Abstract
Background
Multisystem inflammatory syndrome in children (MIS-C) is a post-viral inflammatory vasculopathy characterized by persistent fever, multiorgan dysfunction, significant laboratory markers of inflammation, lack of an alternative diagnosis, and prior SARS-CoV-2 infection or exposure in children and adolescents. The most common early symptoms include a prolonged fever, as well as dermatologic, mucocutaneous, and gastrointestinal symptoms such abdominal pain, vomiting, and diarrhea.
Case presentation
We present a pediatric patient with multisystem inflammatory syndrome with the development of abdominal pain and seizure who was found to have a circumferential wall thickening of the terminal ileum and ileocecal junction in abdominal CT scan. The brain MRI of the patient showed cytotoxic lesions of the corpus callosum (CLOCC) which had hypersignal intensity with a few diffusion restrictions in the splenium of the corpus callosum.
Conclusion
This case is being reported to raise awareness of MIS-C presenting characteristics. Given the rising number of MIS-C patients and a lack of understanding regarding early diagnostic clinical characteristics and therapy, further research into clinical presentations, treatment, and outcomes is urgently needed.
Similar content being viewed by others
Background
Multisystem inflammatory syndrome in children (MIS-C) is a severe and potentially lethal complication of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection that presents with various clinical manifestations [1]. Although the pathophysiology of MIS-C is not well understood, various speculations have been made regarding the immunopathogenesis of this syndrome; such hypotheses include SARS-CoV-2 acting as a superantigen and inducing a cytokine storm, as well as molecular mimicry [2]. This syndrome is characterized by fever in conjunction with clinical and laboratory evidence of multisystem inflammation that leads to cardiovascular, pulmonary, nervous, hematological, and gastrointestinal dysfunction in patients with recent SARS-CoV-2 infection [3].
Gastrointestinal symptoms including abdominal pain, vomiting, and diarrhea are common in patients with COVID-19 infection and MIS-C and may mimic appendicitis due to the involvement of the terminal ileum [4,5,6]. Many patients with terminal ileitis related to SARS-CoV-2 infection have been reported, but few studies have described terminal ileitis as the presenting manifestation of MIS-C.
In this article, we describe a child with MIS-C presenting with fever, clinical and radiologic evidence of terminal ileitis, and seizure who was referred to a tertiary hospital in Iran and improved with medical management.
Case presentation
The patient was an 11-year-old child weighing 38 kg who was admitted to Namazi Teaching Hospital affiliated to Shiraz University of Medical Sciences, Southern Iran with the chief complaint of fever and chills lasting from three days before hospitalization. He had also been suffering from watery diarrhea, several episodes of vomiting, intermittent abdominal pain, and two episodes of abnormal spastic movements in his upper and lower extremities, accompanied by a decreased level of consciousness, each lasting about 10 min. He was a known case of mitral valve regurgitation and had been receiving Propranolol for this condition. The patient had close contact with a COVID-19 patient one month prior to the presentation, but he denied having any previous respiratory symptoms. The patient was not vaccinated against COVID-19 due to the lack of vaccines for pediatric patients in Iran at that time.
On arrival, he seemed ill and was conscious with mild respiratory distress. He had a heart rate of 134 beats per minute, a blood pressure of 98/42 mmHg, a respiratory rate of 40 respirations per minute, and an oral temperature of 38 degrees Celsius; however, he was not dehydrated. His arterial oxygen saturation was 95% on room air. In his physical examination meningeal signs were not detected. On abdominal examination, guarding and generalized tenderness with the greatest intensity in the right lower quadrant was detected. Other physical examinations, including evaluation of the nervous system were normal. Urine culture and urine analysis, blood culture, and stool culture were sent from the patient for evaluation of the source of infection, all of which came back negative. Since the patient did not have any meningeal signs, lumbar puncture was not performed for him and cerebrospinal fluid culture is not available. Based on the working diagnosis of systemic sepsis secondary to suspected appendicitis and COVID-19, the patient was isolated and ceftriaxone (100 mg/kg/day), metronidazole (30–40 mg/kg/day divided into three doses), and phenytoin (loading dose, 20 mg/kg and then 5 mg/kg/day) were prescribed intravenously. His initial abdominopelvic ultrasonography revealed circumferential edematous wall thickening in the terminal ileum and the ileocecal valve with multiple varying size hypoechoic lymph nodes in the right parailiac area, the largest of which measured 27 × 16 mm. The COVID-19 evaluation included Polymerase Chain Reaction (PCR) of the nasopharyngeal sample for SARS-CoV-2, COVID-19 serology, and inflammatory markers for MIS-C (Table 1). On the third day of admission, he developed a maculopapular rash all over his body and redness in his oral mucosa and lips. Table 1 shows the patient's laboratory data during hospitalization.
