Abstract
Background
Emery-Dreifuss Muscular Dystrophy (EDMD) is an uncommon genetic disease among the group of muscular dystrophies. EDMD is clinically heterogeneous and resembles other muscular dystrophies. Mutation of the lamin A/C (LMNA) gene, which causes EDMD, also causes many other diseases. There is inter and intrafamilial variability in clinical presentations. Precise diagnosis can help in patient surveillance, especially before they present with cardiac problems. Hence, this paper shows how a molecular work-out by next-generation sequencing can help this group of disorders.
Case presentation
A 2-year-10-month-old Javanese boy presented to our clinic with weakness in lower limbs and difficulty climbing stairs. The clinical features of the boy were Gower's sign, waddling gait and high CK level. His father presented with elbow contractures and heels, toe walking and weakness of limbs, pelvic, and peroneus muscles. Exome sequencing on this patient detected a pathogenic variant in the LMNA gene (NM_170707: c.C1357T: NP_733821: p.Arg453Trp) that has been reported to cause Autosomal Dominant Emery-Dreifuss muscular dystrophy. Further examination showed total atrioventricular block and atrial fibrillation in the father.
Conclusion
EDMD is a rare disabling muscular disease that poses a diagnostic challenge. Family history work-up and thorough neuromuscular physical examinations are needed. Early diagnosis is essential to recognize orthopaedic and cardiac complications, improving the clinical management and prognosis of the disease. Exome sequencing could successfully determine pathogenic variants to provide a conclusive diagnosis.
Similar content being viewed by others
Background
Emery-Dreifuss Muscular Dystrophy (EDMD) (MIM 310,300 and 310,200) is a rare genetic muscular disease with an estimated incidence of 1–9 in 1,000,000 worldwide [1]. It resembles the most common muscular dystrophy, i.e., dystrophinopathy (Duchenne and Becker muscular dystrophy/ DMD and BMD). EDMD has three patterns of inheritance: X-linked recessive, autosomal recessive, and autosomal dominant [2]. Characteristic of EDMD is the presence of contracture in the neck, elbow, and heels in the patient or their relatives [3]. Mutation of the Lamin A (LMNA) gene that encodes lamin A/C protein, which causes EDMD, also causes a wide range of other diseases [4]. Thus, the overlapping genotype and phenotype similarities with other muscular dystrophies present diagnostic challenges. A precise diagnosis of EDMD is vital because the disease is associated with life-threatening cardiac conditions. Patients have clinical variabilities in disease progression, life expectancies and prognoses. Hence genetic counselling is essential for affected families once the disease has been diagnosed conclusively.
Case presentation
A 2 year-10-month-old male boy of Javanese descent presented to Universitas Gadjah Mada Academic Hospital with weakness of the lower limbs. The boy had an unremarkable birth history from nonconsanguineous parents (Fig. 1). There was no cognitive impairment, no seizure, visual or auditory impairment, and no bowel or bladder dysfunction. He walked at 12 months. Other developmental milestones were also unremarkable. He was vaccinated for age-appropriate immunizations according to the national immunization program guidelines.
The patient was conscious and afebrile on physical examination with no tachycardia or facial dysmorphic features. There was weakness in the proximal of the upper limbs and distal in the lower limbs. Facial weakness was not detected. No obvious calf pseudohypertrophy and no ankle joint contracture were observed (Fig. 2a). Follow-up at six years old, the patient presented wasting in the upper arm, Achilles contracture, and limitation of neck flexor because of neck contractures (Fig. 2b). Muscle strength examination was 4/5 on the upper and 4/5 on the lower limbs, with no cervical weakness. Swaying movements were present on walking. No toe walking was observed. The sensitivity of all digits was intact. Tendon and cutaneous reflexes were normal. He could walk without support, and the Gower sign was positive. The cardiorespiratory examination was unremarkable. No medical treatment had been given.
Laboratory examinations showed an increased creatine kinase (CK) level of 2485 UI/L, increased LDH level at 1078 U/L, haemoglobin count of 11.6 g/dl, white blood cell count of 9200 cells/μL and normal transaminase level. ECG showed no abnormality of conduction with normal p waves. Echocardiography showed normal structure and function of the heart. Significantly, the patient had no cardiac symptoms at this point.
