Abstract
Background
Group A Streptococcus has been recognized as an important human pathogen and it remains among the top ten causes of mortality from an infectious disease. Group A Streptococcus throat carriage plays an important role in the development of infection and transmission to contacts. In Ethiopia, there is little information about screening of children for group A Streptococcus carriage.
Objective
This study was aimed to assess the magnitude of throat carriage, associated factors, and antimicrobial susceptibility pattern of group A Streptococcus among healthy school children in Jigjiga city, Eastern Ethiopia from 12 April to 27 May 2021.
Method
A cross-sectional study was conducted enrolled by simple random sampling. Data on socio-demographic and related characteristics were gathered using pretested structured questionnaire. The throat sample was collected from 462 healthy school children and immediately transported to Jigjiga University Sultan Sheik Hassan referral hospital laboratory for investigation. Identification of group A Streptococcus was done by colony characterstics, gram staining, catalase negativity, bacitracin sensitivity, and Pyrrolidonyl arylamidase tests. Antibiotic susceptibility test was done on Muller-Hinton agar containing 5% sheep blood by modified Kirby-Bauer disc diffusion method. The data were coded, cleaned, and entered onto EpiData Version 3.1 then exported to SPSS version 26.0 for analysis. Bivariate and multivariable logistic regression through adjusted odds ratio (AOR) was used to determine the relationship between culture-positivity rates of GAS and predictor variables. A p-value < 0.05 was taken as statistically significant on multivariable analysis.
Results
The overall prevalence of group A Streptococcus throat culture rate was 10.6% (95%CI; 8.1%—13.7%). Previous family member who had a sore throat, children living with larger families (more than 11 members), and children living with non-immediate families were significantly associated with culture-positivity rates of GAS. Children who live with a family member with a sore throat compared with those who lived with in a family with no history of sore throat (AOR = 2.51; 95%CI 1.09–5.73), children who live with a large family comared to children living in families with less members (AOR = 4.64; 95% CI 1.53–14.1), and children who live with non-immediate families compared to children living with their mothers (AOR = 3.65; 95% CI 1.39 – 9.61), showed significant association with group A Streptococcus carriage rate. Resistance to all other antibiotics tested was low (< 5%). Multidrug resistance was found in 4.1% of isolates.
Conclusion
The present study showed 10.6% throat carriage of group A Streptococcus. Family member with a sore throat, having a large family, and living with non-immediate families have all been identified as independent predictors of carriage prevalence.
Similar content being viewed by others
Introduction
Group A Streptococcus (GAS) has been an important human pathogen since the early days of modern microbiology, and it is still among the top ten causes of mortality from an infectious disease [1]. All diseases caused by GAS are most common in settings of poverty, where living conditions promote transmission of the organism, and prevention and treatment programs are less likely to be present or effective [2].
Group A Streptococcus can infect people of any age, however children are more likely to be infected [3]. Group A Streptococcus is responsible for a wide range of clinical Syndrome, including impetigo and pharyngitis, as well as more serious disorders such as streptococcal toxic shock syndrome (STSS), meningitis, pneumonia, or cellulitis etc. Furthermore, autoimmune diseases such as acute rheumatic fever (ARF) can be triggered by recurrent episodes of GAS infection [4].
According to global disease burden figures in 2005, WHO ranked GAS as the ninth leading cause of human mortality, with the majority of deaths attributable to invasive GAS infections and rheumatic heart disease (RHD) [2]. The prevalence of GAS disease is estimated at > 18.1 million cases with an incidence of > 1.78 million cases per year [1, 5]. The most common infection caused by GAS is pharyngitis in children between 5 and 15 years of age [6]. It is responsible for approximately 15–30% of cases of pharyngitis in children [7]. Failure to eradicate streptococci from the pharynx occurs in about one-third of non-treated cases, giving rise to the carrier status in those individuals [8]. Untreated GAS pharyngitis may trigger ARF and its sequela, RHD, remain important public health problems in low and middle-income countries [1, 9].
The existence of the carrier state is reported to be as high as 15–20% in previous studies [10]. Carriers of GAS may represent a potential source for the acquisition of infections for other children and adults [11, 12]. According to a review article published by Oliver and colleagues in 2018, reported 10.5% and 5.9% pooled prevalence of GAS carriage in children from high-income countries and children from low/middle-income countries, respectively [13]. A few studies have been done in Africa on the GAS carriage which ranged around 9.0% [8].
