Abstract
Background
Surgical site infections (SSIs) in children represent a common and serious postoperative complication. Surgical skin preparation is an essential preventive measure in every surgical procedure. The most commonly used antiseptic agents for surgical skin preparation are chlorhexidine gluconate and iodophors in alcohol-based solutions. In adult patients the use of chlorhexidine-containing antiseptic solutions for preoperative skin preparation has been advocated to reduce SSI rates. Our objective was to conduct a systematic literature review on use of antiseptic agents for surgical skin preparation in children less than 16 years of age.
Methods
A systematic review of MEDLINE, EMBASE, CINAHL and CENTRAL was performed using both MeSH and free text terms and using the relevant Cochrane filter to identify full text randomized trials (RCTs) and comparative observational studies. Interventions of interest were the choice of main agent in antiseptic solutions (chlorhexidine/povidone-iodine/alcohol) compared with each other or with other antiseptic agents. Primary outcome was the reported rate of surgical site infections.
Results
In total 8 studies were included in the review; 2 RCTs and 6 observational studies. Observational studies generally did not primarily investigate the association of different antiseptics with subsequent SSI. The identified randomised controlled trials included only 61 children in total, and were of low quality. Consequently, we did not conduct a formal meta-analysis. Since the publication of a comprehensive systematic review of perioperative measures for the prevention of SSI in 2016, no randomized controlled trials comparing antiseptic agents for surgical skin preparation in paediatric surgery have been conducted.
Conclusion
Robust evidence on the optimal skin antisepsis to reduce SSIs in children is lacking. Direct extrapolation of effects from trials involving adults is not appropriate as physiologic characteristics and risk factors for SSIs differ between adults and children. It is therefore essential to conduct high quality RCT investigating interventions to identify optimal measures to reduce SSI rates in children.
Trial registration
Prospero registration (CRD42020166193).
Similar content being viewed by others
Background
Postoperative surgical site infection (SSI) may be associated with any type of surgical procedure and is a common and serious postoperative complication. SSIs are among the most common hospital acquired infections in children and represent a relevant burden to patients and their families as well as health care systems [1]. With about one quarter of cases SSI is the leading cause for readmissions among children undergoing surgery [2]. According to the World Health Organization (WHO) up to 50% of SSIs could be prevented [3]. Prevention intervention bundles including skin antisepsis have been successfully implemented to reduce SSI rates in children [4]. Although SSI rates in children are lower compared to adult population (2% in children versus 45% in adults after colorectal surgery) [5], the cost of each infection is high at 2000 € per infection [6]. In high-income countries such as the U.S. on average 3.9 million surgical procedures in children and adolescents are performed each year [7]. Assuming a rate of SSI of 2% this results in at least 78,000 cases of SSI each year, not accounting for patient groups and surgical procedures with a significantly higher incidence. Neonates and children with congenital anomalies are known to be especially vulnerable [8,9,10]. Incidence of SSI in this patient group has been reported as high as 17% [8]. The burden of SSIs is even higher in low- and middle-income countries (LMICs) at 24.7% compared with 6.3% in high-income countries [11]. Against this background and the estimated number of 1.7 billion children per year in LMIC in need of surgery, prevention of SSIs is an essential part of improving health care for children globally [12].
Based on multiple systemic literature reviews, the World Health Organization (WHO) [13, 14] as well as the National Institute for Health and Care Excellence (NICE) [15] and the Asia Pacific Society of Infection Control (APSIC) [16] have recently published guidelines on the prevention of SSIs. The majority of literature on SSI prevention reports on data from adult patients. For specific procedures with a high risk of SSI in children, such as in cardiovascular and neurosurgery, it has been shown that intervention bundles can reduce the rate of SSI [17,18,19,20,21,22,23]. Nonetheless, there is little evidence on the influence of optimal individual preventive measures, for example surgical skin preparation agents, in children.