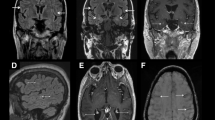
The result of SARS-CoV-2 PCR on the patient’s nasopharyngeal sample came back negative, but the serology for Anti-SARS-CoV-2 IgG was positive, which shows a comparatively remote infection with the virus. Laboratory investigations revealed leukopenia, microcytic anemia, thrombocytopenia, hypoalbuminemia, and elevated levels of liver enzymes, C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), Lactate Dehydrogenase (LDH), and Ferritin. The patient underwent an ultrasound and then a computed tomography (CT) scan of the abdomen, which showed thickening of the terminal ileum and the ileocecal valve and lymphadenopathy in the right iliac region (Fig. 1). The Electroencephalogram (EEG) of the patient showed some paroxysmal generalized epileptic discharges. The brain MRI showed cytotoxic lesions in the corpus callosum with a hypersignal intensity and a few diffusion restrictions in the splenium of the corpus callosum (SCC) in T2 and FLAIR views (Fig. 2). The lung CT scan showed ground-glass opacities (GGO) in the lower parts of both lungs, especially on the right side (Fig. 3). Echocardiography of the patient was normal except for a moderate mitral valve regurgitation.
CLOCC in an 11-year-old boy with MIS-C. Brain MRI shows a lesion in the splenium of the corpus callosum (arrows) which is hypersignal on T2W (a) and FLAIR (b) sequences and was hyposignal on pre-contrast T1W sequence (c). There is no abnormal enhancement after the administration of the contrast agent (d). There is also increased signal intensity on DWI sequences at b 1000 s/mm2 (e) associated with loss of signal on the apparent diffusion coefficient map (f). These findings represent a focus of cytotoxic edema in the splenium of the corpus callosum
Considering the diagnosis of MIS-C, intravenous Methyl Prednisolone (2 mg/kg/day) and Intravenous Immunoglobulin (IVIG) infusion (2 g/kg/day) were started. During the 10 days of hospitalization, fever, diarrhea, and abdominal pain gradually improved, and he was discharged with good general condition. He was prescribed oral Prednisolone (1 mg/kg/day) and Phenytoin (5 mg/kg/day) after discharge from the hospital. Prednisolone was tapered and eventually discontinued after two weeks without any recurrence of symptoms during the follow-up period.
The patient's electroencephalogram was repeated, which was normal; therefore, phenytoin was discontinued since seizures did not recur in the one-month neurologic follow-up. The patient was well in the follow-up period, but no follow-up imaging was done for the patient.
Discussion and conclusion
We presented an 11-year-old boy with MIS-C, who presented with fever and multisystem involvement of the gastrointestinal tract (diarrhea, abdominal pain, terminal ileitis, and elevated liver enzymes), the nervous system (seizure, lesions in SCC), the respiratory system (GGO in the lungs), and the skin (maculopapular rash), with laboratory evidence of inflammation (lymphocytopenia, hypoalbuminemia, and elevated levels of CRP, ESR, Ferritin, and LDH), no other apparent microbial cause of inflammation, and evidence of SARS-CoV-2 infection (positive serology for COVID-19). Both COVID-19 and MIS-C have gastrointestinal manifestations, but terminal ileitis has been reported in a few children with COVID-19 and fewer patients with MIS-C.