Crowding, limited access to hygiene, inadequate medical care, housing quantity and quality, healthcare access and quality, education, or economic advantage are all risk factors for GAS infection or colonization (carriage) [1].
Even though GAS causes significant problems, there is scarcity of information in Ethiopia [14, 15] and only a few investigations on GAS have been conducted in previous years [14], particularly it was untouched in Jigjiga, Eastern Ethiopia. Therefore, this study was designed to assess throat carriage prevalence, associated factors, and antimicrobial susceptibility pattern of GAS among healthy school children in Jigjiga city, Eastern Ethiopia.
Methods and materials
Study area and period
The study was conducted in Jigjiga, a city in the Somali Region of Ethiopia from 12 April to 27 May 2021. Jigjiga is 630 km east far from the capital city, Addis Ababa. It is estimated that 763,509 people live in Jigjiga. There were ninety-nine (99) primary schools in the city of Jigjiga. Of which, 74 private schools, and 25 government schools. A total of 36,507 students are present. Among these, 20,056 were males and 16,451 were females [16].
Study design and population
A cross-sectional study was conducted. School children from governmental and private primary schools who were randomly selected from twenty schools using a lottery system. Written informed consent was obtained from their parents for children aged less than 11 years. Furthermore, children aged 11 years and above were insisted to give a written assent.
Inclusion and exclusion criteria
All students in selected schools aged 7–14 years old who attend the class during the study period were included in the study. All children on antibiotics for the previous two weeks and children with throat infection or any related sign and symptoms of pharyngitis were excluded from the study.
Sample size determination
A single proportion formula was used to determine the sample size for this quantitative study by considering the following assumptions: a prevalence of asymptomatic pharyngeal carriage rate of GAS among healthy school children in Hawassa, southern Ethiopia with a prevalence of 12.2% [15], 95% confidence level, and a margin of allowable error of 5%,. To minimize errors arising from the likelihood of non-compliance, 10% of the sample size was added. The final sample size was 178.
On the other hand, sample size was determined by considering different factor-associated GAS colonization using double population proportion formula with the assumption of a two-sided confidence level of 95%, the margin of error 5%, and power of 80%. Finally, 231 study participants were calculated. So, the sample size for a single population proportion was smaller than the sample size calculated for the second calculated double population proportion, which is a sample size of 231 was used. Because we used a multi-stage sampling method, we introduced a design effect. As a result, 231 was multiplied by 2, resulting a total sample size of 462.
Sampling procedure and sampling technique
From a total of 99 primary schools, twenty (20) schools were chosen at random through a lottery system, from government and private schools. Then the determined sample size for the study was proportionally allocated to each selected school. A simple random sampling method was used to enroll children. In order to include study participants in the study, a multistage sampling technique was used. Samples were taken from children whose parents agree to participate until the sample size attend from each selected school.
Sample collection and transportation methods
Data on the socio-demographic characteristics of the parents/guardians and children, as well as the children's clinical history, were collected using a structured questionnaire adopted from previous studies [15, 17]. Two professional nurses administered the questionnaires, and two trained laboratory personnel used cotton swabs to collect a throat sample from a selected child. The throat swab samples were placed in Amie's transport media. Within two hours, the sample was sent in a cold chain to Jigjiga University Sultan Sheik Hassan referral hospital laboratory for investigation.
Laboratory investigation
The throat sample was cultured on 5% sheep blood agar plates (Himedia, India) by rolling the swab over a small area of the plate and streaking the sample with a sterile loop, then incubated at 37 °C with 5% CO2 atmosphere for 24 h. A catalase test and gram staining were performed on colonies having ß-hemolysis. All catalase-negative and gram-positive cocci were subcultured for 24 h at 37 °C on 5% fresh blood agar plates with a Bacitracin disk in a 5% CO2 atmosphere to differentiate colonies suspected to be S. pyogenes. Any zone of inhibition around the bacitracin disk was a candidate for Pyrrolidonyl arylamidase (PYR) tests, change of color to red /purple was confirmed culture positive for GAS. [18, 19].