Investigation revealed that was no family history of members except for the father. History taking revealed the father presented with motoric disturbances and was diagnosed with acute flaccid paralysis at eight years old. He started toe walking and could not raise his hands against gravity since he was eight years of age, and there was no history of fever. At the time of the clinical presentation, he was easily exhausted and had difficulty climbing stairs. No shortness of breath and no swelling of the feet were found. On physical examination, there was no facial weakness. He could not bend his neck downwards nor sidewards. He had profound contractures of the elbow and heels (Fig. 3). Muscle wasting and weakness were found in scapulohumeroperoneal regions, with no contractures of fingers. He had decreased physiological reflex and no pathological reflex. Lordosis and scoliosis were detected. He has swaying and tiptoeing in his walk. He could ride a motorcycle by himself and was capable of performing his normal occupation. The father had increased CK level at 518 UI/L, but other blood results were within normal limits. Based on the clinical features, we suspected this was a case of EDMD with a differential diagnosis of limb-girdle muscular dystrophy. No NCS-EMG and MRI findings were available of the patient and the father as they did not consent to these procedures.
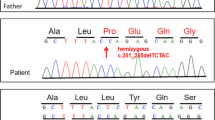
Exome sequencing was carried out on Illumina Hiseq 4000 platform (Illumina, San Diego, CA) at a mean read depth of 100x. Genomic DNA libraries were prepared by Agilent SureSelect Human All Exon V5 Kit (Agilent Technologies, Santa Clara, CA) following the manufacturer's protocol and sequenced through our laboratory at the National University of Singapore [4]; heterozygous variant involving a C to T transition in exon 7 of the LMNA gene (NM_170707: c.C1357T) was found (Fig. 4a). This variant leads to a missense mutation, Arg453Trp (R453W), that was previously reported to cause Autosomal Dominant EDMD. This variant was validated by Sanger sequencing in the patient. Targeted screening of this variant showed presence in the father, confirming this as a familial mutation (Fig. 4b). The variant was also classified as pathogenic based on the ACMG curation guidelines.
Knowing the EDMD phenotypes, we further examine the cardiac function of the father. Electrocardiography showed bradycardia with a heart rate of 40–50 bpm and total atrioventricular block with atrial fibrillation (Fig. 5). Echocardiography showed hypokinetic and dilatation of heart muscles, also thrombus suspicion in the left ventricle. Sinus node dysfunction was suspected; thus, atrioventricular block medication was administered. The cardiologist planned for urgent permanent pacemaker placement and further management of cardiomyopathy.
Discussion and conclusion
Although initially grouped together with the other X-linked muscular dystrophies, most notably the dystrophinopathies forms, EDMD has become accepted as a separate and distinctive type after a thorough clinical evaluation of patients. A triad of presentations characterizes the disease course. The first two characteristic features are the weakness of the proximal muscle in childhood which initially affects the lower extremities, along with elbow flexion contractures and shortening of the Achilles tendon, consequently generating toe walking. Adults with EDMD manifest a waddling gait, lordotic stance and absence of the deep tendon reflexes. The third characteristic consists of arrhythmias, ranging from junctional rhythm (atrial standstill) to atrial fibrillation and even sudden cardiac death [5].
The serum CK level among the affected family members is also increased, although not in the similar range as in DMD or BMD. Other critical differences compared to DMD/BMD are the absence of developmental delay or the calf pseudohypertrophy. In 1966, this disease entity was differentiated and is currently known as X-linked EDMD [6].
EDMD creates a diagnostic challenge due to its similarities in the clinical presentation and laboratory findings with other muscular dystrophies. However, early diagnosis is crucial to prevent early mortality and morbidity from cardiac complications and muscular contractures. It is also essential to provide genetic screening for family members of the patients to determine risk.
In neuromuscular disorder, a nerve conduction study with electromyography is essential to determine which structure is involved. As contracture or spasticity with weakness can be part of an upper motor neuron involvement, an MRI to rule out a central affection or an NCS-EMG with a myopathic pattern to support EDMD may be necessary to support their diagnosis [7]. Moreover, because the father had acute flaccid paralysis since he was eight years old, it is suggested that Charcot Marie Tooth (CMT) should be ruled out by NCS-EMG as some of CMT's variants were thought to be due to LMNA gene mutation. However, as we did not have data on NCS-EMG and MRI of the father of the case index, molecular analysis was performed to resolve the diagnosis for this case.
EDMD has three main genetic patterns: X-linked recessive, autosomal dominant, and autosomal recessive, with the X-linked EDMD arising from emerin gene chromosome mutation (Xq28) being the most common. Autosomal recessive inheritance is extremely rare [8]. The emerin protein is located in the inner nuclear membrane of body cells, predominantly in skeletal and cardiac muscles. Mutation in the emerin gene causes premature termination in mRNA translation, disrupting protein synthesis and eventually nuclear functioning [9]. The autosomal dominant and recessive patterns of EDMD are known to be caused by mutations of the Lamin A/C genes (LMNA) gene on 1q21.2-q21.3. This mutation contributes to the disorder of cardiac and skeletal muscles. Lamin A/C proteins configure the inner nuclear membrane, which plays a significant role in mechanically stabilizing the nuclear envelope and cell signalling. Lamin A/C gene has 12 exons that produce at least four types of RNA via alternate splicing, including lamins A, Aδ10, C and C2. Lamin A and C are intermediate filament proteins. Their defects in the nuclear cells’ mechanical integrity cause disruption in the regulation of tissue-selective transcription alterations and defects in cell proliferation.