Surgical skin preparation is performed in order to reduce bacterial load at the surgical site. The most widely used agents are chlorhexidine gluconate (CHG) and iodophors (i.e. povidone-iodine (PVP)) in alcohol based solutions [24]. While other interventions, such as the use of antiseptic-coated sutures or whole-body washing, are also of interest, surgical skin preparation is particularly relevant as being part of every surgical procedure independent of circumstances (elective vs. emergency procedure), comorbidities and age. Worldwide, antiseptic solutions are expected to be readily available and application is simple and can be carried out without specific training. The literature review conducted by the WHO in November 2016 recommends the use of CHG solutions for adult surgery [14]. Nonetheless, as has been shown in surveys conducted among paediatric surgery units in Great Britain [25], Germany and Switzerland there is no standardization in choice of antiseptic agent for surgical skin preparation in children. Less than 50% of questioned surgeons use CHG in neonates and preterm babies. Although newer evidence suggests both CHG and PVP to be safe alternatives for surgical skin preparation in infants [26], concerns for side effects such as local or systemic toxicity persist [24, 27]. Especially in preterm neonates < 34 weeks and in very low birth weight infants, a patient group particularly at risk of SSI, evidence on safety profiles on antiseptic solutions is equivocal [24].
In response to the lack of evidence in the choice of antiseptic solution for surgical skin preparation in paediatric surgery pointed out by the WHO, we aimed to systematically review the current body of evidence.
Methods
A systematic review of the published literature was conducted and is reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A protocol for this review was prospectively registered with PROSPERO (CRD42020166193).
PICOS (Participants, intervention, comparator, outcomes and study design)
Studies evaluating the interventions of interest in children under 16 years of age undergoing any surgery, including minimally invasive surgery, elective and emergency surgery, were included. Studies of surgical procedures that did not include a visible incision and therefore did not result in the presence of a conventional surgical wound or did not require suturing or closure of the wound were excluded. Studies which included both adults and children were excluded, if the age of included children in trial was ≥ 16 years of age. Interventions and comparators studied were: choice of main agent in antiseptic solutions (chlorhexidine/povidone-iodine/alcohol) compared with each other or with other antiseptic agents. The primary outcome of interest assessed is the SSI rate. All types of SSIs were considered (superficial, deep wound, organ space). Randomized controlled trials (RCTs) and comparative observational studies were considered eligible for inclusion.
Search strategy
Electronic searches were conducted on Feb 29, 2020 and on Dec 8, 2020 using OVID SP on the following databases: MEDLINE (1946-Dec week 4 2020), Excerpta Medica Database (EMBASE) (1974–2020 Dec 8th); Cumulative Index to Nursing and Allied Health Literature (CINHAL) (1988–2019) and Cochrane Central Register of Controlled Trials (CENTRAL). No restrictions on publication language were applied. A comprehensive list of search terms was used, including Medical subject Headings (MesH) (see Additional File 1). References from relevant articles were identified by using the search terms “surgical wound infection”, “surgical site infection”, “SSI”, “child*”, “peadiat*”, “pediat*”, “chlorhexidine*”, “alcohol*”, “ethanol”, and “iodin*”. References were then screened by titles and abstract in order to find relevant studies. The full text of all potentially eligible articles was obtained. Duplicate studies were excluded. Full text articles were screened for eligibility based on the prespecified eligibility criteria. Data was extracted from the selected articles and converted into a tabulated form, including study year, study design, study time, surgical procedure or degree of contamination, mean age, number of patients, type of antiseptic agent, criteria for diagnosis of SSI and SSI rate, duration of follow-up and rate of preoperative prophylactic antibiotic therapy. Quality of RCTs was graded using the van Tulder scale [28]. Quality of the observational studies was graded using the Newcastle–Ottawa Scale [29]. As preliminary literature searches already showed little evidence in paediatric patients no plans for meta-analysis were made before conducting the search. Overall level of evidence was assessed according to Oxford Centre for Evidence-Based Medicine [30].
Results
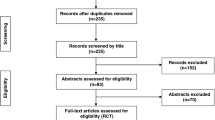
The search identified 991 records (Fig. 1). After removal of duplicates, 885 articles were screened. 855 articles were excluded after screening of titles and abstracts. Thirty full texts were assessed for eligibility. Of those twenty-two were excluded, mostly because of inappropriate age or inappropriate outcome. A total of eight articles remained, of which 2 were RCTs [31, 32] and 6 were observational studies [9, 33,34,35,36,37].