Antimicrobial susceptibility testing
The drug susceptibility test was done by a disk diffusion method by using Muller Hinton Agar (MHA) supplemented with 5% sheep’s blood. Colony suspension was made using normal saline (0.85% NaCl) equivalent to 0.5% McFarland standard from grown overnight colonies (18–24 h) on sheep blood agar plate. The suspension was inoculated to an MHA plate with 5% sheep’s blood using a culture swab and incubated at 5% CO2 for 18 to 24 h. Drug disks containing penicillin (10 IU), erythromycin (15 g), azythromycin (15 g), amoxicillin (10 g), chloramphenicol (30 g), ceftriaxone (30 g), vancomycin (30 g), and tetracycline (10 g) were utilized. The drugs are selected in accordance with the Ethiopian Drug Administration standard treatment guidelines for health centers and Control Authority's and the Clinical Laboratory Standards Institute's (CLSI). Finaly, the zone of inhibition was measured with a ruler, then recorded and compared to the Clinical and Laboratory Standards Institute [20].
Data quality control
The questionnaire was written in English, then translated into Amharic and Somali, and finally back to English to ensure uniformity. The questionnaire was pre-tested before actual data collection begins. For laboratory testing, the sterility of each batch of produced media was determined by incubating 5% of the culture media in a 5% CO2 enriched atmosphere at 37 °C for 24 h before using it. Streptococcus pyogenes (ATCC 12,696) and Streptococcus agalactae (ATCC 13,813) were used as a positive and negative control respectively. Quality assurance in antimicrobial susceptibility was done by repeating the selected tests on the same day as the original.
Method of data analysis
The data was entered and coded into the Epi-Data version 3.1 upon creating the questionnaire template. The entered data was cleaned to ensure the validity of all recorded data. The analysis was then carried out using SPSS version 26.0. Descriptive statistics and frequency tables were used to summarize the data. The magnitude of the association between the different variables with the throat culture positive for GAS was measured by the adjusted odds ratio (AOR) with a 95% confidence interval. Bivariate and multivariable logistic regression analysis was made to obtain the odds ratio and the confidence interval of statistical associations. All variables that are significant at p-value < 0.25 in the bivariate analysis were considered for multivarible analysis. Previous family member who had a sore throat, children living with larger families (more than 11 members), and children living with non-immediate families were adjusted and calculated through adjusted odds ratios to measured the strength of statistical association at 95% confidence intervals and statistical significance was declared at p < 0.05.
Results
Sociodemographic characteristics of study participants
A total of 462 schoolchildren were participated in the study with a response rate of 100%. About 244 (52.8%) of study participants were females. Two hundred seven students (57.8%) were from governmental primary schools, while 195 (42.2%) were from a private primary school in Jigjiga city administration. The study participants' ages range from 7 to 14, with a mean age was of 10.9 years (SD ± 1.89). The majority (89.4%) of the participants live in a city. Two hundred twenty-nine (49.6%) of research participants lived with 6 to 10 family members, while 214 (46.3%) shared a sleeping room with 1 to 3 people. A large percentage of (73.5%) the parents/guardians of the children were married. Mothers were the primary caregivers in 320 (69.3%) of the cases, and 331 (71.6%) of the respondents had acquired formal education. One hundred forty-three (30.9%) of the respondents/caregivers were merchants and 108 (23.4%) were government employees (Table 1).
Clinical history of the study participants
The majority (76%) of the study participants had no history of hospitalization in the previous 5 years and 87.9% of family member had no history sore throat in the last 30 days. Nearly, half of the parents 47.6% claimed that they had never given antibiotics to their children.
Prevalence of group A Streptococcus
Among 462 in healthy school children, 49 (10.6%) (95% CL; 8.1%—13.7%) were confirmed to have GAS in their throats.
Analysis for factors associated with GAS colonization
In bivariate regression analysis; caregivers, monthly income, family size (number of person), sharing a sleeping room, Sore throat (within the last 30 days), and previous family member who had a sore throat showed a significant association at a p-value of < 0.25 and were considered as a candidate for multivariate logistic regression. Through a multivariable analysis: previous family member who had a sore throat, children living with larger families (more than 11 members), and children living with non-immediate families were significantly associated with culture-positivity rates of GAS at p-value < 0.05 after adjusted for confounding factors.
Children who lived with a family member who had a sore throat previously were twice likely to have GAS in their throat compared with those who lived with no family member who had a sore throat (AOR = 2.51; 95%CI 1.09–5.73;). Children living in families with more than 11 members were four times more likely carrying GAS compared to children living in families with less members (AOR = 4.64; 95% CI 1.53–14.1). Children living with non-immediate families were more than three times more likely to have GAS compared to children living with their mothers (AOR = 3.65;95%; CI: 1.39 – 9.61) (Table 2).
