal Escherichia coli meningitis (60%) with the 1 month old of the median age [12]. Unfortunately, we cannot get more details due to the lack of information on neonatal cases. All in all, GBS and Escherichia coli remain the most common causes of BM in the young infants.
Generally, it is difficult to distinguish between bacterial and aseptic meningitis at the early stage of the disease, especially in young infants. The majority of infants with CSF pleocytosis receive broad-spectrum antibiotics while awaiting the results of culture tests. Due to the high mortality and morbidity rates of BM [3, 4], it is imperative to receive prompt and appropriate antibiotics to young infants. But some infants with CSF pleocytosis were finally diagnosed as AM, leading to abuse of antibiotics [7]. Therefore, we aimed to establish a new scoring model with 100% sensitivity to detect BM of young infants with CSF pleocytosis, and with high specificity to avoid unnecessary prolonged antibiotic use and excessive hospitalization for infants with AM.
Several diagnostic prediction models have been developed to assess the likelihood of BM in patients presented with suspected central nervous system infection. Up to now, the frequently reported predictors of the BM included seizures; higher level of peripheral WBC count, peripheral ANC, CSF ANC, CSF protein, CRP; lower lever of CSF glucose or CSF/blood glucose ratio [5,6,7,8,9]. However, the cut-offs of the predictors of each model are different, which may be attributed to applicable age difference and racial heterogeneity. Compared with adults and children, young infants have less neck resistance and meningeal irritation due to the incomplete anterior fontanelle closure and poor neck muscle development, but intrcranial hypertension is more commonly manifested as increased anterior fontanelle tension. In addition to CRP, among the currently available diagnostic biomarkers, PCT previously identified and validated as the best biomarker for distinguishing early between BM and AM in pediatric patients [18]. Thus, in the present study, we chose variables the frequently reported predictors above together with AFP and PCT to generate a new scoring model for young infants suspected with BM. As we all know, CSF WBC are composed of multiple nuclear cells and mononuclear cells. In bacterial meningitis, the increase of CSF WBC is mainly due to the increase of neutrophils in multiple nuclear cells, so we chose CSF ANC rather than CSF WBC as a risk factor. The CSF Gram stain result was also not considered in our regression variable, because a positive Gram stain result already indicates BM by itself, a negative test result barely alters the prior odds of BM.
Our new scoring model included PCT ≥ 3.80 ng/ml, CSF glucose ≤ 1.86 mmol/L and CSF protein ≥ 1269 mg/L. PCT as a predictor of BM was also proposed by Dubos and Mintegi [8, 9]. However, most models did not consider PCT but CRP and or ANC (CSF and or peripheral blood) as predictors [5, 6, 19, 20]. In fact, PCT has shown a better performance than traditional markers (CRP, CSF ANC, CSF protein, etc.) to identify invasive bacterial infection, specifically for meningitis [18, 21]. Similarly, the replacement of peripheral ANC with PCT significantly increased the specificity of the BMS model in Garcia’s study [22]. In our study, peripheral WBC count was significantly lower in BM than in AM, but it wasn’t an independent predictor of BM. One possible explanation for peripheral leucopenia in BM group was related to the pathogens causing BM in young infants. Compared with the Streptococcus pneumonia and Haemophilus influenza in older infants and children, leukopenia was most common in young infants with Escherichia coli and GBS [23, 24]. Another possible explanation was that in our study peripheral WBC was perhaps obtained earlier than in others’ studies. We reviewed the clinical data and found that 76.5%(78/102)of the infants had peripheral blood routine examination within 24 h of fever. CSF protein concentrations were higher in healthy infants than in older infants and children [25], that was why our CSF protein as a predictor of infantile BM was higher than other models applicable to the wide range of ages of the children [5, 6, 8, 9, 19]. We also found lower levels of CSF glucose was predictive of infantile BM, which was in consistence with Bonus [6].
CSF Gram staining is fast, convenient, and well validated for detecting bacteria. In the BM group, there were 6 infants with the positive Gram-stain, who got the risk score ≥ 4 using our prediction model. Therefore, we recommend that broad spectrum antibiotics should be used to infants with CSF pleocytosis and positive Gram-stain until culture results are available. According to the not low risk criterion (score ≥ 1) of our new model, we identified the young infantile BM with 100% (95% CI 91.9%-100%) sensitivity and 60.3% (95% CI 46.4%-72.9%) specificity. Thus, we also recommend that physicians should give antibiotics to young infants with CSF pleocytosis and risk score ≥ 1.