Study characteristics
Study characteristics are summarized in Tables 1 and 2. Two single centre RCTs comparing povidone-iodine with methyl-alcohol and chlorhexidine respectively were identified in the search. Both trials included children as well as adults. Of the six observational studies identified in the search process, two included adults as well as children, one included only neonates and three included children of different age groups. Surgical wounds and procedures were mostly classified as clean or clean contaminated. Only Lubega et al.also incorporated surgical procedures involving dirty or infected wounds [37].
Randomized controlled trials
Meier et al. [38] conducted a single-centre randomized, controlled, open label trial comparing market soap and methyl alcohol with povidone-iodine for preoperative skin preparation with 200 participants of all ages undergoing elective inguinal hernia repair in a developing world country hospital (53 children less than 13 years). It is not reported which type of solution was used (aqueous vs. alcoholic, concentration of povidone iodine). Demographic characteristics in the paediatric group were not reported. The infection rate in children was lower compared to adults, although not significantly so (1.9% vs. 6.5%; p = 0.294). The infection rate between trial arms did not differ significantly (methyl alcohol group 5.1% vs. povidone iodine group 5.9% SSI; p = 1.000). Berry et al. [31] also conducted a single-centre randomized, controlled, open label trial comparing povidone-iodine and chlorhexidine both for surgical scrub (povidone –iodine 10% in alcohol or chlorhexidine 0.5% in alcohol) and skin preparation (7.5% povidone-iodine or 0.5% chlorhexidine in alcohol) in 866 patients. The trial was conducted at Western, General Hospital in Edinburgh between May 1978 and February 1980 and all patients undergoing elective surgery were included in the trial. Surgical procedures were either clean non-abdominal operations or clean/clean-contaminated abdominal or genitourinary interventions. Only eight children less than 15 years of age were recruited. Most likely, because of the small number of children included in the trial, the authors did not further differentiate between adult and paediatric patients. Between trial arms (scrub and skin preparation with povidone-iodine vs. chlorhexidine) there was a significant difference in SSI rate (14.8% PVP vs. 9.8% CHG; p = 0.03) at the time of the patients ‘ discharge after the index surgery. Nevertheless, as there were more infections with CHG compared to PVP in some subgroups, i.e. larger bowel surgery, hernia repair and genitourinary surgery, the authors conclude that there is no clear benefit of CHG over PVP for surgical scrub and skin preparation.
Observational studies
Two prospective and four retrospective observational studies [9, 33,34,35,36,37] were identified in the literature search (Table 2). Of the included studies only one was conducted in a LMIC. Lubega et al. [37] prospectively investigated the rate and risk factors of SSI after emergency surgery in a regional referral hospital in Uganda. All degrees of wound contamination were included and skin antiseptic agents used were PVP and CHG. Only eight children were included in the trial. Further analysis of the paediatric subgroup was not conducted. Overall, multivariate analysis did not show a significant difference between antiseptic agents used. There was only one further observational study recording the incidence of SSIs prospectively. Chiang et al. [36] investigated the clinical significance of positive cranial bone flap cultures and the associated risk of SSI after craniotomies or craniectomies. Both adults and children were included in the study. Unfortunately, the authors do not state how many paediatric patients were included. PVP solution and gel as well as CHG were used for preoperative skin preparation. Although application of PVP gel and solution resulted in significantly less SSI, the authors emphasize that this result may be biased due to the lack of data for some procedures and change of skin preparation policy during the study period. Four retrospective observational studies [9, 33,34,35] investigating risk factors for SSIs in children were identified in the literature search (Table 2). One of these exclusively investigated risk factors in neonates. Rojo et al. conducted a case–control study of 90 surgical in neonates with a mean age of 32.5 gw. CHG was the antiseptic agent used in most cases (PVP (n = 14), CHG (n = 70), other (n = 6)). Neither in this study nor in the other observational studies any significant differences between antiseptic agents used were identified.
