Abstract
Background
There is increasing evidence for early, active rehabilitation to enhance motor function following early brain injury. This is clear for interventions targeting the upper extremity, whereas passive treatment approaches for the lower extremity persist. The purpose of this trial is to evaluate the effectiveness of early, intensive rehabilitation targeting the lower extremity and delivered in a parent-therapist partnership model for children with perinatal stroke.
Methods
We describe a protocol for a waitlist-control, single-blind, mixed methods effectiveness randomized controlled trial, with an embedded qualitative study using interpretative description. Participants are children with perinatal stroke aged eight months to three years with signs of hemiparesis. Participants will be randomly allocated to an immediate ELEVATE (Engaging the Lower Extremity Via Active Therapy Early) intervention group, or a waitlist-control group, who will receive usual care for six months. The ELEVATE intervention involves one hour of training four days per week for 12 weeks, with a pediatric therapist and a parent or guardian each delivering two sessions per week. The intervention targets the affected lower extremity by progressively challenging the child while standing and walking. The primary outcome measure is the Gross Motor Function Measure-66. Secondary outcomes include the Pediatric Quality of Life Inventory™, Young Children's Participation and Environment Measure, and an instrumented measure of spasticity. A cost-effectiveness analysis and qualitative component will explore benefit to costs ratios and parents’ perspectives of early, intensive rehabilitation, and their role as a partner in the rehabilitation, respectively.
Discussion
This study has the potential to change current rehabilitation for young children with perinatal stroke if the ELEVATE intervention is effective. The parent interviews will provide further insight into benefits and challenges of a partnership model of rehabilitation. The mixed methods design will enable optimization for transfer of this collaborative approach into physical therapy practice.
Trial registration
ClinicalTrials.gov NCT03672864. Registered 17 September 2018.
Similar content being viewed by others
Background
Perinatal stroke occurs between 20 weeks gestation and 28 days postnatal life and is the leading cause of unilateral cerebral palsy [1] with an estimated incidence of 1 in 1600–3000 live births. Perinatal stroke may result in life-long motor impairments [1,2,3,4]. For example, the children typically have limitations with walking, which results in abnormal loading of the joints, premature musculoskeletal problems, reduced physical activity, and participation [5, 6]. Further, the sequelae from perinatal stroke can have a profound impact on family well-being [7].
Recent evidence indicates active interventions improve function [8], while passive interventions, such as bracing and botulinum toxin A injections, provide only short-term gains with unknown or suboptimal longer-term outcomes [9,10,11]. Early, intensive rehabilitation for the upper extremity has gained acceptance, but active rehabilitation for the lower extremity remains infrequent with little standardization between therapists and centres [12].
Our preliminary efficacy trial indicated that the ELEVATE (Engaging the Lower Extremity Via Active Therapy Early) intervention resulted in significantly greater improvements in gross motor function compared to a control group receiving usual care [13]. Children less than three years old with perinatal stroke tolerated one hour of intensive lower extremity activity, four days/week for twelve weeks.
Sufficient intensity of neurological rehabilitation is important [14], but it is often neither feasible nor cost-effective for physical therapists to administer high-intensity training for a prolonged period. Achieving this intensity is often better achieved by children’s parents or guardians, especially with very young children [15, 16]. Involvement of the family is essential for enhancing child outcomes [17], but there are limited reports of parents’ experiences with conducting home rehabilitation programs, especially as partners in therapy [18]. Furthermore, while family centered care advocates for parent-clinician partnerships, clinicians are cautioned against ‘downloading’ professionally driven interventions to families [19]. This study is a concurrent mixed methods design; the qualitative study will explore the parents’ perspectives of the intervention and their experiences as a partner in rehabilitation.
The purpose of this trial is to evaluate the effectiveness of early, intensive rehabilitation delivered using a parent-therapist partnership model for children with perinatal stroke. The trial was designed in collaboration with parents and therapists to build on our findings in the laboratory and determine if the intervention is effective in a real-world setting. We hypothesize that, as compared to a control group receiving usual care, young children with perinatal stroke and unilateral cerebral palsy (CP) who receive intensive lower extremity training delivered in a parent-therapist partnership will demonstrate greater improvement in gross motor function.
