Abstract
Background
Regional variation in cost of neonatal intensive care for extremely preterm infant is not documented. We sought to evaluate regional variation that may lead to benchmarking and cost saving.
Methods
An analysis of a Canadian national costing data from the payor perspective. We included all liveborn 23–28-week preterm infants in 2011–2015. We calculated variation in costs between provinces using non-parametric tests and a generalized linear model to evaluate cost variation after adjustment for gestational age, survival, and length of stay.
Results
We analysed 6932 infant records. The median total cost for all infants was $66,668 (Inter-Quartile Range (IQR): $4920–$125,551). Medians for the regions varied more than two-fold and ranged from $48,144 in Ontario to $122,526 in Saskatchewan. Median cost for infants who survived the first 3 days of life was $91,000 (IQR: $56,500–$188,757). Median daily cost for all infants was $1940 (IQR: $1518–$2619). Regional variation was significant after adjusting for survival more than 3 days, length of stay, gestational age, and year (pseudo-R2 = 0.9, p < 0.01). Applying the model on the second lowest-cost region to the rest of the regions resulted in a total savings of $71,768,361(95%CI: $65,527,634–$81,129,451) over the 5-year period ($14,353,672 annually), or over 11% savings for the total program cost of $643,837,303 over the study period.
Conclusion
Costs of neonatal intensive care are high. There is large regional variation that persists after adjustment for length of stay and survival. Our results can be used for benchmarking and as a target for focused cost optimization, savings, and investment in healthcare.
Similar content being viewed by others
Table of contents summary
A national data analysis evaluated regional differences in cost of neonatal intensive care for preterm infants and the potential cost saving in benchmarking better performers.
What is known on this subject
Neonatal intensive care for extremely premature infants (< 29 weeks) is prolonged and expensive. Regional variation has not been described in this population and can assist in cost reduction by learning from high performers.
What this study adds
There is a wide regional variation in the remarkably high cost of neonatal intensive care that suggests a potential for benchmarking and focused cost savings.
Background
Prematurity affects almost one in ten newborns [1], with 1 % of all newborns being extremely preterm (born before 29 weeks, or weighing less than 1500 grams [2, 3]). These fragile infants are often hospitalized for many weeks in a neonatal intensive care unit (NICU), requiring prolonged respiratory support, parenteral nutrition, and undergo many interventions such as ultrasonograms, surgeries, and blood tests. The complex care for this population involves multiple specialists in a level 3 (high acuity) NICU for several months. The intensive care provided is reflected in its high cost [4,5,6]. Extremely preterm infants accounted for some of the highest patient expenditures in hospitals [7, 8].
In recent years, support for infants born at 23 and 24 weeks gestational age, previously thought to be unviable, has become common in tertiary NICUs [2, 9, 10]. Indeed, most of these extremely preterm infants are resuscitated, with the majority surviving and being discharged home [2]. This has “pushed the envelope” for neonatal viability. Indeed, in many jurisdictions, it is standard practice to provide life support to newborns born at 23 or more weeks of gestation [9, 10].
Costs for providing care to this most vulnerable group have been uncertain [11,12,13,14]. Understanding these costs is important for health policy makers and planners in allocation decisions [15, 16]. As well, it has broad applicability since cost is considered a component of quality within the Institute of Healthcare Improvement’s (IHI) Quadruple Aim of Healthcare Quality [17]. Previous work with cost effectiveness analyses (CEAs) has estimated the cost-effectiveness of NICU care in various situations [18,19,20,21,22,23,24,25,26]. For example, neonatal resuscitation at 23 weeks had an estimated cost-utility of $15,134 to $22,256 per Quality-Adjusted Life Year (QALY) [19]. Variation in total cost can also affect the cost-effectiveness of the intervention.
As with all high-cost interventions, there is frequently wide variation in overall amounts. In this situation of extreme expense, documenting regional variation can help sites streamline processes and improve performance by learning from high performers. Thus, we sought to evaluate the cost and cost variation of care for these fragile preterm infants.
Methods
Data source
We used data from the Canadian Institute for Health Information (CIHI) database, a Canadian national agency responsible for the collection and analysis of health information. We received information on total cost of the neonatal stay from birth to discharge home or death, subcategorized by gestational age, province, and year. CIHI data is subject to quality checks, with ≥98% correlation with patient charts in multiple studies [27, 28]. Costing components are detailed in CIHI indicator library [29].
