Abstract
Background
Screening of critical congenital heart disease (CCHD) using pulse oximetry is a routine procedure in many countries, but not in Indonesia. This study aimed to evaluate the feasibility of implementing CCHD screening with pulse oximetry for newborns in Yogyakarta, Indonesia.
Methods
A cross-sectional study was conducted at four hospitals in Yogyakarta, Indonesia. Newborns aged 24–48 hours who met the inclusion criteria were screened on the right hand and left or right foot using a pulse oximeter. Positive results were indicated by: either (1) SpO2 level < 90% in one extremity, (2) SpO2 level of 90–94% in both right hand and either foot on three measurements conducted 1 hour apart, or (3) a saturation difference > 3% between the upper and lower extremity on three measurements conducted 1 hour apart. Positive findings were confirmed by echocardiography.
Results
Of 1452 newborns eligible for screening, 10 had positive results and were referred for echocardiographic evaluation. Of those, 8 (6 per 1000 live birth, 8/1452) had CCHD. Barriers found during screening processes were associated with hospital procedures, equipment, healthcare personnel, and condition of the newborn.
Conclusion
Pulse oximetry screening might be feasible to be implemented within the routine newborn care setting for CCHD in Indonesia. In order to successfully implement pulse oximetry screening to identify CCHD in Indonesia, the barriers will need to be addressed.
Similar content being viewed by others
Background
Congenital heart disease (CHD) is the most common congenital abnormality in newborns [1] with a reported incidence of 4 to 50 per 1000 live births [2, 3]. Approximately 25% of CHD are classified as critical congenital heart disease (CCHD), that are often lethal and require immediate transcatheter or surgical intervention in the first year of life [4]. Furthermore, CHD is responsible for over 260,000 deaths annually worldwide [5] with a CCHD associated mortality count of 34.8% in developing countries [6]. Challenges primarily exist in early detection of CCHD, with some CCHD newborns prematurely sent home before diagnosis, since they may appear healthy at first. This challenge is considerably noticeable in resource limited settings.
In Indonesia, approximately 2.5 per 1000 live births suffer from CHD [7]. A significant delay in CHD diagnosis is seen in 6 out of 10 cases, most with severe complications [8]. Additionally, one-third of the newborns with CCHD were not detected before discharge [9]. Pulse oximetry screening for CCHD has been recommended and widely implemented in many countries, leading to a significant reduction in mortality among newborns with CCHD. Furthermore, unnecessary costs related to complications due to late diagnosis of CCHD can be avoided [10]. Studies on the feasibility of pulse oximetry screening to detect CCHD have been conducted in low- and middle-income country setting including South Africa [11], India [12], Sri Lanka [13] and Brazil [14]. Despite the importance shown in the immediate detection of CCHD, no screening program has been implemented in Indonesia, contributing to the often presentation of late and even terminal cases at tertiary hospitals. Therefore, this study aimed to evaluate the feasibility of CCHD screening using pulse oximetry and provide relatable evidence for local and national policymakers in implementing pulse oximetry screening program in Indonesia.
Methods
A cross-sectional study was conducted at four hospitals in Yogyakarta, Indonesia from August 1st, 2021, to November 30th, 2021. The hospitals were: Dr. Sardjito a class A, tertiary referral hospital; JIH a class B, general hospital; and Sadewa and Sakina Idaman, both class Cs maternal and neonatal care specialty hospitals. All seemingly healthy newborns were included, and those born at < 35 weeks’ gestation age, prenatally diagnosed with CHD, carrying dysmorphic features or signs of cardiovascular abnormalities such as cyanosis, cardiac murmur or those with abnormal vital signs were excluded [15, 16].
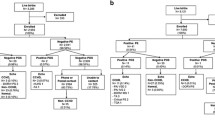
Pulse oximetry screening was performed using the American Academy of Pediatrics (AAP) standardized algorithm by measuring oxygen saturation of the right hand and the left or right foot between 24 and 48 hours of age or before 24 hours of age if the baby is discharged early. Screening of CCHD was considered negative or passed if measurement of SpO2 was > 95% for both the right hand and right or left foot, with a difference of < 3% between the right hand and either foot. No further cardiac evaluation was performed in these subjects unless indicated by subsequent clinical condition(s). Screening was considered positive or failed if at least one of the following: (1) SpO2 level < 90% in one extremity, (2) SpO2 level of 90–94% in both right hand and either foot on three measurements conducted 1 hour apart, or (3) a saturation difference > 3% between the upper and lower extremity on three measurements conducted 1 hour apart [17]. Subjects failing the screening were referred to Dr. Sardjito Hospital for echocardiographic evaluation. The algorithm of study is presented in Fig. 1.