Antibiotic susceptibility testing
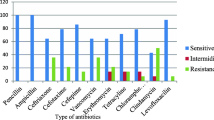
To determine the antimicrobial susceptibility patterns of isolates, those 49 bacterial isolates were tested for eight different antimicrobials. Majority of them were susceptible to amoxicillin 44 (89.8%), azithromycin 45 (91.8%)), ceftriaxone 47 (95.9%), chloramphenicol 46 (93.9%), erythromycin 46 (93.9%), penicillin 49 (100%), and vancomycin 45 (91.8%) (Table 3). Moreover, two (4.1%) of the 49 isolates were drug-resistant to two drugs, particularly amoxacillin, and eryomycin/vancomycin.
Discussion
In the present study the overall pharyngeal carriage rate of GAS was 10.6%. (95% CI; 8.1%—13.7%). A carrier rate comparable to ours has been recorded in Ethiopia (9.7% and 12.2%, respectively) [14, 15], United Arab Emirates (10%) [21], Nepal (10.8%) [22], and Yemen (12.8%) [23].
In contrast, the prevalence was lower than those reported in Uganda (16.0%) [24], Egypt (16%) [25], India (23.1%) [17], Argentina (14.2%) [26] and Brazil (14.0%) [27]. Our results showed a higher carriage rate than Gabon's 5.8% [27] and Nepal's 5.0% [28]. The possible explanation for the variation might be due hygiene, sample size, seasonal change, and geography, and socio-demographic [15, 23,24,25].
Children who lived with a family member who had a sore throat were twice as likely to colonize group A Streptococcus (GAS) than children who did not have a primary case or sore throat in their household. In Melbourne, Australia, researchers discovered that when a primary case is present, the likelihood of subsequent infection within a family increases by 1.8 times [29]. In addition, according to a study conducted in Nepal, one out of every five children is affected when a family member has already been infected [22].
Children from families with more than eleven members had a four-fold greater chance of carrying GAS than children from families with fewer members. Similarly, research in Hawassa found that children living in families with more than five members were more than 10 times more likely to be carriers of GAS than children living in families with fewer members. [15]. According to another study from Iraq, the carrier rate among children living in homes with more than six individuals is two times higher than among children living in families with fewer members [30]. According to the findings of these investigations, there is a substantial link between the carrier rate and the number of family members. The explanation for this could be crowding simply increases risk of disease (and colonization) transmission. Unlike a study by Asrat Anja [15], in this study Children living with non-immediate families were more than three times more likely to have GAS than children living with their immediate family. Crowding and poor hygiene, therefore, increases the chance of the transmission of GAS [31].
All GAS isolates were sensitive to penicillin in our study. The same high activity of penicillin had been reported in many countries, namely Ethiopia [14, 15, 32], Uganda [24], India [17], Nepal [22, 28] and Argentina [26]. Our findings reveal that amoxicillin (89.8%) and ceftriaxone (95.9%) have slightly reduced sensitivity against GAS when compared to recently published publications in Senegal and Ethiopia [15, 33]. For patients allergic to penicillin, erythromycin and other macrolides were suggested as first-line alternatives [6]. The isolates displayed the same level of sensitivity to erythromycin and azithromycin (93.9%). The isolates also showed 93.9% and 91.8% sensitivity to chloramphenicol and vancomycin, respectively. A comparable result was reported from Ethiopia [14, 15, 32] and other parts of the world [17, 24,25,26, 33, 34]. About 2(4.1%) of isolated GAS isolates were showed multiple drug resistant. A comparable result was reported from Hawwasa, Ethiopia [15].
Limitation of the study
Since the study was a cross-sectional study conducted over a short period, the impact of environmental or seasonal factors on variations in prevalence could not be determined. Some of the children identified as being colonized could have been ill (not colonized). In addition, this study was unable to performed ASO titer due to a lack of resources.
Conclusion
The present study showed a significant throat carriage of GAS in the Jigjiga city school children population. Children with a sore throat in the family, children from a large family, and children from non-immediate families have all been identified as independent predictors of the throat culture positive for GAS. The vast majority of GAS isolates cultured from the Jigjiga city school children were found to be sensitive to Penicillin, amoxicillin, ceftriaxone, chloramphenicol, erythromycin, azithromycin, and vancomycin.