We included all newborn deliveries at 23–28 weeks gestational age, between January 1st, 2011 and December 31st, 2015. This represented years when 23-week infants began to be frequently supported in NICUs across Canada. There is usually a long delay in data availability as a result of extensive quality and audit checks precluding more current information.
We did not include Quebec as they do not submit data to CIHI. As well, the Canadian territories (Yukon, Northwest, and Nunavut) and the province of Prince Edward Island were excluded because of small numbers of deliveries and incomplete cost data. We also excluded stillbirths.
We used the province-submitted total cost for each patient for the complete neonatal hospital stay from birth to discharge home or death, including all hospital transfers. This excluded physician compensation. Which is not included in the database. Costing data is collected in the national database, CIHI, from the provinces using a standardized costing method [30]. This reflects the complete cost to the payor—the Ministries of Health—thereby providing a public perspective. Costing followed CIHI’s standardized approach [31,32,33]. Cost was adjusted to the published Canadian Healthcare Consumer Price Index [34] in 2011 Canadian dollars.
Statistical analysis
Sunnybrook Hospital Research Ethics Board and CIHI approved the study protocol.
We calculated means, 95% confidence intervals [95%CI], medians, interquartile ranges [IQR] and standard deviations (SD) for each patient group. We compared groups using the Mann-Whitney-Wilcoxon test and Kruskal-Wallis test for non-normally distributed data. For variance, we used the Fligner-Killeen test for variance of multiple, non-normally distributed variables. For trends, we calculated the coefficient of determination (r2). We evaluated regional variation by adjusting for gestational age, length of stay, and year, using a multivariate analysis of cost. Length of stay was added to the multivariate analysis to correct for variation in hospitalization practices and discharge criteria variations. We calculated confidence intervals for each coefficient, pseudo-R2 and Akaike Information Criterion (AIC) to assess the model’s robustness. We repeated the model with cost data on infants who survived the first 3 days to accurately capture the cost impact of NICU stay, eliminating those who were too ill to survive or those who may have been withdrawn of life support. We also eliminated extreme outliers by calculating Cook’s D. Analyses were performed in R statistical language v4 and SPSS v21.
Results
We analysed the costs for 6932 extremely preterm infants from 2011 to 2015 (Table 1). There were 5033 infants who survived more than 3 days. The absolute numbers of births for the 23–28-week age group was relatively constant year to year. The proportion of 23- and 24-week infants related to the total of 28 weeks and under was stable and ranged from 22.3–25.4% during the years of study (p = 0.5). Ontario accounted for 50.3% of all infant data, and Alberta, British Columbia, and Ontario together accounted for to 83% of the infants in all ages. For 23-week infants, Ontario accounted for 56% of the cohort. The proportion of 23-week infants was stable during the study years.
Length of stay
The median length of stay (LOS) was 41 days (IQR: 1–77). Ontario had the lowest median LOS (29 days, IQR: 1–66) and Nova Scotia had the highest median LOS of 77 days (IQR: 53–106). (Table 1) For infants who survived more than 3 days, the median LOS was 61 days (IQR: 34–90) and ranged from 51 days (IQR: 27–82) in Ontario to 88 days (IQR: 64–126) in Newfoundland.
Cost
The median total cost was $66,668 (IQR: $4920–$125,551). This ranged from $48,144 in Ontario (IQR: $2807–$90,619) to $122,526 in Saskatchewan (IQR: $8288–$273,699). The lowest costing for the entire regional cohort was in Ontario, with median cost of $48,144 (IQR: $2807–$90,619), and the second lowest was in New Brunswick, with median cost of $72,956 (IQR: $33,265–$89,216). Figure 1 demonstrates the regional variation in cost for the entire cohort by gestational age. For infants who survived more than 3 days, the median total cost was $91,137 (IQR: $56,596–$188,757). The median daily cost was $1940 (IQR: $1515–$2619) and ranged from $1661 in New Brunswick (IQR: $1325–$2567) to $2696 in Saskatchewan (IQR: $1958–$3420). The median daily cost for infants who survived more than 3 days was $1805 (IQR: $1392–$2419) and ranged from $1567 in New Brunswick (IQR: $1252–$2325) to $2764 in Saskatchewan (IQR: $1931–$3436). There was a small increase in the median total cost over the years of the study (r2 = 0.043 p < 0.001).