Terminal ileitis is the inflammation of the end-portion of the ileum that may present acutely as abdominal pain, mainly in the right lower quadrant with or without diarrhea, chronic bowel obstruction symptoms, and gastrointestinal bleeding that could mimic acute appendicitis [7]. TI usually occurs in association with a wide range of etiologies, including inflammatory bowel diseases (Crohn's disease and, to a lesser frequency, ulcerative colitis), taking nonsteroidal anti-inflammatory drugs, intestinal ischemia, eosinophilic enteritis, neoplasms (lymphoma), vasculitis, spondyloarthropathy, lymphoid hyperplasia, and infectious agents. Bacterial pathogens such as Mycobacterium tuberculosis, Yersinia, Salmonella, and Clostridium difficile have been reported as infectious agents causing TI [8,9,10,11]. Viral pathogens such as Cytomegalovirus can also cause TI [12]. In two case reports, TI was recently reported in two children (11and 12 years old) with SARS-CoV-2 infection who presented with acute abdomen [13, 14].
Only a few cases of TI in children with MIS-C have been reported. In a short report from a single center in the UK, eight children with COVID-19 and symptoms of atypical appendicitis whose imaging studies confirmed TI were reported. Three patients developed Systemic Inflammatory Response Syndrome; two of them were initially planned for appendectomy, but the decisions for the surgical intervention were subsequently overturned due to hemodynamic instability or a positive SARS-CoV-2 PCR. Ultimately, all children improved without any surgical interventions [15]. In a single-center study from the USA, 34 out of 35 (97%) children with MIS-C had gastrointestinal symptoms, and 19 out of 35 had moderate to severe abdominal symptoms that warranted radiographic imaging. In four out of seven patients for whom CT scan of the abdomen were done, terminal ileitis was detected. In two out of fourteen patients for whom abdominal ultrasonography was performed, mild ileal thickening was reported. In one patient with bowel obstruction symptoms who underwent ileocolic resection, there was a 6 cm mass in the ileocolic pedicle and a 3 cm span of granular, thickened terminal ileal mucosa. All other patients improved with medical therapy, including Corticosteroids, Aspirin, Infliximab, and at least one dose of IVIG [3].
In a study by Yack-Corrales et al. 1010 pediatric patients with COVID-19 or MIS-C were evaluated for acute abdomen and appendicitis. Even though 34 patients were diagnosed with appendicitis, no case of terminal ileitis was found in that large cohort; therefore, such diagnoses are extremely rare [4].
In another cohort study conducted on 32 children with MIS-C and left ventricular dysfunction or cardiogenic shock admitted to 15 hospitals in France and Switzerland, two patients underwent emergency laparotomy for suspected appendicitis; however, they were finally diagnosed with mesenteric lymphadenitis [16]. In another short report from South Africa, two of 23 children with MIS-C underwent operation for suspected appendicitis. The etiology of acute abdomen was not explained in the report [17].
Seizure was an indicative symptom of neurologic involvement in this patient. There was hypersignal intensity with a few diffusion restrictions in the splenium of the corpus callosum (SCC) detected in his brain MRI. There is a wide range of etiologies leading to lesions in SCC, including callosal malformations, disorders of myelination (X-linked adrenoleukodystrophy and hereditary Krabbe's disease), tumors, hypoglycemia, ischemia due to hypoxia, epilepsy itself, diffuse axonal injury secondary to trauma, Marchiafava–Bignami disease secondary to chronic alcohol abuse and vitamin B12 deficiency, post-shunt decompression in chronic hydrocephalus, certain antiepileptic drugs, encephalopathy due to Hemolytic Uremic Syndrome, infectious agents (Influenza virus, Rotavirus, Mumps virus, Escherichia coli, Adenovirus, Aspergillus, Mycobacterium tuberculosis), AIDS dementia complex, Mild Encephalitis/Encephalopathy with Reversible isolated SCC lesion (MERS), Posterior Reversible Encephalopathy Syndrome (PRES), and Multiple Sclerosis (MS) [18,19,20]. The inflammation of brain tissue or vessels is the most probable etiology of lesions in SCC in this patient. We postulate that the cytotoxic lesion of the corpus callosum in the index case, was secondary to the systemic inflammation from SARS-CoV-2 infection, resulting in Multisystem Inflammatory Syndrome in Children.