Quality of evidence
The quality of the included RCTs was graded using the van Tulder scale [28] (Table 3) and Newcastle–Ottawa Scale [29] for observational studies (Table 4). It has to be noted, that some of the studies [31, 33]included in this review have been conducted in the 80ies. At this time infection control strategies were not yet implemented widely. There have been significant changes in outcome definitions as well as changes in products and surgical methodology since. Therefore, transferability of results is limited. The trial on preoperative skin preparation conducted by Meier et al. [38] only included 53 paediatric patients with an unusual cut-off age for paediatric patients (less than 13 years). It is not clear what the mean age in this group of patients was. Moreover, subgroup analysis was not carried out, due to low rate of wound infections in this group. As the trial was conducted in a LMIC with the goal to identify cost-effective measures to reduce SSI rates the selection of antiseptic agents is not representative for general paediatric surgery. Berry et al. [31] compared the use of PVP and CHG for surgical scrub and skin preparation. As the authors point out it is not clear whether differences in outcome are due to one or both interventions. Furthermore, only 1% of patients recruited can be considered paediatric. Thus, the significance of the results for this patient group is highly questionable. The observational studies identified in the search included a multitude of different antiseptic agents for surgical skin preparation. All studies included iodine-based solutions for surgical skin preparation. Four of six studies included CHG as alternative antiseptic agent. Overall patients were not allocated evenly to either surgical skin preparation with iodine or CHG (see Table 2). Furthermore, degree of contamination, type of surgical procedures and rate of prophylactic antibiotic therapy were not comparable among studies. All in all, the quality of evidence of included RCTs and observational studies was low to moderate and was not sufficient in order to conduct a meta-analysis. Level of evidence of the studies according to Oxford Centre for Evidence-Based Medicine was low (see Table 5) and does not allow for a recommendation of choice of antiseptic for preoperative skin preparation above level C [30].
Discussion
This systematic literature review confirms the lack of evidence on strategies for surgical skin preparation in children. In particular, there are no data to support the use of specific agents for high-risk groups, such as neonates and especially preterm babies.
Establishing safe and effective surgical care for children is a critical but neglected area within global surgery [7]. Simple preventive measures, such as optimal surgical hand and skin preparation and appropriate antibiotic prophylaxis have shown to significantly lower the rate of SSI independent of available resources and surgical procedure [39, 40]. Implementing and investigating standardized perioperative management programs in children does not only reduce postoperative morbidity and associated health-care costs. Standardized surgical skin preparation is an important part in the framework of antibiotic stewardship programs and as such can support adherence to other integral parts of perioperative management, such as antibiotic prophylaxis. Moreover, as patients experiencing SSI generally require antibiotic treatment, reducing the rate of SSI in children decreases the need for antibiotic treatment postoperatively.
Importantly, evidence from the adult population to paediatric perioperative management is not readily transferable. Although some may argue that school aged children and adolescents may be comparable to adult patients, this is definitely not the case in neonates, infants and toddlers. Skin absorption and fragility, wound healing and skin microbiome change depending on age and environment [41]. Children have a different spectrum of comorbidities, physiological characteristics and therefore also have different risk factors for SSIs [42]. Interventions proven to be effective in adults do not necessarily have the same impact in children and could possibly have unexplored adverse effects [43]. Skin toxicity and systemic absorption have been of special concern for many agents in use in neonatal surgery, such as chlorhexidine, povidone-iodine and alcoholic preparations [24]. Our findings underline the ongoing dilemma on choice of antiseptic agents for skin preparation in children. Not only in paediatric surgery [25], but also in neonatal skin care and before catheter insertion in children a variety of agents and concentrations are in use [44,45,46]. Ideally antiseptic agents used for skin preparation in children should have an antimicrobial effect, should have a quick onset of action and long residual effect without any or minimal toxic effects on the skin and the organ systems [24]. In adult patients, the latest literature review on the use of antiseptic solutions conducted by NICE in 2019 included 28 RCTs [15]. Like the WHO in 2016 [14], the authors conclude that alcoholic solutions are more effective in reducing the risk of SSIs and that specifically alcohol-based chlorhexidine gluconate has the lowest risk of SSIs occurring postoperatively. Both reviews state that evidence is of moderate to low quality. The RCTs including children in this field are of low quality [31, 38]. The observational studies available did not investigate differences in SSI rate between antiseptic agents as their primary objective [9, 33,34,35,36,37].
Antiseptic solutions have been investigated in other fields of skin antisepsis in children [24]. CHG has been recommended by the WHO for umbilical cord care in community and primary care settings in developing countries [47] and has shown to be superior to other agents in this setting [48]. Daily washings with CHG for critically ill children and application of CHG-containing solutions before central venous catheter insertion are other examples in which CHG has shown to be effective in the paediatric population [49, 50]. All in all, there is substantial evidence suggesting that CHG containing antiseptic skin preparations are effective in paediatric infection prevention and control [24]. However, comparative research to evaluate different agents is extremely rare.