The present study provides useful baseline information on the GAS carriage rate and resistance trend among healthy school children in this study area. Further study is important to understand the epidemiological features and to give the therapeutic strategies for public health problems due to antibiotic resistance.
Availability of data and materials
The data sets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
- AOR:
-
Adjusted Odds Ratio
- ASO:
-
Antistreptolysin O
- AST:
-
Antibiotic Susceptibility Test
- CLSI:
-
Clinical Laboratory Standard Institute
- COR:
-
Crude Odds Ratio
- GAS:
-
Group A Streptococcus
- MHA:
-
Mueller Hinton Agar
References
Ralph A, Carapetis J. Group A streptococcal diseases and their global burden. Curr Top Microbiol Immunol. 2012;368:1–27.
World Health Organization. The current evidence for the burden of group A streptococcal diseases. World Health Organization; 2005.
Efstratiou A, Lamagni T. Epidemiology of Streptococcus pyogenes. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center © The University of Oklahoma Health Sciences Center; 2016.
Ralph AP, Carapetis JR, Group a streptococcal diseases and their global burden. Host-pathogen interactions in streptococcal diseases. 2012. 1–27.
Barth DD, Moloi A, Mayosi BM, Engel ME. Prevalence of group A Streptococcal infection in Africa to inform GAS vaccines for rheumatic heart disease: a systematic review and meta-analysis. Int J Cardiol. 2020;307:200–8.
Bisno A, Gerber M, Gwaltney J, Kaplan E, Schwartz R, Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis. 2002;35(2):113–25.
Platt M, Vicario S, Marx J. Rosen’s emergency medicine: Concepts and clinical practice. 7th ed. Philadelphia: Mosby Elsevier; 2010. p. 57.
Engel ME, Mayosi BM. Clinical and epidemiological aspects of streptococcus pyogenes pharyngitis and carriage in Africa: streptococcus pyogenes in Africa. SA Heart. 2013;10(2):434–9.
Matthys J. There are still problems in identifying who will develop complications of sore throat in primary care. BMJ (Clin Res Ed). 2014;348:g299.
Henningham A, Barnett TC, Maamary PG, Walker MJ. Pathogenesis of group A streptococcal infections. Discov Med. 2012;13(72):329–42.
Bessen DE. Population biology of the human restricted pathogen, streptococcus pyogenes. Infect Genet Evol. 2009;9(4):581–93.
Sanyahumbi AS, Colquhoun S, Wyber R, Carapetis JR. Global disease burden of group A Streptococcus. Streptococcus pyogenes: basic biology to clinical manifestations. University of Oklahoma Health Sciences Center; 2016.
Oliver J, MalliyaWadu E, Pierse N, Moreland NJ, Williamson DA, Baker MG. Group A Streptococcus pharyngitis and pharyngeal carriage: a meta-analysis. PLoS Negl Trop Dis. 2018;12(3):e0006335.
Abdissa A, Asrat D, Kronvall G, Shitu B, Achiko D, Zeidan M, et al. Throat carriage rate and antimicrobial susceptibility pattern of group A Streptococci (GAS) in healthy Ethiopian school children. Ethiop Med J. 2011;49(2):125–30.
Anja A, Beyene G, Daka D. Asymptomatic pharyngeal carriage rate of Streptococcus pyogenes, its associated factors and antibiotic susceptibility pattern among school children in Hawassa town, southern Ethiopia. BMC Res Notes. 2019;12(1):564.
Jigjiga City Administration Education Bureau. General school information. Jigjiga: Jigjiga City Administration Education Bureau; 2020.
Mukundan A, Vijayakumar S. Pharyngeal carriage of Group A streptococci among school children. J Int Med Dent. 2017;4(1):18–26.
Brooks GF, Butel JS, Morse SA. Jawetz, Melnick, & Adelberg's medical microbiology: Lange Medical Books/McGraw-Hill. Medical Pub. Division; 2004.
Cheesbrough M. District laboratory practice in tropical countries, part 2: Cambridge university press. 2006.
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. Wayne: CLSI supplement; 2020.
Ali Al Shamisi FH. The Prevalence of Streptococcus Pyogenes and its emm Gene Types among School Children in AI Ain, UAE. 2016.
Prajapati A, Rai S, Mukhiya R, Karki A. Study on carrier rate of Streptococcus pyogenes among the school children and antimicrobial susceptibility pattern of isolates. Nepal Med Coll J. 2012;14:169–71.