From the genetic aspect, two hotspot mutations of LMNA have been reported: (1) Arg453Trp/R453W, consistently identified in EDMD, and (2) Arg482Trp/Gln/Leu(R482W/Q/L), consistently identified in patients presenting with partial lipodystrophy (FPLD). Mutations leading to striated muscle laminopathy (EDMD/LGMD1B/DCM-CD) are distributed all along the LMNA gene [10] (Fig. 4). As one of the most frequent mutations that are responsible for 16% of AD-EDMD cases, the exchange of arginine 453 by tryptophan (R453W) causes an abnormal nuclear phenotype [11, 12]. Therefore, this mutation is not uncommon. The R453W is a hot spot mutation previously associated with, and some phenotypes reported can be seen in Table 1.
AD-EDMD has been reported with a broader clinical spectrum and higher frequency of de novo mutations than the X-linked form. Later and mild involvement of contracture in LGMD1B, which were re-classified as EDMD2, can contribute to its delayed diagnosis [32].
Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy in childhood. For discriminating EDMD from DMD patients, DMD children will have the following features: (a) delay in the acquisition of walking, which usually happens between 16 and 18 months; (b) early pseudohypertrophy of calves; (c) no elbow retractions; (d) CK values up to 100 times the maximum normal value; and (e) increased values of transaminases, which are never observed in patients with EDMD from either emerin or lamin A/C gene defects [33]. Moreover, the neurological examination of the father highlighted both muscular and cardiac characteristics making possible the suspicion of EDMD in our patient. In Indonesia, this is the second case of EDMD reported [14]. Moreover, our case is the first muscular dystrophy report from Indonesia using the whole-exome sequencing approach showing the utility of this approach together with clinical manifestation and usual diagnostic tests for establishing a diagnosis. However, it should be noted that many institutions worldwide do not have access to genetic testing. Although rare, there could be under-reporting of such cases due to a lack of precise molecular diagnosis because of clinical heterogeneity of this disease. This study highlights the importance of comprehensive genetic screening together with clinical features and usual diagnostic tests to further investigate suspicious cases that could resemble some form of DMD.
Most the EDMD cases are sporadic cases. Therefore, presenting the same symptoms in two family members is unusual. Early presentation of EDMD in a boy would be easily mistaken as DMD. EDMD can be inherited by an X-linked pattern, which further shows similarity with DMD. Furthermore, precise diagnosis is essential, as attested in this case, where severe cardiac involvement in the father could be detected and treated earlier.
We report a mutation in the LMNA gene underlying an autosomal dominant form of EDMD. EDMD phenotypes resemble the more common form of muscular dystrophy, i.e. dystrophinopathies (DMD/BMD), and may also be inherited in an x-linked inheritance pattern. EDMD should be considered when diagnosing a child with a clinical suspicion of DMD. Early diagnosis, intervention, targeted management, and counselling are crucial to increasing the health and life quality of EDMD patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Autosomal Dominant
- AV:
-
Atrio Ventricular
- AVB:
-
Atrio Ventricular Block
- BMD:
-
Becker Muscular Dystrophy
- CK:
-
Creatine Kinase
- DCM-CD:
-
Familial dilated cardiomyopathy with conduction systems disease
- DMD:
-
Duchenne Muscular Dystrophy
- ECG:
-
Electrocardiography
- EDMD:
-
Emery-Dreifuss Muscular Dystrophy
- ICD:
-
Implantable Cardioversion Defibrillator
- LGMD:
-
Limb-Girdle Muscular Dystrophy
- LGMD1B:
-
Limb-Girdle Muscular Dystrophy Type 1B
- LMNA:
-
Lamin A/C
- N/A:
-
Not available
- PM:
-
Pacemaker
- VVIR:
-
Ventricular pacing, ventricular sensing, inhibition response and rate-adaptive, used for ventricular pacemakers
References
The Portal of Rare Diseases and Orphan Drugs. http:/www.orpha.net/consor/cgi-bin/OC Exp.php?Lng=GB&Expert=261. Accessed 29 June 2021.
Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, et al. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat Genet. 1994;8(4):323–7.