Methods
Experimental design
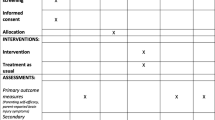
This is a three-centre (Edmonton [primary site], Calgary, and Ottawa), mixed methods study in which a qualitative study is embedded in a waitlist-control, single-blind randomized controlled trial (RCT). The children will be randomly allocated to either the Immediate Intervention Group (treatment) or the Waitlist-control Group (usual care) with follow-up (Fig. 1). The Immediate Intervention Group will undergo the three-month ELEVATE intervention immediately following randomization. The Waitlist-control Group will delay treatment for six months before initiating the ELEVATE intervention. The primary time point of interest is at six months in the study (Fig. 2). All children will undergo monthly assessments for 12 months in the study and will also be assessed at age four years of age to evaluate longer-term effects of training.
Experimental Design with Measurement Time Points. Participants are randomly allocated to either receive the ELEVATE intervention immediately (Immediate Group) or delay the intervention for 6 months (Waitlist-control Group) before they are offered the optional ELEVATE training. Children undergo monthly assessments for 12 months (including 9 months of follow-up for the Immediate Group and 3 months of follow-up for the Waitlist-control Group). Outcome measures are obtained as indicated by arrows and stars. GMFM-66: Gross Motor Function Measure-66; PSAD: Portable Spasticity Assessment Device; PedsQL CP: The Pediatric Quality of Life Inventory™ module for cerebral palsy; YC-PEM: Young Children's Participation and Environment Measure; RUQ: Resource Use Questionnaire
Inclusion criteria
-
children ages 8 months to 3 years
-
medical history and physical exam consistent with perinatal stroke
-
hemiparesis in the upper and/or lower extremity
-
agreement from parent/guardian to adhere to the training and testing schedule
Exclusion criteria
-
bilateral motor impairment
-
epileptic seizures that could interfere with training
-
cognitive, behavioural or developmental impairments that preclude participation in the protocol
-
botulinum toxin A injections or surgery in the lower extremities within the previous six months
-
concurrent casting during the intervention phase (including constraint-induced movement therapy with casting)
-
diagnosis associated with neurological/developmental regression
-
parent unable to communicate (verbal and written) in English or French
Randomization and stratification
Participants will undergo baseline assessments before randomization (Fig. 1). The randomization sequence was generated in Edmonton by a statistician. We used a permuted block design with a block size of 2–4. Randomization was stratified by city to ensure that the numbers allocated to the intervention and control groups will be roughly equal at each site. Their assignment is concealed until baseline assessments are complete, and are made available to the study sites through Research Electronic Data Capture (REDCap).
Intervention
ELEVATE [13] will be administered one hour/day, four days/week, for twelve weeks. The ELEVATE intervention follows principles of motor learning and neuroplasticity [14], with high repetition of child-initiated activity. The activities will focus on weight-bearing on the feet with the aim to maximize recruitment of multiple, lower extremity muscle groups, especially on the affected side. Training may include activities such as kicking, squatting, navigating obstacles and walking on ramps and stairs. Children will train in soft-soled slippers, without braces or orthotics, to enhance active use of muscles throughout the lower extremity. Children who are not yet walking will be encouraged to walk with support while incorporating activities that increase weight bearing through the affected lower extremity and challenge their balance. As children begin to walk independently, their mobility will be further challenged by walking at different speeds, changing walking direction, and walking on unstable or more physically-demanding surfaces. The aim for intervention sessions is an hour of activity, which is anticipated to result in approximately 1000–3000 steps (as measured by step counters; Actigraph, FL), depending on the child’s age and ability [13]. Small ankle and foot weights (110 g and 20 g increments, respectively) will be used to increase work of the affected lower extremity, once children tolerate one hour of training [20]. Children with hemiparesis resulting from perinatal stroke typically walk asymmetrically, and adding ankle and foot weights to the affected lower extremity increases asymmetry of walking, which is necessary to induce learning [21]. Once children demonstrate more symmetrical gait with the weights, the amount of weight is increased to induce further learning [22].