Appleberry et al. reported a case with status epilepticus in the context of MIS-C with post-ictal cerebral edema. The patient received pulse steroids and IVIG which resulted in improvement of his condition; however, long-term neurologic deficits such as dysphagia and developmental regression persisted [21]. Fortunately, the neurologic manifestations of our case were resolved after proper treatment with pulse steroids and IVIG.
The occurrence of seizure secondary to the cytotoxic lesion of the corpus callosum and severe abdominal tenderness secondary to TI as the presenting manifestations in this patient urged us to report him to convey an essential best practice message. Physicians should consider the possibility of cytotoxic lesion of the corpus callosum and TI in MIS-C patients with seizure and severe acute abdomen symptoms, perform timely imaging studies, and start proper medical treatment as soon as possible to avoid unnecessary surgical interventions and complications.
Limitations
It would be better if the patient repeated his brain MRI and abdominopelvic sonography to evaluate the reversibility of such findings, but follow-up was achieved by clinical findings and EEG alone. CSF examination was also not performed for the patient since he did not have any meningeal signs.
Availability of data and materials
Not applicable.
Abbreviations
- MIS-C:
-
Multisystem inflammatory syndrome in children
- CLOCC:
-
Cytotoxic lesions of the corpus callosum
- SCC:
-
Splenium of the corpus calusom
- GGO:
-
Ground glass opacity
- IVIG:
-
Intravenous immunoglobulin
- TI:
-
Terminal ileitis
References
Rowley AH. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453–4.
Buonsenso D, Riitano F, Valentini P. Pediatric Inflammatory Multisystem Syndrome Temporally Related With SARS-CoV-2: Immunological Similarities With Acute Rheumatic Fever and Toxic Shock Syndrome. Front Pediatr. 2020;11(8):574. https://doi.org/10.3389/fped.2020.00574 (PMID:33042918;PMCID:PMC7516715).
Sahn B, Eze OP, Edelman MC, Chougar CE, Thomas RM, Schleien CL, Weinstein T. Features of Intestinal Disease Associated With COVID-Related Multisystem Inflammatory Syndrome in Children. J Pediatr Gastroenterol Nutr. 2021;72(3):384–7. https://doi.org/10.1097/MPG.0000000000002953 (PMID:32969960;PMCID:PMC7901530).
Yock-Corrales A, Lenzi J, Ulloa-Gutiérrez R, Gómez-Vargas J, Antúnez-Montes OY, Rios Aida JA, Del Aguila O, Arteaga-Menchaca E, Campos F, Uribe F, Hernández Díaz R, Buitrago AP, Londoño LMB, Kozicki V, Brizuela M, Buonsenso D. Acute Abdomen and Appendicitis in 1010 Pediatric Patients With COVID-19 or MIS-C: A Multinational Experience from Latin America. Pediatr Infect Dis J. 2021. https://doi.org/10.1097/INF.0000000000003240.
Tullie L, Ford K, Bisharat M, et al. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020;4:e19.
- Mirahmadizadeh A, Borazjani R, Ebrahimi M, Hashemizadeh Haghighi L, Kamali K, et al. COVID-19 Presented with Gastrointestinal Manifestations in an 11-Days-Old Neonate: A Case Report and Review of the Literature, Arch Pediatr Infect Dis. 8(3):e104508. https://doi.org/10.5812/pedinfect.104508.
Goulart RA, Barbalho SM, Gasparini RG, de Carvalho AC. Facing Terminal Ileitis: Going Beyond Crohn’s Disease. Gastroenterology Res. 2016;9(1):1–9. https://doi.org/10.14740/gr698w (Epub 2016 Mar 8. PMID: 27785317; PMCID: PMC5051106).
Kim DH, Cheon JH. Intestinal Behcet’s Disease: A True Inflammatory Bowel Disease or Merely an Intestinal Complication of Systemic Vasculitis? Yonsei Med J. 2016;57(1):22–32. https://doi.org/10.3349/ymj.2016.57.1.22.