As shown in this review evidence on the optimal choice of antiseptic solutions for preoperative skin preparation in children is scarce and of low quality. Though some may argue that it is lengthy and expensive to conduct randomized trials on perioperative measures in the paediatric population, it is necessary and as demonstrated by Renko et al. [51] possible. This RCT investigating the efficacy of different sutures supports the use of more costly triclosan-coated sutures because of their efficacy in reducing SSIs in this vulnerable population. While the efficacy of skin antiseptics may be comparable and their costs are generally low, their safety profiles may differ, influencing selection. Furthermore, even small efficacy advantages may translate into a large number of SSIs prevented. New innovative designs, such as point-of-care cluster randomization may allow for the efficient yet robust evaluation of perioperative care, including skin antiseptics, aiming to prevent common post-operative complications for paediatric surgical interventions.
Conclusion
The findings of this study confirm the lack of evidence on choice of antiseptic agent for surgical site preparation in children. Direct transfer of evidence from the adult population to paediatric surgery is not appropriate due to important differences in possible toxic local and systemic side effects, spectrum of comorbidities and type of surgery in children. Therefore, it is essential to conduct high quality RCT investigating interventions to identify optimal measures to reduce SSI rates in children.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APSIC:
-
Asia Pacific Society of Infection Control
- CDC:
-
Centers for Disease Control and Prevention
- CENTRAL:
-
Cochrane Central Register of Controlled Trials
- CHG:
-
Chlorhexidine gluconate
- CINHAL:
-
Cumulative Index to Nursing and Allied Health Literature
- EMBASE:
-
Excerpta Medica Database
- Gw:
-
Gestational week
- IQR:
-
Interquartile range
- LMICs:
-
Low- and Middle-income Countries
- Max. :
-
Maximal
- Min.:
-
Minimal
- NICE:
-
National Institute for Health and Care Excellence
- n.s.:
-
Not stated
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PVP:
-
Povidone-iodine
- RCT :
-
Randomized controlled trial
- SSI:
-
Surgical site infection
- U.S.:
-
United States
- WHO:
-
World Health Organization
References
Zingg W, Hopkins S, Gayet-Ageron A, Holmes A, Sharland M, Suetens C, et al. Health-care-associated infections in neonates, children, and adolescents: an analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect Dis. 2017;17(4):381–9.
Kulaylat AN, Rocourt DV, Tsai AY, Martin KL, Engbrecht BW, Santos MC, et al. Understanding readmissions in children undergoing surgery: A pediatric NSQIP analysis. J Pediatr Surg. 2018;53(7):1280–7.
World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection Geneva2018 [Available from: https://www.ncbi.nlm.nih.gov/books/NBK536404/.
Toltzis P, O’Riordan M, Cunningham DJ, Ryckman FC, Bracke TM, Olivea J, et al. A statewide collaborative to reduce pediatric surgical site infections. Pediatrics. 2014;134(4):e1174–80.
Feng C, Sidhwa F, Cameron DB, Glass C, Rangel SJ. Rates and burden of surgical site infections associated with pediatric colorectal surgery: insight from the National Surgery Quality Improvement Program. J Pediatr Surg. 2016;51(6):970–4.
Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J. 2004;1(4):247–73.
Rabbitts JA, Groenewald CB. Epidemiology of Pediatric Surgery in the United States. Pediatr Anesth. 2020;30(10):1083–90.
Davenport M, Doig CM. Wound infection in pediatric surgery: A study in 1,094 neonates. J Pediatr Surg. 1993;28(1):26–30.
Bucher BT, Guth RM, Elward AM, Hamilton NA, Dillon PA, Warner BW, et al. Risk factors and outcomes of surgical site infection in children. J Am Coll Surg. 2011;212(6):1033-8.e1.
Sharma LK, Sharma PK. Postoperative wound infection in a pediatric surgical service. J Pediatr Surg. 1986;21(10):889–91.
GlobalSurg C. Surgical site infection after gastrointestinal surgery in children: an international, multicentre, prospective cohort study. BMJ Glob Health. 2020;5(12): e003429.
Mullapudi B, Grabski D, Ameh E, Ozgediz D, Thangarajah H, Kling K, et al. Estimates of number of children and adolescents without access to surgical care. Bull World Health Organ. 2019;97(4):254–8.