Parent therapist partnership
This effectiveness trial is designed to deliver the ELEVATE intervention by parents and therapists working in partnership. The partnership was considered an ideal model because it provides a high dose of intervention while supporting parents as partners in the rehabilitation process [23]. Physical therapists or physical therapy assistants working in public or private outpatient pediatric therapy centres will provide the intervention. The parent and therapist will each deliver two days of intervention per week. The parent will attend the intervention sessions with the therapist, thereby providing opportunities for observation and discussion. This will include reinforcement of the training principles, feedback on training and opportunities for progressions. Parents will be provided with a training manual describing suggested training activities [20].
Intervention fidelity across centres
All treating therapists will be trained by the Principal Investigator and research physical therapist before the trial. Training will include background on activity-dependent neuroplasticity and an overview of the efficacy of early intensive rehabilitation. Practical training will include video examples and hands-on practice with young children. Treatment fidelity will be assessed throughout the trial using step-counts during intervention sessions, monthly video documentation, and biweekly meetings with staff at each site. Additional training sessions will be provided if training therapists indicate low confidence with the intervention.
Outcomes
Primary outcome measure
Gross motor function
The Gross Motor Function Measure-66 (GMFM-66) is the primary outcome measure, a criterion-referenced observational measure for assessing gross motor function in children with CP [24]. It has been validated for children > 0.5 years old and is a gold standard for measuring gross motor function in children with CP [25]. The assessing therapists will be pediatric physical therapists from the community trained by study personnel to administer the GMFM-66 and are masked to the child’s group assignment. All parents will be informed not to disclose the child’s group assignment to the assessing therapist. All assessments will be videotaped for reference. The GMFM-66 will be administered twice at baseline and monthly thereafter, for a total of 14 measurements per child. The two measures at baseline will be averaged to provide a more reliable estimate.
Secondary outcome measures
Quality of life
Quality of life of the participants and their parents will be quantified at entry to the study, and again every 3 months using the Pediatric Quality of Life Inventory™, module for cerebral palsy (PedsQL CP) [26]. The PedsQL CP will be administered via online self-report through REDCap, or over the phone if respondents require assistance. The PedsQL is reliable and sensitive [26] and has been used extensively for this population. The children in this study are too young to self-report, so a parent proxy will be used. The scales in the CP module used are those recommended for children up to four years old [26]. The PedsQL also has a 36-item scale to measure the impact of a child’s health condition on the family, called Family Impact Module (PedsQL FIM). The scale has been widely used for parents of children with chronic health conditions, including perinatal stroke [7]. Three scores will result from this module, which reflect the parents’ health-related quality of life, family functioning and a total score.
Resource use and costing
Costs related to lower extremity therapy as well as resources used for treatment and management of the child’s cerebral palsy will be included over the study period, similar to a previously reported protocol [27]. The child will be the unit of analysis and utilization incurred by the participant, caregivers and/or the healthcare system will be assigned to the child for analysis. The Resource Use Questionnaire (RUQ) is a tool validated for use in participants with neurodevelopmental disabilities [28, 29] and will be used to collect information regarding resource use and out-of-pocket costs (e.g. physical or occupational therapy, materials and equipment, child-focussed recreation activities, etc.).
The RUQ will be administered at baseline, six months, the end of the one year study period and at the four-year-old follow up. The total cost per participant per month will be calculated and used in extrapolation. For the healthcare system perspective, costs will include physical or occupational therapy, child-focused recreation activities and additional services related to the child’s care paid for by the public healthcare system. For the societal perspective, costs will include all costs for the healthcare system perspective as well as parent lost productivity and parent out-of-pocket costs. For analysis using the societal perspective, a human capital approach [30] will be used to monetize lost caregiver productivity. Parent reports on the RUQ will include out-of-pocket costs for additional services, child-focused recreation activities, and materials and equipment. Lost productivity and out-of-pocket costs will be summed for the family of each participant to obtain total lost productivity and out of pocket costs for each participant over the study period.
The cost of the ELEVATE intervention will be estimated from the compensation to therapists including training, administration of the ELEVATE intervention and the cost of any materials and supplies associated with the intervention.