Algorithm of study protocol (Adapted from the protocol in Ewer et al. [17])
Screening was performed by a healthcare worker, which included either a doctor, nurse, or midwife in charge. The measurement was written manually in case report form. Training of healthcare workers was conducted prior to the study to avoid variability in screening procedures. To compensate for the variability of available oximeter types among hospitals, we performed an agreement correlation before recruitment using three types of pulse oximeters: Massimo (Massimo Corporation, Irvine, CA, USA), Mindray (Mindray Cooperation, Nanshan, Shenzhen, China) and fingertip.
Data were analyzed using STATA version 12.1 (StataCorp, College Station, Texas, USA) and presented appropriately. Descriptive statistics were presented as numbers and percentages, mean or medians.
A semi-structured interview on barriers experienced by the medical personnel throughout the screening process was also conducted. The qualitative data were then reviewed, defined and presented thematically based on the common barriers.
Ethics
The Medical and Health Research Ethics Committee, of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia has approved this study (230/UN.1/FKKMK.3/IKA.2/TU/PT.01.04/2021). Informed consent to participate in the study was obtained from the parents or legal guardians of participants.
All experiment protocols involving humans were in accordance with national/international/institutional guidelines or the Declaration of Helsinki.
Results
Throughout the study period, there were 2631 newborns delivered at the four selected hospitals. From 118 newborns who were ineligible, 89 were < 35-weeks’ gestation age, 10 passed away, 14 were prenatally confirmed with CHD and 5 had dysmorphic features. A total of 1452 (57.7%) from the remaining 2513 eligible newborns were then screened (Fig. 2). Of the 1452 babies screened, 7 babies had positive results at the first screening and only 5 (0.3%) needed a second screening. Two of the babies passed the second screening. The third screening results were positive for all of the remaining three babies. Of those, 10 had positive results and were referred for further echocardiography confirmation, finally resulting in 8 (0.6%) subjects with CCHD. The screening was performed within a period of ≤24 hours after birth in 855 subjects (59%) and after 24 hours in 597 (41%) subjects.
Oxygen supplementation was promptly administered in cases where newborns were visibly bluish or when desaturation of SpO2 levels or signs of respiratory distress were apparent. These frequently occurred soon after birth (less than 24 hours) and was most commonly caused by asphyxia, pulmonary hypertension of the newborn, or other pulmonary problems. The healthcare workers did not include these neonates for screening.
Most screening was performed using the standardized pulse oximetry (Massimo). The agreement among Massimo and Fingertip pulse oximetry was 0.815, while the agreement among Massimo and Mindray pulse oximetry was 0.943.
The baseline characteristics of the eligible newborns are presented in Table 1. Echocardiography results performed in the 10 newborns with positive screening are shown in the Table 2.
Main barriers during process of pulse oximetry screening are shown in Table 3.
Discussion
This study explored the feasibility of implementing CCHD screening with pulse oximetry for 1452 newborns in Yogyakarta, Indonesia. The results of the study indicate that pulse oximetry screening might be feasible to be implemented within the routine newborn care setting for CCHD in Indonesia.
The prevalence of CCHD in our study was 6 of 1000 live births, with positive CCHD screens occurring in 8/1452 (0.6%) of newborns. This was higher than previously reported in Sri Lanka 0.16% (14/8718), [13], India 0.16% (3/1855) [12], Turkey 0.12% (12/10,200) [18], Morocco 0.06% (5/8013) [19], the Netherlands 0.02% (5/23,959) [20], New Zealand 0.02% (3/16,644) [21], South Africa 0.01% (1/1001) [11] and the United States (US) 0.01% (1/6745) [16].
