Abstract
Background
Langerhans cell histiocytosis (LCH) is a heterogeneous disease with diverse clinical manifestations. Abdominal organ involvement is rare. Early diagnosis and active treatment are needed. The purpose of this article is to enable readers to have a better knowledge of LCH and prevent misdiagnosis.
Case presentation
A 2-month-old boy had diarrhea, hematochezia, and a rash, and was diagnosed as having a cow milk protein allergy (CMPA). He was given an amino acid-based formula, but there was no sign of improvement in his condition.
The patient then had a skin biopsy and was diagnosed as having multisystem Langerhans cell histiocytosis (MS-LCH). The general condition of the child deteriorated after the first two doses of chemotherapy, and the child died.
Conclusions
MS-LCH is a protracted and progressive condition with poor prognosis. Early diagnosis and treatment are essential for survival. If a child has chronic diarrhea and hematochezia in the presence of a characteristic rash, the pediatrician should consider the possibility of this disease to avoid misdiagnosis.
Similar content being viewed by others
Background
Langerhans cell histiocytosis (LCH) was considered to be a monoclonal dysplasia of Langerhans cells [1], but it is now believed to be the clonal proliferation of dendritic cells originating from bone marrow, with the infiltration of inflammatory cells. These dendritic cells are CD1a/CD207 positive. LCH is a broad spectral heterogeneous group of diseases with diverse clinical manifestations. Gastrointestinal tract (GIT) involvement in LCH has been very infrequently reported. GI manifestations of LCH are quite variable and unspecific. Bloody stools, intractable diarrhea, protein-losing enteropathy, constipation, abdominal pain, vomiting, malabsorption, and even intestinal perforation are some of the reported symptoms. Prognosis of children with gastrointestinal tract (GIT) involvement in LCH has been poor. Singhi et al. [2] reported 12 patients, including 2 children. One pediatric patient was lost to follow-up; however, the other patient was found to have concurrent systemic disease with skin and bone marrow involvement. The child died 1 year after initial diagnosis. Yadav SP et al. [3] reported 2 children and reviewed 37 other cases of LCH with GIT involvement, More than 50% patients died within 18 months from diagnosis. So early diagnosis and active treatment are needed. The purpose of this paper is to strengthen clinicians’ understanding of LCH.
Case presentation
A 2-month-old boy was admitted to the hospital with a history of diarrhea, hematochezia, and rashes since his birth on June 4, 2019. His birth weight was 3.8 kg, and he weighed 6 kg when he was 2 months old (WHO Z score [4] 0–2). Initially, he was diagnosed as suffering from a cow milk protein allergy (CMPA) as he was being fed a cow milk protein-based formula. He was changed to an amino acid-based formula, but after 1 month there was no improvement. Physical examination revealed generalized rashes on the face, trunk, limbs, scalp, perineum, and palms (Fig. 1). The rashes were erythematous and papular, with scab formation. Several of them had become confluent and coalesced to form larger lesions. Yellow exudates and scab formation could be seen in the external auditory canals bilaterally, and superficial ulceration and yellowish white secretions were seen in the gums of the oral cavity (Fig. 2). The cervical, axillary, and groin lymph nodes could be palpated. A routine blood examination showed white blood cell 14.44 × 109/L, red blood cell 3.92 × 1012/L, hemoglobin 112 g/L, and high-Sensitive C-reactive Protein 27.24 mg/L. A liver function test showed albumin 24.0 g/L, and the TBNK cell findings were total T cell (CD3+) 33.8%, CD4/CD8 4.45, and B lymphocyte (CD19+) 45.1%. A brain computed tomography scan showed decreased density of the unerupted incisors and canines (Fig. 3). The dermal histopathology results were obtained by the microscope OLYMPUS BX53 and cellSens Standard softwareas and as follows: S-100 (+), CD1a (+), CD68 (+), Langerin (+), CD20 (−), Ki-67 (+ 40%), CD21 (−), CD23 (−), CD3 (focal +), Bcl-2 (focal +), CK-pan (−), EMA (−), and HMB45 (−) (Fig. 4). The patient was diagnosed as having multisystem LCH (MS-LCH) (Grade IV). He was then transferred to the pediatric oncology department for chemotherapy. The general condition of the child deteriorated after the first 2 weeks of VP solution (prednisone and vinblastine), and he died.