Participation
The Young Children's Participation and Environment Measure (YC-PEM) will be used to assess participation in three settings: home, daycare/preschool, and the community [31]. This questionnaire, completed by a parent or caregiver, was developed for children aged 0–5 years. The YC-PEM will be administered at baseline and every three months throughout the twelve-month study period and may be administered over the phone with research staff or via online self-report through REDCap.
Spasticity
We will use a newly developed device for measuring spasticity called the Portable Spasticity Assessment Device (PSAD, Nordic Neurostim, Denmark) [32, 33] to determine if the intervention reduces contracture or spasticity at the ankle, as seen in other active therapies for the lower extremities [32, 34]. Ankle joint range of motion, mechanical resistance caused by contracture and reflex-mediated spasticity can be measured using this device. We focus on the ankle, since this is the lower extremity joint very commonly affected by spasticity and is often the target of passive approaches including botulinum toxin, serial casting and orthoses [35]. Contracture, defined here as increased stiffness in joint movement cause by changes in intrinsic soft-tissue properties, will be reflected by the torque during slow stretch without any muscle activity. Spasticity will be indicated by the joint angle at which reflex EMG is generated in a fast stretch, similar to the angle of catch in the Tardieu Scale. This measure will be used at two study sites, because we only have two devices.
Reporting of co-interventions
During the six-month control period for the Waitlist-control Group and the follow-up period for both groups, parents will be asked to report any rehabilitation for their child’s lower extremity including the nature, duration and frequency. This will provide clarity on usual care, and the variations between sites.
Sample size
The effect size on the primary outcome measure is estimated at 0.91, based on data from a cohort of children trained by their parents in our preliminary efficacy trial, as this is the more conservative estimate compared to the 1.67 effect size of children trained by a physical therapist. Twenty children per group are required to achieve statistical power of 0.8 and significance of 0.05. To account for cluster randomization with an estimated intra-cluster correlation coefficient of 0.02, a design effect of 1.4 is considered, which results in a total sample size of 56.
Data management and analysis
Data will be entered in REDCap and analyzed at the primary study site. The primary time point of interest is at six months (Fig. 2), so change scores from baseline to six months will be compared between the ELEVATE intervention and control groups. Multiple linear regression models may be developed to assess the effect of variables (e.g. age at time of intervention) for each outcome. We will assess model fit and provide estimates with 95% confidence intervals.
Cost-effectiveness analysis (CEA)
In the CEA, the ratio of the difference in mean cost between the ELEVATE and Waitlist-control groups to the difference in effectiveness scores (change in GMFM-66) between groups will be used to estimate an incremental cost-effectiveness ratio (ICER) from the publicly funded healthcare payer and societal perspectives. The mean effectiveness for each group will be compared using patient level regression. We will use Ordinary least squares (OLS) to assess the difference in effectiveness and the difference in cost between groups. We will control for a set of covariates including study site, age and baseline GMFM-66 scores. The mean cost per participant for each group will also be compared using participant-level regression. A bootstrapped analysis will be conducted for the CEA and results presented on a cost-effectiveness acceptable curve (CEAC).
Trial monitoring
The trial is overseen by Health Canada because the PSAD is approved for investigational testing. The trial is monitored by the Quality Management in Clinical Research unit at the University of Alberta. The Research Ethics Board 3, University of Alberta, approved this study and the researchers are responsible for reporting protocol amendments and adverse events. Annual ethics renewals of the protocol are monitored by this Board.
Qualitative study
Design
Interpretative Description methodology [36] will be used as the methodological framework for the concurrent qualitative study to gain insight into the perspective of parents participating in a partnership with community therapists to administer the ELEVATE intervention. Interpretative Description has been chosen because this study intends to generate clinically relevant knowledge that is rich in description and interpretation and can be applied to improve clinical practice.
Sample selection
Participants will be recruited from the multi-centre RCT and sampling will be purposive, to reflect the expected variation in experiences of participants [37]. We aim to interview parents with diverse backgrounds and experiences including individuals with varied socioeconomic status, multiple and single child families and single parent families. Sampling decisions will be made iteratively as information about participants’ experiences is explored and areas where further information is required becomes evident. Hence, a definitive sample size is not defined a priori but will be evaluated on an ongoing basis as data sufficiency becomes apparent or adequate to provide deep insight into the research question [38, 39]. We estimate an approximate sample size of 12–15 for the study, based on previous qualitative studies of parent experiences with home-based rehabilitation for children with CP [18].