A meta-analysis of 21 studies involving 457,202 participants concluded that pulse oximetry is a highly specific and moderately sensitive test for detection of CCHD with very low false-positive rates [22]. Pulse oximetry screening has been successfully implemented in high-income countries and has led to a significant reduction in CCHD related deaths. A study showing results from a 6 years evaluation (2007–2013) after implementation of pulse oximetry screenings across the United States found a 33.4% (95% CI: 10.6–50.3%) reduction in CCHD deaths per 100,000 births, with a further potential reduction of 120 infant deaths per year from CCHD [23].
Several countries that have already conducted CCHD screening programs with pulse oximetry such as the US, China, the Netherlands, and the United Kingdom have indicated the practice combined with clinical assessment is beneficial and cost-effective [10]. Through the program, costs for treating complications due to the late diagnosis of CCHD can be avoided. A US study reported the screening program saves 20 infants annually, with an equivalent cost of $40,385 per life-year gained under base case assumptions that each screening would cost $6.28 per newborn [24].
Meanwhile, low- and middle-income countries still must face several barriers to be able to execute the pulse oximetry screening program. One study in Morocco revealed barriers such as the tendency to discharge healthy newborns before 24 hours, and the difficulty in confirming positive screening results due to the lack of available echocardiographs in several hospitals [19]. Other reported challenges in implementing pulse oximetry screening include acceptance of the program, timing of screening and significance of false positives rate, and response to positive screen results [25].
In our study, we classified barriers found during screening into four concerns. The first concerns involve hospital procedures or workflow. The AAP recommends screenings should be done within 24–48 hours of age. Adversely, most subjects in our study were screened before 24 hours due to the relatively short postnatal length of stay for healthy babies decided in hospital procedures. The timing of screening should be considered since it will influence the screening results. A previous study revealed that the measurement of saturation before 24 hours of age will increase the false positive or false negative rate [20]. The transition from fetal to neonatal circulation and stabilization of systemic oxygen saturation levels might explain this finding. A New Zealand study revealed that a midwifery-led maternity setting characterized by early discharge, influenced the time of testing, effecting saturation levels [21].
The second barrier involves the scarcity of standardized neonatal pulse oximeters, with devices readily available in neonatal intensive care units or observation rooms for monitoring sick newborns, but not in postnatal wards. Only some pulse oximeters were equipped with tightly fixed sensors using Velcro or rubber fasteners which are easier to use compared to finger-type devices where the pulses tend to be difficult to detect and take longer to read. Owing to limited sources, some hospitals even resorted to using adult probes for newborns. The type of probe can affect the effectiveness of examinations. The US Food and Drugs Administrator (FDA) stated three recommendations in using a pulse oximeter: (1) be aware to the factors that can affect the accuracy of a pulse oximeter reading, (2) understand the particular brand and sensor by referring to the device labelling or manufacture’s website, and (3) always consider accuracy limitations when using a pulse oximeter to assist in diagnosis and treatment. Knowing these recommendations is important in understanding the risk of measurement inaccuracy and providing the highest outcomes [26]. Nevertheless, a recent study revealed that a pulse oximetry device provided good accuracy in ruling out hypoxemia in comparison to saturation reading by arterial blood gas sample [27].
The third barrier is related with the condition of the baby. During our study, some newborns were constantly crying or moving, posing a challenge in the application and assessment of pulse oximetry. It is recommended that infants should be fully awake but settled during the screening process, since deep sleep may result in hypoventilation and low saturation results [28]. A previous study in New Zealand showed that newborns that were asleep or unsettled during screening were less likely to have positive results than those who were awake but settled [21].
The fourth and last of the barriers is the lack of healthcare personnel in the postnatal ward. The healthcare providers were occupied with other clinical duties and sometimes forgot the screening protocol. Some did not consider the pulse oximetry measurement to be within the scope of their practice due to low motivation because no incentive was given. A study in South Africa reported that most of the nurses involved in the study were satisfied with the purpose and aim of the study, but they do not have enough time to do the screening since their workloads were already heavy [11]. A study in New Zealand stated that most of midwives agreed that pulse oximetry screening was beneficial, but their already heavy workload prevented them from routinely performing screens. This was one of their concerns regarding the implementation of pulse oximetry as a universal screening program [29].