Emery AE. Emery-Dreifuss muscular dystrophy - a 40 year retrospective. Neuromuscul Disord. 2000;10(4–5):228–32. https://doi.org/10.1016/s0960-8966(00)00105-x (PMID: 10838246).
Tomar S, Moorthy V, Sethi R, Chai J, Low PS, Hong STK, et al. Mutational spectrum of dystrophinopathies in Singapore: Insights for genetic diagnosis and precision therapy. Am J Med Genet Part C Semin Med Genet. 2019;181(2):230–44.
Pillers DAM, Von Bergen NH. Emery–dreifuss muscular dystrophy: A test case for precision medicine. Appl Clin Genet. 2016;9:27–32.
Emery AE, Dreifuss FE. Unusual type of benign x-linked muscular dystrophy. J Neurol Neurosurg Psychiatry. 1966;29(4):338–42.
Mercuri E, Counsell S, Allsop J, Jungbluth H, Kinali M, Bonne G, Schwartz K, Bydder G, Dubowitz V, Muntoni F. Selective muscle involvement on magnetic resonance imaging in autosomal dominant Emery-Dreifuss muscular dystrophy. Neuropediatrics. 2002;33(1):10–4.
Madej-Pilarczyk A, Marchel M, Ochman K, Cegielska J, Steckiewicz R. Low-symptomatic skeletal muscle disease in patients with a cardiac disease – Diagnostic approach in skeletal muscle laminopathies. Neurol Neurochir Pol. 2018;52(2):174–80.
Ostlund C, Worman H. Nuclear envelope proteins and neuromuscular diseases. Muscle Nerve. 2003;27(393–406).
Bonne G, Yaou RB, Béroud C, Boriani G, Brown S, De Visser M, et al. 108th ENMC International Workshop, 3rd Workshop of the MYO-CLUSTER project: EUROMEN, 7th International Emery-Dreifuss Muscular Dystrophy (EDMD) Workshop, 13–15 September 2002, Naarden, The Netherlands. Neuromuscul Disord. 2003;13(6):508–15.
Roblek M, Schuchner S, Huber V, Ollram K, Vlcek-Vesely S, Foisner R, et al. Monoclonal antibodies specific for disease-associated point-mutants: Lamin A/C r453w and r482w. PLoS One. 2010;5(5):e10604.
Håkelien AM, Delbarre E, Gaustad KG, Buendia B, Collas P. Expression of the myodystrophic R453W mutation of lamin A in C2C12 myoblasts causes promoter-specific and global epigenetic defects. Exp Cell Res. 2008;314(8):1869–80.
Fan Y, Tan D, Song D, Zhang X, Chang X, Wang Z, et al. Clinical spectrum and genetic variations of LMNA -related muscular dystrophies in a large cohort of Chinese patients. J Med Genet. 2021;58(5):326–33.
Bernasconi P, Carboni N, Ricci G, Siciliano G, Politano L, Maggi L, et al. Elevated tgf b2 serum levels in emery-dreifuss muscular dystrophy: Implications for myocyte and tenocyte differentiation and fibrogenic processes. Nucleus. 2018;9(1):337–49.
Lin HT, Liu X, Zhang W, Liu J, Zuo YH, Xiao JX, et al. Muscle magnetic resonance imaging in patients with various clinical subtypes of LMNA-related muscular dystrophy. Chin Med J (Engl). 2018;131(12):1472–9.
Lee Y, Lee JH, Park HJ, Choi YC. Early-onset LMNA-associated muscular dystrophy with later involvement of contracture. J Clin Neurol. 2017;13(4):405–10.
Niebroj-Dobosz I, Sokołowska B, Madej-Pilarczyk A, Marchel M, Hausmanowa-Petrusewicz I. Dysfunctional lamins as mediators of oxidative stress in emery-dreifuss muscular dystrophy. Folia Neuropathol. 2017;55(3):193–8.
Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, et al. Muscular dystrophy-associated sun1 and sun2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014;10(9):e1004605.
Scharner J, Brown CA, Bower M, Iannaccone ST, Khatri IA, Escolar D, et al. Novel LMNA mutations in patients with Emery-Dreifuss muscular dystrophy and functional characterization of four LMNA mutations. Hum Mutat. 2011;32(2):152–67.
Deconinck N, Dion E, Yaou RB, Ferreiro A, Eymard B, Briñas L, et al. Differentiating Emery-Dreifuss muscular dystrophy and collagen VI-related myopathies using a specific CT scanner pattern. Neuromuscul Disord. 2010;20(8):517–23.