Data collection
Data will be collected through individual, semi-structured interviews at the start of intervention in the RCT and immediately following the intervention. Parents who choose not to participate in the RCT will be offered the opportunity to participate in a single interview to explore the barriers to participating in intensive rehabilitation. The interviews will follow a semi-structured interview guide (Supplementary File 1) at each time point. All interviews will be de-identified, audio recorded and transcribed verbatim.
Data management and analysis
Thematic analysis will be used to organize and describe the data in rich detail. An inductive approach to thematic analysis will be used; themes will be strongly linked to the data [40], while interpreting findings with the intent to apply them to the practice of physical therapy. Data analysis will run concurrently with data collection to ensure that sampling and data collection are informed by emergent results. The coding process will involve interpretative thinking rather than simply recording and analyzing [41]. Field notes from interviews, including observations and interviewer impressions, will not be included in coding but will assist with interpreting transcripts.
Rigour and credibility
The quality of this study will be enhanced by keeping a detailed record of activities, regular team meetings and ongoing reflexivity around the information being collected and the team members’ roles as clinician-researchers [42]. Detailed documentation will involve journaling and field notes following interviews. Modifications to the semi-structured interview guide will be documented as the team of researchers come to a deeper understanding of parents’ experiences throughout this study. Data analysis will be conducted collaboratively with at least two members of the research team.
Discussion
Perinatal stroke results in life-long consequences for children and families. By intervening early, limited health care resources can be directed to children at an age when the effects are potentially largest. This study engages parents as partners so they become familiar with the principles of the approach. We anticipate that parent involvement will facilitate longer-term outcomes that will potentially enhance child motor development beyond the study period. We hypothesize that early improvements in gross motor function will enhance child outcomes and improve child and family quality of life. In addition, economic benefits may extend beyond our study period, for example, by delaying or avoiding the need for orthoses and botulinum toxin A injections, which we aim to capture at the four-year-old follow-up. Understanding parents’ experiences with the parent-therapist partnership model will allow for refinement of this approach to rehabilitation and more effective translation to clinical settings. Finally, partnering with key knowledge users, including families and pediatric clinicians, aims to bring about faster and more seamless adoption of the intervention if the results demonstrate effectiveness.
Availability of data and materials
Not applicable. Results from this trial will be uploaded to clinicaltrials.gov upon completion.
Abbreviations
- ELEVATE:
-
Engaging the Lower Extremity Via Active Therapy Early
- CP:
-
Cerebral Palsy
- RCT:
-
Randomized Controlled Trial
- REDCap:
-
Research Electronic Data Capture
- GMFM-66:
-
Gross Motor Function Measure-66
- PedsQL CP:
-
Pediatric Quality of Life Inventory™ module for cerebral palsy
- YC-PEM:
-
Young Children's Participation and Environment Measure
- PSAD:
-
Portable Spasticity Assessment Device
References
Dunbar M, Kirton A. Perinatal stroke: mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet Child Adolesc Heal. 2018;2(9):666–76. https://doi.org/10.1016/S2352-4642(18)30173-1.
Mineyko A, Kirton A. The black box of perinatal ischemic stroke pathogenesis. J Child Neurol. 2011;26(9):1154–62. https://doi.org/10.1177/0883073811408312.
Haak P, Lenski M, Hidecker MJC, Li M, Paneth N. Cerebral palsy and aging. Dev Med Child Neurol. 2009;51(suppl. 4):16–23. https://doi.org/10.1111/j.1469-8749.2009.03428.x.
Turk MA. Health, mortality, and wellness issues in adults with cerebral palsy. Dev Med Child Neurol. 2009;51(suppl. 4):24–9. https://doi.org/10.1111/j.1469-8749.2009.03429.x.