Indonesia has a large annual live birth rate, at 5 million per year with around 62.7% deliveries commonly assisted by midwives. As many as 79% of women gave birth at health care centers, with around 16% giving birth at home [30]. Nevertheless, Indonesia still lacks any national program for CCHD screening [8]. Pulse oximetry fulfils the criteria for mass screening. It is very effective, low cost and can significantly reduce morbidities and mortality by providing earlier detection of CHD. However, to achieve these goals optimally in a setting where resources are limited is challenging, though not impossible. These goals require extensive standardized training for healthcare providers who work directly in childbirth and newborn care (midwives, nurse, and general practitioner), the measurement protocols need formal regulations and the involvement of policy makers such as health ministries and the pediatric cardiology society to make pulse oximetry screening a recommendation in the standard care of newborns. Further, in order to develop an appropriate system for home birth, the timing of administration of pulse oximetry might need to be altered since a community midwife leaves approximately several hours after an uncomplicated home birth. Extensive training for community midwives and providing each midwife with a handheld pulse oximeter also need to be conducted. However, in order to make this approach works, an appropriate regional system to support the use of pulse oximetry in individual home births should be developed.
In order to optimize the impact of pulse oximetry screening in low-middle income countries, Zheleva et al. summarized several recommendations to be considered including the assessment of referral CHD health services, assessment of birth delivery center processes and staff training needs, financial burden and implementation of CCHD screening process as part of the overall patient care continuum [31]. In principle and practice, pulse oximetry screening is relatively simple, inexpensive, and easy to implement. However, screening is just one step in a lifelong continuum of management for the child diagnosed with CCHD and their family. If a child has a positive screening for CCHD, they require immediate access to definitive diagnosis, safe transportation, and surgical and interventional cardiology services. Newborn screening cannot save as many lives as it should if high quality cardiac services are not available to the child and family following a positive screen. It will help detect cases, but many will not survive or will live a life with serious disability.
The major limitations of our study involve the high proportion of newborns who were not screened over the study period due to the many aforementioned reasons and there was no report provided on parent’s acceptance of and uptake of the pulse oximetry screening for CCHD. This study was also limited by the use of three different types of pulse oximeters from three different manufacturers as well as the use of an adult sensor. Despite the limitations, our study is among the first reports of the feasibility of CCHD screening using pulse oximetry in Indonesia and provides the local evidence of barrier perspectives from healthcare workers during the screening process. The findings can be used as available local evidence for policymakers before recommending needed changes to the national screening program.
Conclusions
Pulse oximetry screening might be feasible to be implemented within the routine newborn care for detection of CCHD in Indonesia. In order to successfully implement pulse oximetry screening to identify CCHD in Indonesia, the barriers will need to be addressed.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AAP:
-
American Academy of Pediatric
- ASD:
-
Atrial Septal Defect
- CHD:
-
Congenital Heart Disease
- CCHD:
-
Critical Congenital Heart Disease
- DORV:
-
Double Outlet Right Ventricle
- PDA:
-
Patent Ductus Arteriosus
- PFO:
-
Patent Foramen Ovale
- TGA:
-
Transposition of the Great Aorta
- VSD:
-
Ventricle Septal Defect
References
Bernier P, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26–34. https://doi.org/10.1053/j.pcsu.2010.02.005.
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–7. https://doi.org/10.1016/j.jacc.2011.08.025.
Mahle WT, Newburger JW, Matherne GP, Smith FC, Hoke TR, Koppel R, et al. On behalf of the American Heart Association congenital heart defects Committee of the Council on cardiovascular disease in the young, council on cardiovascular nursing, and interdisciplinary council on quality of care and outcomes research; and the American Academy of Pediatrics section on cardiology and cardiac surgery, and committee on fetus and newborn. Role of pulse oximetry in examining newborns for congenital heart disease: a scientific statement from the American Heart Association and American Academy of Pediatrics. Pediatrics. 2009;124(2):823–36. https://doi.org/10.1542/peds.2009-1397.
Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5):1502–8. https://doi.org/10.1542/peds.2012-3435.
Zimmerman MS, Smith AGC, Sable C, Echko MM, Wilner LB, Olsen HE, et al. Global, regional, and national burden of congenital heart disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. 2020;4(3):185–200. https://doi.org/10.1016/S2352-4642(19)30402-X.
Bah MNM, Sapian MH, Jamil MT, Alias A, Zahari N. Survival and associated risk factors for mortality among infants with critical congenital heart disease in a developing country. Pediatr Cardiol. 2018;39:1389–96. https://doi.org/10.1007/s00246-018-1908-6.