Othman AM, Assayaghi RM, Al-Shami HZ, Saif-Ali R. Asymptomatic carriage of Streptococcus pyogenes among school children in Sana’a city, Yemen. BMC Res Notes. 2019;12(1):339.
Nayiga I, Okello E, Lwabi P, Ndeezi G. Prevalence of group a streptococcus pharyngeal carriage and clinical manifestations in school children aged 5–15 yrs in Wakiso District, Uganda. BMC Infect Dis. 2017;17(1):248.
Abd El-Ghany SM, Abdelmaksoud AA, Saber SM, Abd El Hamid DH. Group A beta-hemolytic streptococcal pharyngitis and carriage rate among Egyptian children: a case-control study. Ann Saudi Med. 2015;35(5):377–82.
Delpech G, Sparo M, Baldaccini B, Pourcel G, Lissarrague S, Allende LG. Throat carriage rate and antimicrobial resistance of Streptococcus pyogenes in rural children in Argentina. J Prev Med Public Health. 2017;50(2):127.
Bélard S, Toepfner N, Arnold B, Alabi AS, Berner R. β-hemolytic streptococcal throat carriage and tonsillopharyngitis: a cross-sectional prevalence study in Gabon. Central Africa Infection. 2015;43(2):177–83.
Manandhar A, Shah Y, Shrestha J. Study on the prevalence of beta haemolytic Streptococcus among school children. J Nepal Paediatric Soc. 2013;33(1):45–7.
Danchin MH, Rogers S, Kelpie L, Selvaraj G, Curtis N, Carlin JB, et al. Burden of acute sore throat and group A streptococcal pharyngitis in school-aged children and their families in Australia. Pediatrics. 2007;120(5):950–7.
Saleh MMS. Carriage state of gaβhs among yemeni schoolchildren and the upper limit of normal for aso in different population groups. Iraqi J Sci. 2010;51(1):63–70.
Faruq Q, Rashid A, Ahmed J, Waiz A, Haque K, Rouf M, et al. Prevalence of streptococcal sorethroat in the school children of Dhaka. Bangladesh Med Res Counc Bull. 1995;21(3):87–94.
Zegeye N, Asrat D, Woldeamanuel Y, Habte A, Gedlu E, Tønjum T, et al. Throat culture positivity rate and antibiotic susceptibility pattern of beta-hemolytic streptococci in children on secondary prophylaxis for rheumatic heart disease. BMC Infect Dis. 2016;16(1):510.
Camara M, Dieng A, Boye CSB. Antibiotic susceptibility of streptococcus pyogenes isolated from respiratory tract infections in dakar, senegal. Microbiol Insights. 2013;6:MBI. S12996.
Bobia AA, Blaj OA, Oancea D, Iulia-Cristina B, Radu-Vasile B, Delia-Ioana H, et al. The prevalence of beta hemolytic streptococcus in a children’s tertiary care hospital in Timisoara. Cent Eur J Clin Res. 2019;2(1):73–8.
Acknowledgements
We acknowledged Haramaya University Colleges of Health and Medical Sciences Institutional Health Research Ethical Review Committee for giving the ethical clearance. We also thank study participants and all individuals who have in one way or another contributed to the completion of this research.
Funding
This research data collection finance was covered by Haramaya university postgraduate directorate.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted out after receiving ethical approval from Haramaya University College of Health Science and Medical Sciences Institutional Health Research Ethics Review Committee (IHRERC). This study was conducted in accordance with the Declaration of Helsinki. Each child's parent/guardian has been sufficiently informed of the study's purpose and the importance of their participation by the data collectors and/or the investigator. Written, Informed, voluntary, and signed consent were taken from all parents/guardians and assent were taken from the child before commencing the study. All parents/guardians were consulted about the benefit of the treatment by a pediatrician who works at Jigjiga University Sultan Shiek Hassen Referral Hospital and the drugs were given free of charge.
Consent for publication
Not applicable.
Conflicts of interests
There were no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barsenga, S., Mitiku, H., Tesfa, T. et al. Throat carriage rate, associated factors, and antimicrobial susceptibility pattern of group A Streptococcus among healthy school children in Jigjiga City, Eastern Ethiopia. BMC Pediatr 22, 227 (2022). https://doi.org/10.1186/s12887-022-03294-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03294-2