Horstmann HM, Hosalkar H, Keenan MA. Orthopaedic issues in the musculoskeletal care of adults with cerebral palsy. Dev Med Child Neurol. 2009;51(suppl. 4):99–105. https://doi.org/10.1111/j.1469-8749.2009.03417.x.
Murphy KP. Cerebral palsy lifetime care - Four musculoskeletal conditions. Dev Med Child Neurol. 2009;51(suppl. 4):30–7. https://doi.org/10.1111/j.1469-8749.2009.03431.x.
Bemister TB, Brooks BL, Dyck RH, Kirton A. Parent and family impact of raising a child with perinatal stroke. BMC Pediatr. 2014;14:182. https://doi.org/10.1186/1471-2431-14-182.
Novak I, Morgan C, Fahey M, et al. State of the evidence traffic lights 2019: systematic review of interventions for preventing and treating children with cerebral palsy. Curr Neurol Neurosci Rep. 2020;20(2):3. https://doi.org/10.1007/s11910-020-1022-z.
Love SC, Valentine JP, Blair EM, Price CJ, Cole JH, Chauvel PJ. The effect of botulinum toxin type A on the functional ability of the child with spastic hemiplegia a randomized controlled trial. Eur J Neurol. 2001;8(5):50–8.
Narayanan UG. Management of children with ambulatory cerebral palsy: an evidence-based review. J Pediatr Orthop. 2012;32 Suppl 2:S172-81. https://doi.org/10.1097/BPO.0b013e31825eb2a6.
Tedroff K, Granath F, Forssberg H, Haglund-Akerlind Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev Med Child Neurol. 2009;51(2):120–7. https://doi.org/10.1111/j.1469-8749.2008.03189.x.
Papavasiliou AS. Management of motor problems in cerebral palsy: a critical update for the clinician. Eur J Paediatr Neurol. 2009;13(5):387–96. https://doi.org/10.1016/j.ejpn.2008.07.009.
Hurd C, Livingstone D, Brunton K, Smith A, Gorassini M, Watt MJ, Andersen J, Kirton A, Yang JF. Early, Intensive, Lower Extremity Rehabilitation Shows Preliminary Efficacy After Perinatal Stroke: Results of a Pilot Randomized Controlled Trial. Neurorehabil Neural Repair. 2022;36(6):360-70. https://doi.org/10.1177/15459683221090931.
Kleim J, Jones T. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech, Lang Hear Res. 2008;51:S225–39.
Sakzewski L, Reedman S, McLeod K, et al. Preschool HABIT-ILE: Study protocol for a randomised controlled trial to determine efficacy of intensive rehabilitation compared with usual care to improve motor skills of children, aged 2–5 years, with bilateral cerebral palsy. BMJ Open. 2021;11(3):e041542. https://doi.org/10.1136/bmjopen-2020-041542.
Eliasson AC, Nordstrand L, Ek L, et al. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral-cerebral palsy; an explorative study with randomized design. Res Dev Disabil. 2018;72:191–201. https://doi.org/10.1016/j.ridd.2017.11.006.
Kruijsen-Terpstra AJA, Ketelaar M, Boeije H, et al. Parents’ experiences with physical and occupational therapy for their young child with cerebral palsy: a mixed studies review. Child Care Health Dev. 2014;40(6):787–96. https://doi.org/10.1111/cch.12097.
Novak I. Parent experience of implementing effective home programs. Phys Occup Ther Pediatr. 2011;31(2):198–213. https://doi.org/10.3109/01942638.2010.533746.
An M, Palisano RJ, Yi C HWI, Chiarello LA, Dunst CJ, Gracely EJ. Effects of a collaborative intervention process on parent-therapist interaction: a randomized controlled trial. Phys Occup Ther Pediatr. 2019;39(3):259–75. https://doi.org/10.1080/01942638.2018.1496965.
Hurd C, Livingstone D, Brunton K, Teves M, Zewdie E, Smith A, Ciechanski P, Gorassini MA, Kirton A, Watt MJ, Andersen J, Yager J, Yang JF. Early Intensive Leg Training to Enhance Walking in Children With Perinatal Stroke: Protocol for a Randomized Controlled Trial. Phys Ther. 2017;97(8):818-25. https://doi.org/10.1093/ptj/pzx045.
Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ. Locomotor adaptation. Prog Brain Res. 2011;191:65–74. https://doi.org/10.1016/B978-0-444-53752-2.00013-8 (Elsevier B.V).
Damiano DL, Stanley CJ, Bulea TC, Park HS. Motor learning abilities are similar in hemiplegic cerebral palsy compared to controls as assessed by adaptation to unilateral leg-weighting during gait: Part I. Front Hum Neurosci. 2017;11:49. https://doi.org/10.3389/fnhum.2017.00049.
Harniess PA, Gibbs D, Bezemer J, Purna BA. Parental engagement in early intervention for infants with cerebral palsy – a realist synthesis. Child Care Health Dev. 2021. https://doi.org/10.1111/cch.12916.
Russell DJ, Avery LM, Walter SD, et al. Development and validation of item sets to improve efficiency of administration of the 66-item Gross Motor Function Measure in children with cerebral palsy. Dev Med Child Neurol. 2009;52(2):e48-54. https://doi.org/10.1111/j.1469-8749.2009.03481.x.
Wei S, Wang SJ, Liao YG, Hong Y, Xu XJ, Shao XM. Reliability and validity of the GMFM-66 in 0- to 3-year-old children with cerebral palsy. Am J Phys Med Rehabil. 2006;85(2):141–7. https://doi.org/10.1097/01.phm.0000197585.68302.25.
Varni JW, Burwinkle TM, Berrin SJ, Sherman SA, Artavia K, Malcarne VL, Chambers HG. The PedsQL in pediatric cerebral palsy: reliability, validity, and sensitivity of the Generic Core Scales and Cerebral Palsy Module. Dev Med Child Neurol. 2006;48(6):442-9. https://doi.org/10.1017/S001216220600096X.
Berrigan P, Hodge J, Kirton A, Moretti ME, Ungar WJ, Zwicker JD. Protocol for a cost-utility analysis of neurostimulation and intensive camp-based therapy for children with perinatal stroke and hemiparesis based on a multicentre clinical trial. BMJ Open. 2021;11(1):e041444. https://doi.org/10.1136/bmjopen-2020-041444.
Tsiplova K, Ungar WJ, Flanagan HE, et al. Types of services and costs of programs for preschoolers with autism spectrum disorder across sectors: a comparison of two canadian provinces. J Autism Dev Disord. 2019. https://doi.org/10.1007/s10803-019-03993-3.
Ungar WJ, Tsiplova K, Millar N, Smith IM. Development of the Resource Use Questionnaire (RUQ-P) for families with preschool children with neurodevelopmental disorders: validation in children with autism spectrum disorder. Clin Pract Pediatr Psychol. 2018;6(2):164–78. https://doi.org/10.1037/cpp0000226.
Canadian Agency for Drugs and Technologies in Health. CADTH methods and guidelines service line: CADTH methods and guidelines. 4th edn. Canada: Guidelines for the Economic Evaluation of Health Technologies; 2017.
Khetani MA. Validation of environmental content in the young children’s participation and environment measure. Arch Phys Med Rehabil. 2015;96(2):317–22. https://doi.org/10.1016/j.apmr.2014.11.016.
Lorentzen J, Kirk H, Fernandez-Lago H, et al. Treadmill training with an incline reduces ankle joint stiffness and improves active range of movement during gait in adults with cerebral palsy. Disabil Rehabil. 2017;39(10):987–93. https://doi.org/10.1080/09638288.2016.1174745.
Yamaguchi T, Hvass Petersen T, Kirk H, et al. Spasticity in adults with cerebral palsy and multiple sclerosis measured by objective clinically applicable technique. Clin Neurophysiol. 2018. https://doi.org/10.1016/j.clinph.2018.07.004.
Willerslev-Olsen M, Lorentzen J, Nielsen JB. Gait training reduces ankle joint stiffness and facilitates heel strike in children with Cerebral Palsy. NeuroRehabilitation. 2014;35(4):643–55. https://doi.org/10.3233/NRE-141180.