BPS, Ministry of Health (Kemenkes) et al. Indonesia Demographic and Health Survey 2017. Jakarta, Indonesia: BKKBN, BPS, Kemenkes, and ICF 2018. Available at http://dhsprogram.com/pubs/pdf/FR342/FR342.pdf. Accessed 10 Des 2021.
Murni IK, Wirawan MT, Patmasari L, Sativa ER, Arafuri N, Nugroho S, et al. Delayed diagnosis in children with congenital heart disease: a mixed-method study. BMC Pediatr. 2021;21:191. https://doi.org/10.1186/s12887-021-02667-3.
Fixler DE, Xu P, Nembhard WN, Ethen MK, Canfield MA. Age at referral and mortality from critical congenital heart disease. Pediatrics. 2014;134(1):e98–e105. https://doi.org/10.1542/peds.2013-2895.
Narayen I, te Pas A, Blom N, van den Akker-van MM. Cost-effectiveness analysis of pulse oximetry screening for critical congenital heart defects following homebirth and early discharge. Eur J Pediatr. 2019;178(1):97–103. https://doi.org/10.1007/s00431-018-3268-x.
Niekerk AMV, Cullis RM, Linley LL, Zuhlke L. Feasibility of pulse oximetry pre-discharge screening implementation for detecting critical congenital heart lesions in newborns in a secondary-level maternity hospital in the western cape, South Africa: the “POPSICLe” study. S Afr Med J. 2016;106(8):817–21. https://doi.org/10.7196/samj.2016.v106i8.10071.
Gopalakrishnan S, Karmani S, Pandey A, Singh N, Kumar JR, Praveen R, et al. Pulse oximetry screening to detect critical congenital heart diseases in asymptomatic neonates. Med J Armed Forces India. 2021;77(2):214–9. https://doi.org/10.1016/j.mjafi.2020.09.004.
Gamhewage NC, Perera KSY, Weerasekera M. Effectiveness of newborn pulse oximetry screening for the identification of critical congenital heart disease in a tertiary care hospital in Sri Lanka. Sri Lanka J Child Health. 2021;50(4):699–703. https://doi.org/10.4038/sljch.v50i4.9890.
de Araujo JSS, Regis CT, Gomes RG, Mourato FA, Mattos S. Impact of telemedicine in the screening for congenital heart disease in a center from Northeast Brazil. J Trop Pediatr. 2016;62(6):471–6. https://doi.org/10.1093/tropej/fmw033.
Schwartz BN, Hom LA, Von Kohorn I, Becker J, Cuzzi SS, Martin GR, et al. Newborn pulse oximetry screening at a community hospital: an 8-year experience. Pediatrics. 2021;143(3):e2020049847. https://doi.org/10.1542/peds.2020-049847.
Bradshaw E, Cuzzi S, Kiernan S, Nagel N, Becker J, Martin G. Feasibility of implementing pulse oximetry screening for congenital heart disease in a community hospital. J Perinatol. 2012;32(9):710–5. https://doi.org/10.1038/jp.2011.179.
Ewer AK, Martin GR. Newborn pulse oximetry screening: which algorithm is best? Pediatrics. 2016;138(5):e20161206. https://doi.org/10.1542/peds.2016-1206.
Özalkaya E, Akdağ A, Sen I, Cömert E, Melek YH. Early screening for critical congenital heart defects in asymptomatic newborns in Bursa province. J Matern Fetal Neonatal Med. 2015;29(7):1–3. https://doi.org/10.3109/14767058.2015.1035642.
Slitine NEI, Bennoui F, Sable CA, Martin GR, Hom LA, Fadel A, et al. Pulse oximetry and congenital heart disease screening: results of the first pilot study in Morocco. Int J Neonatal Screen. 2020;6(3):53. https://doi.org/10.3390/ijns6030053.
Narayen IC, Blom NA, Geloven N, Blankman EIM, van den Broek AJM, Bruijn M, et al. Accuracy of pulse oximetry screening for critical congenital heart defects after home birth and early postnatal discharge. J Pediatr. 2018;197:29–35. https://doi.org/10.1016/j.jpeds.2018.01.039.