Adamek HE, Schantzen W, Rinas U, Goyen M, Ajaj W, Esser C. Ultra-high-field magnetic resonance enterography in the diagnosis of ileitis (Neo-)terminalis: a prospective study. J Clin Gastroenterol. 2012;46(4):311–6. https://doi.org/10.1097/MCG.0b013e31822fec0c.
Bojic D, Markovic S. Terminal ileitis is not always Crohn’s disease. Ann Gastroenterol. 2011;24(4):271–5.
Dilauro S, Crum-Cianflone NF. Ileitis: when it is not Crohn’s disease. Curr Gastroenterol Rep. 2010;12(4):249–58. https://doi.org/10.1007/s11894-010-0112-5.
Wajsman R, Cappell MS, Biempica L, Cho KC. Terminal ileitis associated with cytomegalovirus and the acquired immune deficiency syndrome. Am J Gastroenterol. 1989;84(7):790–3 PMID: 2545093.
- Periyakaruppan M, Kumar S, Kandasamy S, Thiagarajan A, Sangaralingam T, Ganapathy N. COVID abdomen: SARS-CoV-2 infection presenting as 'acute abdomen' in a child. Crit Care Emerg Med. 2020. https://doi.org/10.21203/rs.3.rs-35865/v1.
Aslan N, Yildizdas D, Sinanoglu MS. A Pediatric COVID19 Case with Suspected Acute Abdomen, Hyperferritinemic Sepsis and Developing MIS-C and Pancreatitis. Indian J Pediatr. 2021;88:288. https://doi.org/10.1007/s12098-020-03544-0.
Tullie L, Ford K, Bisharat M, Watson T, Thakkar H, Mullassery D, Giuliani S, Blackburn S, Cross K, De Coppi P, Curry J. Gastrointestinal features in children with COVID-19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020;4(7):e19 (Epub 2020 May 20).
Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation. 2020;142(5):429.
Webb K, Abraham DR, Faleye A, McCulloch M, Rabie H, Scott C, Cape Town MISC-Team. Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. 2020;4(10):e38.
Kazi AZ, Joshi PC, Kelkar AB, Mahajan MS, Ghawate AS. MRI evaluation of pathologies affecting the corpus callosum A pictorial essay. Indian J Radiol Imaging. 2013;23(4):321–32.
akanashi J, Barkovich AJ, Yamaguchi K, Kohno Y. Influenza-associated encephalitis/encephalopathy with a reversible lesion in the splenium of the corpus callosum: A case report and literature review. Am J Neuroradiol. 2004;25:798–802.
Malhotra HS, Garg RK, Vidhate MR, Sharma PK. Boomerang sign: Clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012;15:151–7.
- Appleberry HC, Begezda A, Cheung H, Zaghab-Mathews S, Mainali G. Report of a Child With Febrile Status Epilepticus and Post-COVID Multi-System Inflammatory Syndrome. Child Neurol Open. 2021;8:2329048X211027725. https://doi.org/10.1177/2329048X211027725. PMID: 34285930; PMCID: PMC8261841.
Acknowledgements
The authors would like to thank the patient's parents for giving consent to publish the data to the scientific community, so that we can better diagnose and treat MIS-C.
Funding
This article has not received any financial support.
Author information
Authors and Affiliations
Contributions
The disease was diagnosed by SSH. MD, AD, and AA took care of the patient during the hospitalization and follow-up of the patient. MD, GP, AD, MK, and AA contributed to the draft of the paper and literature research. SSH contributed to the writing and review of the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The informed consent of the child's parents has been obtained for the publication of this report. Institutional approval from The Research Ethics Committee of Shiraz University of Medical Sciences has been obtained to publish. File number:( IR.sums.med.rec.1400.269).
Consent for publication
The patient’s parents have signed a written consent form to publish the case report if the patient remains anonymous to public.
Competing interests
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Davoodi, M., Pouladfar, G., Kadivar, M.R. et al. Terminal ileitis and cytotoxic lesion of corpus callosum as the presenting features of Multisystem inflammatory syndrome in children (MIS-C): a case report. BMC Pediatr 23, 15 (2023). https://doi.org/10.1186/s12887-022-03707-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03707-2