We also assessed the role of BMS and MSE scoring models in the present study. Their areas under the ROC curve were 0.64 and 0.82, respectively, which were smaller than our new scoring model of 0.93. Most prediction models were developed to accurately identify patients with BM. Because missing BM will have devastating consequences, only 100% sensitivity seems good enough. But, the sensitivity of the BMS model was 90.9%, lower than 100% sensitivity of MSE model and our model. Meanwhile, BMS results were found to be negative for a few children with BM in other studies [26, 27]. That slightly lower sensitivity of BMS model might be partly attributed to the lack of inflammatory indicators such as PCT or CRP as predictors. In addition to emphasizing 100% sensitivity, higher specificity can add value in a clinical setting. On the other hand, the specificity of our model was higher than that of the other two models. The possible reason was that a higher CSF protein level threshold in our model could better discriminate between young infants with BM and those with AM. We also identified complication using the not low risk criterion of our new model, presenting a sensitivity of 77.1% and a specificity of 38.9%, whose performance was less excellent than in identifying the BM in young infants.
Several limitations of our analyses should be considered. First, this was only a retrospective study and was not evaluated in a prospective study. Second, blood glucose before lumbar puncture was not always timely registered, leading to the lack of a more appropriate predictor of CSF/blood glucose ratio. Furthermore, Distribution of main pathogens of BM varies in other regions and would alter the performance of the prediction models. Since our study was realized in a single hospital in an east China population, one may question the generalizability of the findings.
Conclusion
We presented a new scoring model, appearing sufficiently accurate to permit the timely diagnosis of BM in young infants with CSF pleocytosis. This simple prediction model is more appropriate for young infants and it has a better performance than BMS and MSE models.
Availability of data and materials
All analyzed datasets are available from the corresponding author upon request.
Abbreviations
- BM:
-
Bacterial Meningitis
- BMS:
-
Bacterial Meningitis Score
- MSE:
-
Meningitis Score for Emergencies
- LP:
-
Lumbar Puncture
- CSF:
-
Cerebrospinal Fluid
- GBS:
-
Group B Streptococcus
- AM:
-
Aseptic Meningitis
- AFP:
-
Anterior Fontanel Pressure
- WBC:
-
White Blood Cell
- ANC:
-
Absolute Neutrophil Count
- CRP:
-
C-Reactive Protein
- PCT:
-
Procalcitonin
- ROC:
-
Receiver Operating Characteristic
- IQR:
-
Interquartile Range
- CI:
-
Confidence Interval
- T:
-
Temperature
References
Okike IO, Ribeiro S, Ramsay ME, Heath PT, Sharland M, Ladhani SN. Trends in bacterial, mycobacterial, and fungal meningitis in England and Wales 2004–11: an observational study. Lancet Infect Dis. 2014;14(4):301–7.
Davis LE. Acute Bacterial Meningitis. Continuum (Minneap Minn). 2018;24(5):1264–83.
Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. 2015;42(1):29–45.
Ramasamy R, Willis L, Kadambari S, Kelly DF, Heath PT, Nadel S, et al. Management of suspected paediatric meningitis: a multicentre prospective cohort study. Arch Dis Child. 2018;103(12):1114–8.
Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics. 2002;110(4):712–9.
Bonsu BK, Ortega HW, Marcon MJ, Harper MB. A decision rule for predicting bacterial meningitis in children with cerebrospinal fluid pleocytosis when gram stain is negative or unavailable. Acad Emerg Med. 2008;15(5):437–44.
Chavanet P, Schaller C, Levy C, Flores-Cordero J, Arens M, Piroth L, et al. Performance of a predictive rule to distinguish bacterial and viral meningitis. J Infect. 2007;54(4):328–36.
Dubos F, Moulin F, Raymond J, Gendrel D, Bréart G, Chalumeau M. Distinction between bacterial and aseptic meningitis in children: refinement of a clinical decision rule. Arch Pediatr. 2007;14(5):434–8.
Mintegi S, García S, Martín MJ, Durán I, Arana-Arri E, Fernandez CL, et al. Clinical Prediction Rule for Distinguishing Bacterial From Aseptic Meningitis. Pediatrics. 2020;146(3):e20201126.
Nigrovic LE, Malley R, Kuppermann N. Meta-analysis of bacterial meningitis score validation studies. Arch Dis Child. 2012;97(9):799–805.
Rees CA, Cruz AT, Freedman SB, Mahajan P, Uspal NG, Okada P, et al. Application of the Bacterial Meningitis Score for Infants Aged 0 to 60 Days. J Pediatric Infect Dis Soc. 2019;8(6):559–62.