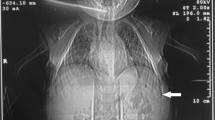
Brain CT scan of this child. The CT scan showed no obvious abnormal changes in the morphology and density of brain tissues, and the bilateral frontotemporal parietal subarachnoid space slightly widened. The position, size and density of cerebral ventricles and cisterns were not abnormal, and the midline structure was not displaced. The density of unerupted incisors and canine teeth were found decreased (white arrow)
Dermal pathology showed: S-100 (+), CD1a (+), CD68 (+), Langerin (+), CD20 (−), Ki-67 (+ 40%), CD21 (−), CD23 (−), CD3 (focal +), Bcl-2 (focal +), CK-pan (−), EMA (−), HMB45 (−). a H-E staining of dermal pathology (× 40); b Immunohistochemical staining for CD1a (× 40); c Immunohistochemical staining for Langerin (× 40); d Immunohistochemical staining for S-100 (× 40). The resolution: a:1360*1024;b:1360*1024;c:1360*1024;d:1360*1024; These figures have not been processed to improve resolution. The scale bar of every microscopy image is 400
Discussion and conclusions
LCH can occur in any age group but it is mostly seen in children [5], and the incidence rate is 4.0–5.4/1,000,000. The pathogenesis of the disease remains unclear [6]. The lesion may involve multiple organs such as the bones (80%), skin (33%), pituitary (25%), liver, spleen, lungs, hematopoietic system (15%), lymph nodes (5–10%) and central nervous system (2–4%).
LCH rarely affects the digestive tract [7], but diarrhea is one of the first symptoms when it does. Up to now, there are only 5 literature reports about LCH with gastrointestinal involvement, and the age of 4 cases in these reports were younger than 2 years old. The clinical symptoms of LCH with gastrointestinal involvement include: nausea, vomiting, abdominal pain, diarrhea, hematochezia, constipation, intestinal obstruction, intussusception, and intestinal perforation. It can also occur in the distal ileum, making it difficult to differentiate from appendicitis.
Seborrhea or an eczema-like rash is seen in 86% of patients with LCH digestive tract involvement [8]. Therefore, a typical erythematous rash may be a clue to a diagnosis of LCH, and it is vital that it is differentiated from CMPA.
Bone, especially maxillofacial skeleton, is the organ most commonly involved in LCH. LCH occurs in the mandible more than the maxilla. The first symptoms of LCH with mandibular invasion are maxillofacial swelling, pain, restricted mouth opening, ulceration of gums or mucous membranes, teeth mobility or loss. In this case, superficial ulceration and yellowish white secretions were seen in the gums of the oral cavity, and surrounding mucosa was swollen. In order to assess the presence of skull and mandible invasion, the patient also underwent head CT examination. No skull involvement was observed, but the density of unerupted front teeth and canine teeth decreased, providing clues and basis for the diagnosis of LCH. In addition, in this patient, bilateral serous otitis was also a clue to a diagnosis of LCH.
It still needed to be differentiated from other diseases in this patient. 1) Late vitamin K deficiency. In this case, the child had the symptom of hematochezia, but his coagulation function was normal. And the child took formula milk, thus the diagnosis of vitamin K deficiency was not considered. 2) Intestinal infection. The child showed symptoms of diarrhea and hematochezia, but no white blood cells were found in routine stool test, and no pathogenic bacteria were cultured in stool microbiological test, so infectious diseases could be excluded. 3) Early-onset inflammatory bowel disease (IBD). The child had diarrhea and hematochezia symptoms, and the weight gain was not ideal. Therefore, the possibility of early-onsite IBD should be taken into consideration. However, since the parents of the child refused to undergo colonoscopy, early-onset IBD could not be completely excluded. Thus, LCH with GIT involvement was considered as final diagnosis based on the monistic explanation.
European guidelines say that a diagnosis of LCH should mainly depend on the histopathology of a biopsy. In this case, Langerhans cells were found in the skin biopsy, and the positive immunohistochemical findings of Langerin, CD1a, and S-100 proteins established the diagnosis of LCH [9]. Currently, LCH is divided into two groups, namely single system LCH (SS-LCH), with single organ or system involvement, and MS-LCH, with the involvement of two or more organs or systems. This patient was a 2-month-old infant with skin, lymph node, ear, and oral lesions, recurrent diarrhea, hematochezia, and decreased liver albumin. Therefore, this case was diagnosed as MS-LCH (Grade IV).