Krarup LH, Kristensen PK, Strand L, Bredtoft SL, Mechlenburg I, Nordbye-Nielsen K. Ankle contractures are frequent among children with cerebral palsy and associated with lower gross motor function and degree of spasticity. Acta Paediatr Int J Paediatr. 2021;110(7):2171–8. https://doi.org/10.1111/apa.15804.
Thorne S. Interpretive Description Qualitative Research for Applied Practice. 2nd ed. Routledge; 2016.
Thorne S, Kirkham SR, Flynn-Magee KO. The Analytic Challenge in Interpretive Description. 2004.
Fossey E, Harvey C, Mcdermott F, Davidson L. Understanding and evaluating qualitative research. Aust New Zeal J Psychiatry. 2002;36(6):717–32.
O’Reilly M, Parker N. “Unsatisfactory Saturation”: A critical exploration of the notion of saturated sample sizes in qualitative research. Qual Res. 2012;13(2):190–7. https://doi.org/10.1177/1468794112446106.
Patton MQ. Qualitative Research & Evaluation Methods: Integrating Theory and Practice. 4th ed. California: SAGE Publications Inc.; 2015.
Bazeley P. Qualitative Data Analysis Practical Strategies. 1st ed. California: SAGE Publications Inc.; 2013.
Finlay L. Outing” the Researcher: The Provenance, Process, and Practice of Reflexivity. 2002.
Acknowledgements
Biostatistics and REDCap access have been supported by the Women and Children’s Health Research Institute. We thank Donna Livingstone, PT, Rhina Delgado, OT, Catherine Bangel, Erin Coffin, and Simone Hunter, PT, for contributing to the design of this study.
Health Canada investigational testing approval
#289,280 (for ActiGraph), and #293,166 (for Portable Spasticity Assessment Device).
Funding
Funding is provided by the Brain Canada Foundation (a federally associated funding body that provides financial support for the conduct of the study through peer review) through the Improving Health Outcomes and Quality of Life Multi-Investigator Research Initiative program, 2017, awarded to J. Yang. Additional funding partners include the Women and Children’s Health Research Foundation, the Faculty of Rehabilitation Medicine, University of Alberta, and the Vi Riddell Rehabilitation Program, Calgary. Graduate studentships provided to C. Hurd through Alberta Innovates, and the Stollery Children’s Hospital Foundation through the Women and Children’s Health Research Institute.
Author information
Authors and Affiliations
Contributions
CLH: conception and design, planning at primary site (Edmonton), procurement of student funding, wrote initial draft, edited and approved the manuscript. MB: design, coordinator at primary site for study, edited and approved the manuscript. CMD: design and coordination for Calgary site, planning for data collection, edited and approved the manuscript. EGC: design, site-lead for Calgary, participated in procurement of funds, planning for study, edited and approved the manuscript. HA: design, coordination and recruitment planning for Ottawa site, edited and approved the manuscript. LP: design and lead for qualitative component of the study, edited and approved the manuscript. JDZ: design and lead for the economic analysis, assisted with procurement of funds, edited and approved the manuscript. AM: design and site-lead for Ottawa, participated in procurement of funds, planning for study, edited and approved the manuscript. MJW: design, assisted with procurement of funds, planning for recruitment in Edmonton, edited and approved the manuscript. JA: design, assisted with procurement of funds, planning for recruitment in Edmonton, edited and approved the manuscript. AK: design, assisted with procurement of funds, provided personnel assistance for Calgary site including recruitment, edited and approved the manuscript. JFY: conception, design, lead PI for study including procurement of funds, planning and coordination for the study, assisted with writing, edited and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Health Research Ethics Review Board #3, University of Alberta, Edmonton, AB, Canada, Pro#00078553. Written informed consent was obtained from a parent or guardian for all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary File 1.
Semi-structured interview guide. This document contains the semi-structured interview guide for interviews conducted with parents before and after the ELEVATE intervention.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hurd, C.L., Barnes, M., Diot, C.M. et al. Parent-therapist partnership to ELEVATE gross motor function in children with perinatal stroke: protocol for a mixed methods randomized controlled trial. BMC Pediatr 22, 480 (2022). https://doi.org/10.1186/s12887-022-03525-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03525-6