Shinjoh M, Yamaguchi Y, Furuichi M, Yaginuma M, Takahashi T, Iwata S. Recent trends in pediatric bacterial meningitis in Japan, 2016–2018 – S. agalactiae has been the most common pathogen. J Infect Chemother. 2020;26(10):1033–41.
Okike IO, Johnson AP, Henderson KL, Blackburn RM, Muller-Pebody B, Ladhani SN, et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin Infect Dis. 2014;59(10):e150–7.
Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017;140(1):e20170476.
Romain AS, Cohen R, Plainvert C, Joubrel C, Béchet S, Perret A, et al. Clinical and Laboratory Features of Group B Streptococcus Meningitis in Infants and Newborns: Study of 848 Cases in France, 2001–2014. Clin Infect Dis. 2018;66(6):857–64.
Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Estimates of the Burden of Group B Streptococcal Disease Worldwide for Pregnant Women, Stillbirths, and Children. Clin Infect Dis. 2017;65(suppl_2):S200–19.
Basmaci R, Bonacorsi S, Bidet P, Biran V, Aujard Y, Bingen E, et al. Escherichia Coli Meningitis Features in 325 Children From 2001 to 2013 in France. Clin Infect Dis. 2015;61(5):779–86.
Dubos F, Korczowski B, Aygun DA, Martinot A, Prat C, Galetto-Lacour A, et al. Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children: a European multicenter case cohort study. Arch Pediatr Adolesc Med. 2008;162(12):1157–63.
De Cauwer HG, Eykens L, Hellinckx J, Mortelmans LJ. Differential diagnosis between viral and bacterial meningitis in children. Eur J Emerg Med. 2007;14(6):343–7.
Oostenbrink R, Moons KG, Derksen-Lubsen AG, Grobbee DE, Moll HA. A diagnostic decision rule for management of children with meningeal signs. Eur J Epidemiol. 2004;19(2):109–16.
Chaudhary S, Bhatta NK, Lamsal M, Chaudhari RK, Khanal B. Serum procalcitonin in bacterial & non-bacterial meningitis in children. BMC Pediatr. 2018;18(1):342.
Garcia S, Echevarri J, Arana-Arri E, Sota M, Benito J, Mintegi S. Outpatient management of children at low risk for bacterial meningitis. Emerg Med J. 2018;35(6):361–6.
Kotzbauer D, Travers C, Shapiro C, Charbonnet M, Cooley A, Andresen D, et al. Etiology and Laboratory Abnormalities in Bacterial Meningitis in Neonates and Young Infants. Clin Pract. 2017;7(2):943.
Georget-Bouquinet E, Bingen E, Aujard Y, Levy C, Cohen R. Group B streptococcal meningitis’clinical, biological and evolutive features in children]. Arch Pediatr. 2008;15(Suppl 3):S126–32.
Thomson J, Sucharew H, Cruz AT, Nigrovic LE, Freedman SB, Garro AC, et al. Cerebrospinal Fluid Reference Values for Young Infants Undergoing Lumbar Puncture. Pediatrics. 2018;141(3):e20173405.
Dubos F, De la Rocque F, Levy C, Bingen E, Aujard Y, Cohen R, et al. Sensitivity of the bacterial meningitis score in 889 children with bacterial meningitis. J Pediatr. 2008;152(3):378–82.
Tuerlinckx D, El Hayeck J, Van der Linden D, Bodart E, Glupczynski Y. External validation of the bacterial meningitis score in children hospitalized with meningitis. Acta Clin Belg. 2012;67(4):282–5.
Acknowledgements
We thank all the participants who took part in our study, and medical record managers for their supports.
Funding
Suzhou Municipal Health Commission, Grant/Award Number: SZS2020310.
Maternal and children’s health research project of Jiangsu Province, Grant/Award Number: F202108.
Author information
Authors and Affiliations
Contributions
J.T., X.K., and F.C. conceptualized the study concept and design. Y.Y. did the statistical analysis. J.W. and T.S. wrote the main manuscript text. Y.J. and Y.B. interpreted the data. All authors analyzed the data, drafted the manuscript, and had final approval of the submitted and published versions.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Children’s Hospital of Soochow University approved the study. All authors are in agreement with the content of the manuscript. A written informed consent was taken from the parents and caregivers. This procedure was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, J., Shi, T., Yue, Y. et al. Development a prediction model for identifying bacterial meningitis in young infants aged 29–90 days: a retrospective analysis. BMC Pediatr 23, 69 (2023). https://doi.org/10.1186/s12887-022-03813-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03813-1