Allegranzi B, Zayed B, Bischoff P, Kubilay NZ, de Jonge S, de Vries F, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288–303.
Allegranzi B, Bischoff P, de Jonge S, Kubilay NZ, Zayed B, Gomes SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e276–87.
NICE. Effectiveness of preoperative skin antiseptics in the prevention of surgical site infection. Surgical site infection: prevention and treatment. London: National Institute for Health and Care Excellence (UK); 2019.
Ling ML, Apisarnthanarak A, Abbas A, Morikane K, Lee KY, Warrier A, et al. APSIC guidelines for the prevention of surgical site infections. Antimicrob Resist Infect Control. 2019;8:174.
Adler AL, Martin ET, Cohen G, Jeffries H, Gilbert M, Smith J, et al. A comprehensive intervention associated with reduced surgical site infections among pediatric cardiovascular surgery patients, including those with delayed closure. Journal of the Pediatric Infectious Diseases Society. 2012;1(1):35–43.
Jha P, Woodward C, Pietz C, Manzo J, Gardner HM. Sternal wound infection prevention project for delayed sternal closure in congenital heart surgery. World Journal for Pediatric and Congenital Hearth Surgery. 2018;9 (2):NP22.
Kalangu KKN, Esene IN, Dzowa M, Musara A, Ntalaja J, Badra AK. Towards zero infection for ventriculoperitoneal shunt insertion in resource-limited settings: a multicenter prospective cohort study. Childs Nerv Syst. 2020;36(2):401–9.
Ryan SL, Sen A, Staggers K, Luerssen TG, Jea A. A standardized protocol to reduce pediatric spine surgery infection: A quality improvement initiative: Clinical article. J Neurosurg Pediatr. 2014;14(3):259–65.
Schaffzin JK, Simon K, Connelly BL, Mangano FT. Standardizing preoperative preparation to reduce surgical site infections among pediatric neurosurgical patients. J Neurosurg Pediatr. 2017;19(4):399–406.
Waseem H, Zaman R, Mazzamurro R, Fisher A, Bhowmik S, Bauer D. Use of a preoperative bundle to prevent pediatric neurosurgical infections. J Neurosurg. 2017;126(4):A1430.
Woodward CS, Son M, Taylor R, Husain SA. Prevention of Sternal Wound Infection in Pediatric Cardiac Surgery: A Protocolized Approach. World Journal for Pediatric and Congenital Hearth Surgery. 2012;3(4):463–9.
Sathiyamurthy S, Banerjee J, Godambe SV. Antiseptic use in the neonatal intensive care unit - a dilemma in clinical practice: An evidence based review. World J Clin Pediatr. 2016;5(2):159–71.
Ng AL, Jackson C, Kazmierski M. Evaluation of Antiseptic Use in Pediatric Surgical Units in the United Kingdom-Where Is the Evidence Base? Eur J Pediatr Surg. 2016;26(4):309–15.
Carr S, Gogal C, Afshar K, Ting J, Skarsgard E. Optimizing skin antisepsis for neonatal surgery: A quality improvement initiative. Journal of Pediatric Surgery. 2022.
Aitken J, Williams FLR. A systematic review of thyroid dysfunction in preterm neonates exposed to topical iodine. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2014;99(1):F21–8.
van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine (Phila Pa 1976). 2003;28(12):1290–9.
Wells G, Shea B, O'Connell D, Peterson j, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. ᅟ. 2000;ᅟ.
OCEBM Levels of Evidence Working Group*. “The Oxford Levels of Evidence 2”. Oxford Centre for Evidence-Based Medicine.; [Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
Berry AR, Watt B, Goldacre MJ, Thomson JW, McNair TJ. A comparison of the use of povidone-iodine and chlorhexidine in the prophylaxis of postoperative wound infection. J Hosp Infect. 1982;3(1):55–63.
Meier DE, Nkor SK, Aasa D, OlaOlorun DA, Tarpley JL. Prospective randomized comparison of two preoperative skin preparation techniques in a developing world country. World J Surg. 2001;25(4):441–3.
McCray E, Martone WJ, Wise RP, Culver DH. Risk factors for wound infections after genitourinary reconstructive surgery. Am J Epidemiol. 1986;123(6):1026–32.