Cloete E, Gentles TL, Webster DR, Davidkova S, Dixon LA, Alsweiler JM, et al. Pulse oximetry screening in a midwifery-led maternity setting with high antenatal detection of congenital heart disease. Acta Paediatr. 2020;109(1):100–8. https://doi.org/10.1111/apa.14934.
Plana MN, Zamora J, Suresh G, Fernandez-Pineda L, Thangaratinam S, Ewer AK. Pulse oximetry screening for critical congenital heart defects (review). Cochrane Database Syst Rev. 2018;3(3):1–77. https://doi.org/10.1002/14651858.CD011912.pub2.
Abouk R, Grosse S, Ailes E, Oster M. Association of US state implementation of newborn screening policies for critical congenital heart disease with early infant cardiac deaths. JAMA. 2017;318(21):2111–8. https://doi.org/10.1001/jama.2017.17627.
Peterson C, Grosse SD, Oster ME, Olney RS, Cassell CH. Cost-effectiveness of routine screening for critical congenital heart disease in US newborns. Pediatr. 2015;132(3):e595–603. https://doi.org/10.1542/peds.2013-0332.
Kluckow M. Barriers to the implementation of newborn pulse oximetry screening: a different perspective. Int J Neonatal Screen. 2018;4(1):4. https://doi.org/10.3390/ijns4010004.
FDA: Pulse oximeter accuracy and limitations FDA safety communication https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication (2021). Accessed 19 January 2022.
Harskamp RE, Bekker L, Himmelreich JCL, Clercq LD, Karregat EPM, Sleeswijk ME, et al. Performance of popular pulse oximeters compared with simultaneous arterial oxygen saturation or clinical-grade pulse oximetry: a cross-sectional validation study in intensive care patients. BMJ Open Respir Res. 2021;8(1):e000939. https://doi.org/10.1136/bmjresp-2021-000939.
Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, et al. Strategies for implementing screening for critical congenital heart disease. Pediatr. 2012;128(5):e1259–67. https://doi.org/10.1542/peds.2011-1317.
Ward K, Dixon L, Cloete E, Gentles T, Bloomfield F. Health professionals’ views of newborn pulse oximetry screening in a midwifery-led maternity setting. “It’s a good thing to do, but fund it!”. Midwifery. 2020;81:102593. https://doi.org/10.1016/j.midw.2019.102593.
Badan Penelitian dan Pengembangan Kesehatan Kementrian Kesehatan RI. Riset Kesehatan Dasar (RISKESDAS) 2018. Jakarta: 2018. http://repository.bkpk.kemkes.go.id/3514/1/Laporan%20Riskesdas%202018%20Nasional.pdf. Accessed 11 June 2022.
Zheleva B, Nair SM, Dobrzycka A, Saarinen A. Consideration for newborn screening for critical congenital heart disease in low- and middle-income countries. Int J Neonatal Screen. 2020;6(2):49. https://doi.org/10.3390/ijns6020049.
Acknowledgements
We gratefully acknowledge all nurses, doctors, and especially Dr. Ekawaty Luthfia Haksari, Dr. Setya Wandita, Dr. Alifah Anggraini, Dr. Elysa Nur Safrida, Dr. Desy Rusmawatiningtyas, Dr. Nini Rahmani Azis, Dr. Dwikisworo Setyowireni, Dr. Braghmandita Widya I, Dr. Kristia Hermawan, and Dr. Sari Kusumastuti for helping in data collection. We also thank Erik C. Hookom, BA, MEd, TEFL and Dr. Endy W Putra for reviewing the draft manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception or the design of the study. IKM, DP, LP were major contributors in writing the manuscript. DP collected the data. IKM, DP, LP analyzed the data. All authors interpreted the data. All authors read, critically revised and approved the final manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Medical and Health Research Ethics Committee, of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia has approved this study (230/UN.1/FKKMK.3/IKA.2/TU/PT.01.04/2021). Informed consent to participate in the study was obtained from the parents or legal guardians of participants.
All experiment protocols involving humans was in accordance with national/international/institutional guidelines or the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Murni, I.K., Wibowo, T., Arafuri, N. et al. Feasibility of screening for critical congenital heart disease using pulse oximetry in Indonesia. BMC Pediatr 22, 369 (2022). https://doi.org/10.1186/s12887-022-03404-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03404-0