Park YE, Hayashi YK, Goto K, Komaki H, Hayashi Y, Inuzuka T, et al. Nuclear changes in skeletal muscle extend to satellite cells in autosomal dominant Emery-Dreifuss muscular dystrophy/limb-girdle muscular dystrophy 1B. Neuromuscul Disord. 2009;19(1):29–36. https://doi.org/10.1016/j.nmd.2008.09.018.
Niebroj-Dobosz I, Marchel M, Madej A, Sokolowska B, Hausmanowa-Petrusewicz I. Circulating autoantibodies to troponin I in Emery-Dreifuss muscular dystrophy. Acta Myol. 2008;27(JULY):1–6.
Astejada MN, Goto K, Nagano A, Ura S, Noguchi S, Nonaka I, et al. Emerinopathy and laminopathy clinical, pathological and molecular features of muscular dystrophy with nuclear envelopathy in Japan. Acta Myol. 2007;26(3):159–64.
Golzio PG, Chiribiri A, Gaita F. “Unexpected” sudden death avoided by implantable cardioverter-defibrillator in Emery-Dreifuss patient. Europace. 2007;9(12):1158–60.
Sanna T, Dello Russo A, Toniolo D, Vytopil M, Pelargonio G, De Martino G, et al. Cardiac features of Emery-Dreifuss muscular dystrophy caused by lamin A/C gene mutations. Eur Heart J. 2003;24(24):2227–36.
Vytopil M, Benedetti S, Ricci E, Galluzzi G, Dello Russo A, Merlini L, et al. Mutation analysis of the lamin A/C gene (LMNA) among patients with different cardiomuscular phenotypes. J Med Genet. 2003;40(e132):1–5.
Colomer J, Iturriaga C, Bonne G, Schwartz K, Manilal S, Morris GE, et al. Autosomal dominant Emery-Dreifuss muscular dystrophy: A new family with late diagnosis. Neuromuscul Disord. 2002;12(1):19–25.
Bower M, Meriggioli MN, Zammit PS, Felice K, Ellis JA, Brown CA, et al. Novel and recurrent EMD mutations in patients with Emery-Dreifuss muscular dystrophy, identify exon 2 as a mutation hot spot. J Hum Genet. 2011;56(8):589–94. https://doi.org/10.1038/jhg.2011.65.
Sewry CA, Brown SC, Mercuri E, Bonne G, Feng L, Camici G, et al. Skeletal muscle pathology in autosomal dominant Emery-Dreifuss muscular dystrophy with lamin A/C mutations. Neuropathol Appl Neurobiol. 2001;27(4):281–90.
Bonne G, Mercuri E, Muchir A, Urtizberea A, Bécane HM, Recan D, et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann Neurol. 2000;48(2):170–80.
Di Barletta MR, Ricci E, Galluzzi G, Tonali P, Mora M, Morandi L, et al. Different mutations in the LMNA gene cause autosomal dominant autosomal recessive Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2000;66(4):1407–12.
Zhu Y, Zhang H, Sun Y, Li Y, Deng L, Wen X, et al. Serum Enzyme Profiles Differentiate Five Types of Muscular Dystrophy. Dis Markers. 2015;2015:543282.
Achmad C, Zada A, Affani M, Iqbal M, Martanto E, Purnomowati A, et al. A novel de novo mutation in lamin A/C gene in emery dreifuss muscular dystrophy patient with atrial paralysis. J Atr Fibrillation. 2017;9(6):1–4.
Acknowledgements
We are thankful to the patient and his family for their collaboration. We are also grateful to all staff and nurses caring for the patient.
Funding
The NUS-UGM-Tahir Foundation grant supported this study. The funding body did not influence study design, data analysis, data interpretation, and manuscript writing.
Author information
Authors and Affiliations
Contributions
KI wrote, designed the study and edited the manuscript. S supervised and reviewed the manuscript. FNA and GA collected and analyzed the clinical data. RA wrote the manuscript, collected and analyzed the clinical data. NI, NPS, and G collected data, edited and revised the manuscript. GT performed and analyzed the genetic experiments. PSL designed the study, analyzed the genetic data, reviewed, edited and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethical Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital approved this study (KE/FK/1081/EC/2020). The authors attest that full and informed consent was obtained from the father as a patient and a parent.
Consent for publication
Written informed consent was obtained from the father as a patient and a parent for the publication of this case report, images, and all information contained in it.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iskandar, K., Sunartini, Astari, F.N. et al. Autosomal dominant Emery-Dreifuss muscular dystrophy caused by a mutation in the lamin A/C gene identified by exome sequencing: a case report. BMC Pediatr 22, 601 (2022). https://doi.org/10.1186/s12887-022-03662-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03662-y