Bashyal RK, Chu JY, Schoenecker PL, Dobbs MB, Luhmann SJ, Gordon JE. Complications after pinning of supracondylar distal humerus fractures. J Pediatr Orthop. 2009;29(7):704–8.
Rojo R, Fanjul M, Garcia-Casillas MA, Corona C, Tardaguila AR, Zornoza M, et al. Surgical wound infections in newborns: analysis of risk factors. Cir Pediatr. 2012;25(3):129–34.
Chiang HY, Steelman VM, Pottinger JM, Schlueter AJ, Diekema DJ, Greenlee JDW, et al. Clinical significance of positive cranial bone flap cultures and associated risk of surgical site infection after craniotomies or craniectomies Clinical article. J Neurosurg. 2011;114(6):1746–54.
Lubega A, Joel B, Justina Lucy N. Incidence and Etiology of Surgical Site Infections among Emergency Postoperative Patients in Mbarara Regional Referral Hospital, South Western Uganda. Surgery Research and Practice. 2017;2017 (no pagination).
Meier DE, Nkor SK, Aasa D, OlaOlorun DA, Tarpley JL. Prospective randomized comparison of two preoperative skin preparation techniques in a developing world country. World J Surg. 2001;25(4):441–3.
Allegranzi B, Aiken AM, Zeynep Kubilay N, Nthumba P, Barasa J, Okumu G, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before–after, cohort study. Lancet Infect Dis. 2018;18(5):507–15.
Ntumba P, Mwangi C, Barasa J, Aiken A, Kubilay Z, Allegranzi B. Multimodal approach for surgical site infection prevention – results from a pilot site in Kenya. Antimicrobial resistance and infection control. 2015;4(Suppl 1):P87-P.
Prescott SL, Larcombe DL, Logan AC, West C, Burks W, Caraballo L, et al. The skin microbiome: impact of modern environments on skin ecology, barrier integrity, and systemic immune programming. The World Allergy Organization journal. 2017;10(1):29.
Bucher BT, Guth RM, Elward AM, Hamilton NA, Dillon PA, Warner BW, et al. Risk factors and outcomes of surgical site infection in children. Journal of the American College of Surgeons. 2011;212(6):1033–8e.1.
Contopoulos-Ioannidis DG, Baltogianni MS, Ioannidis JP. Comparative effectiveness of medical interventions in adults versus children. The Journal of pediatrics. 2010;157(2):322–30 e17.
Datta MK, Clarke P. Current practices in skin antisepsis for central venous catheterisation in UK tertiary-level neonatal units. Arch Dis Child Fetal Neonatal Ed. 2008;93(4):F328.
Tamma PD, Aucott SW, Milstone AM. Chlorhexidine use in the neonatal intensive care unit: results from a national survey. Infect Control Hosp Epidemiol. 2010;31(8):846–9.
Shah D, Tracy M. Skin antisepsis survey in Australia-New Zealand neonatal nurseries. J Paediatr Child Health. 2013;49(7):601–2.
WHO. Recommendations on Postnatal Care of the Mother and Newborn Geneva: World Health Organization; 2013 [Executive summary]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK190090/.
Imdad A, Bautista RMM, Senen KAA, Uy MEV, Mantaring Iii JB, Bhutta ZA. Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database of Systematic Reviews. 2013(5).
Milstone AM, Elward A, Song X, Zerr DM, Orscheln R, Speck K, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–106.
NP O'Grady M Alexander LA Burns EP Dellinger J Garland SO Heard et al 2011 Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 52 9 1087 1099
Renko M, Paalanne N, Tapiainen T, Hinkkainen M, Pokka T, Kinnula S, et al. Triclosan-containing sutures versus ordinary sutures for reducing surgical site infections in children: a double-blind, randomised controlled trial. Lancet Infect Dis. 2017;17(1):50–7.
Acknowledgements
Not applicable.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: IB, US, JB.; data collection: IB; analysis and interpretation of results: IB and JB; manuscript preparation: IB, revision of manuscript for important intellectual content: JB and US. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bielicki, I., Subotic, U. & Bielicki, J.A. Systematic literature review on surgical site preparation in paediatric surgery. BMC Pediatr 22, 455 (2022). https://doi.org/10.1186/s12887-022-03502-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03502-z