Some patients with SS-LCH may have spontaneous regression of the disease. However, in most patients with MS-LCH [10], especially infants, the clinical course is protracted and progressive, with poor prognosis [11]. Yadav SP et al. [3] pointed out that the mortality rate of patients with gastrointestinal involvement in LCH was 55.5%, which was higher compared with 7% of patients with single-system involvement and 40% of patients with multi-system involvement. In retrospect, the gastrointestinal tract should be considered as a high-risk organ that affects prognosis. Almost 40% of patients required second-line therapy and/or hepatocyte transplantation, suggesting a poor prognosis for gastrointestinal involvement in LCH [3]. For this reason, early diagnosis and treatment are essential.
In this particular case, the patient was misdiagnosed as having CMPA initially and was fed with amino acid milk formula for 1 month, which delayed his receiving the correct treatment and may have contributed to his eventual death. It is hoped that this case report will improve the clinical knowledge of LCH and help prevent misdiagnosis.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- LCH:
-
Langerhans cell histiocytosis
- MS-LCH:
-
Multisystem Langerhans cell histiocytosis
- SS-LCH:
-
Single system-Langerhans cell histiocytosis
- CMPA:
-
Cow Milk Protein Allergy
- CT:
-
Computed Tomography
References
Yi W, Chen WY, Yang TX, Lan JP, Liang WN. Langerhans cell sarcoma arising from antecedent langerhans cell histiocytosis: a case report. Medicine. 2019;98(10):e14531.
Singhi AD, Montgomery EA. Gastrointestinal tract langerhans cell histiocytosis: a clinicopathologic study of 12 patients. Am J Surg Pathol. 2011;35(2):305–10.
Yadav SP, Kharya G, Mohan N, et al. Langerhans cell histiocytosis with digestive tract involvement. Pediatr Blood Cancer. 2010;55(4):748–53.
WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85.
Baron Gonzalez de Suso L, Olmedilla Jodar M, Perez Alonso V, Melgar Bonis A. Langerhans cell histiocytosis presenting as enterocolitis and shock in neonate. J Pediatr Hematol Oncol. 2019;41(3):e155–7.
Thacker NH, Abla O. Pediatric Langerhans cell histiocytosis: state of the science and future directions. Clin Adv Hematol Oncol. 2019;17(2):122–31.
Ufuk F, Herek D, Calli DN. Gastrointestinal: extensive abdominal involvement as an initial presentation of Langerhans' cell histiocytosis. J Gastroenterol Hepatol. 2019;34(7):1129.
Shevachut C, Pornpun S, Thirachit C. Gastrointestinal tract involvement of Langerhans cell Histiocytosis: concerning point of diagnosis. J Health Sci Med Res. 2020;39(1):67–72.
Vasiliki T, Loizos P, Vassilios P, et al. Long-term outcome, clinical course and treatment approaches of paediatric langerhans cell histiocytosis: a greek reference Centre report. Acta Paediatr. 2021;00:1–8.
Dhar S. Sahana M, Subhra Dhar, et al. Langerhans cell histiocytosis in children: a retrospective case series of 126 cases. Pediatr Dermatol. 2020;37:1085–9.
Tuysuz G, Yildiz I, Ozdemir N, et al. Langerhans cell Histiocytosis: single center experience of 25 years. Mediterran J Hematol Infect Dis. 2019;11(1):e2019035.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding
No external funding received to conduct this study.
Author information
Authors and Affiliations
Contributions
LXT conceived the idea and conceptualised the study. PZ and WL collected the data and analysed the data. LXT drafted the manuscript, then ZCY reviewed the manuscript and substantively revised it. All authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
I confirm that I have read the Editorial Policy pages. The First Hospital of Jilin University, Changchun, China approved the study, and the institutional guidelines for the care and treatment of patients were followed rigorously. Informed written consent was obtained from the patient’s parents for publication of this case report and the accompanying images.
Consent for publication
The parents/guardians gave their written consent for their child’s personal or clinical details along with any identifying images to be published in this study.
Competing interests
The authors declare that they have no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lv, X., Wang, L., Pi, Z. et al. Langerhans cell histiocytosis misdiagnosed as cow protein allergy: a case report. BMC Pediatr 21, 469 (2021). https://doi.org/10.1186/s12887-021-02921-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-021-02921-8