There was wide variation between regions even within similar age groups (Fig. 1). For example, median total costs for 25-week infants in Saskatchewan were as high as $273,698 while in Ontario the median was $78,565, and in New Brunswick it was $57,356, a 4.8-fold difference. We examined for regional cost variation for infants born at 28-week gestation (Fig. 2), a typically more stable population, with fewer complications of NICU stay. The median costs in Ontario were $40,524, in Manitoba they were $80,829, and in Saskatchewan they were $116,113, a 2.9-fold difference. There was wide regional variation in cost for every gestational age when compared to the entire cohort. The variation in costs of hospitalization between the regions for each age group were significant (p < 0.001). In a multivariate analysis using a generalized model, fitted to its Gamma distribution, and after elimination of extreme outliers, we demonstrated a persistent regional variation in cost of care after adjustment for length of stay, survival more than 3 days, gestational age, and year of study (n = 6890). For example, for 28-week infants, the adjusted variation was up to 1.87-fold in cost. This model was robust, demonstrated by a pseudo-R2 = 0.93, p < 0.001.
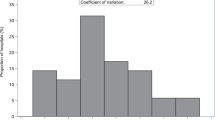
Regional variation in 28-week infants who survived the first 3 days of life, representing a relatively more mature and more stable preterm populations in our cohort. Kruskal-Wallis test for variance. CA – Combined, included provinces, AB- Alberta, BC- British Columbia, MB- Manitoba, NB- New Brunswick, NL- Newfoundland and Labrador, NS- Nova Scotia, ON- Ontario, SK- Saskatchewan
Using the model to estimate potential cost savings, we applied the lowest cost to the other regions in the cohort. The total cost saving calculated was $87,801,982 (95%CI: $95,783,981–$83,810,983) over the 5 years, representing 13.6% saving of the total budget of $643,837,303 over the same timeframe, or $17,560,396 annually.
For a more achievable benchmark [35],we applied the second-lowest cost region to the other regions in the cohort. This resulted in a total cost savings of $71,768,361 (95%CI: $65,527,634–$81,129,451) over the 5-year period. This represented 11.15% of the total budget of, or $14,353,672 annually.
Discussion
We evaluated all extremely premature infants born in Canada from 2011 through 2015. We demonstrated high overall cost for premature infants and their complications. There was up to 8-fold regional variation in cost. The effects persisted even after adjustment for differences in survival, gestational age, length of stay, and year of birth. We found that overall, the median cost of care was $66,668 and for infants who survived more than 3 days median cost was $91,000. This did not change significantly over the study period. We also found that the median length of stay for the entire cohort was 41 days and did not change over time. Moreover, we found that significant savings could be achieved with benchmarking to lower cost regions. In a recent cost evaluation study, Rios et al. [36] reported the cost of tertiary NICU care using a predictive model, estimating the cost of the age group of < 29 week infants at $100,423 (IQR: $56,800–$159,358) and a mean daily cost of $1964. Our study differed in focusing on regional differences and the inclusion of the different age groups and stay at different level of hospital units.
Our study has several strengths. First, we used a reliable, quality-standardized, national-level dataset that includes cost and gestational age. Second, our study reflects data from time periods when infants born at 23 weeks gestation began to be routinely supported. Third, our findings follow the patient care pathway in the complete hospitalization from birth to discharge home or death. This includes hospital transfers to higher and lower acuity sites, thus providing the cost of care for the infant prolonged stay, at the provincial level, from the payor perspective. Fourth, our cost modelling shows robust, significant variation after adjustment for several variables.
Healthcare spending in Canada is determined regionally, where each province is responsible for most of its own healthcare services [37]. The coverage and costing are influenced by local healthcare policies in the context of local economies, and by differences in clinical practices, as well as medical decisions. Notably, regional differences in healthcare costs were demonstrated previously in other areas of healthcare [18,19,20,21,22,23] but not in NICU patients.
International reports through the World Health Organization (WHO) and the Organization for Economic Cooperation and Development (OECD) have compared national outcomes and financial performance in healthcare for many years. Regional variation has previously been reported in various healthcare expenditures [38,39,40,41] at the national level, both in per capita calculations and in relationship to GDP. National comparisons are fraught with difficulties in comparing like elements. In contrast, regional comparisons can often be more standardized. Indeed, regional cost differences have been demonstrated in cancer care [38], cochlear implants [42], tuberculosis care [43], and long-term care [44]. The latter, for example, demonstrated 5-fold variation in regional cost in the same country [44]. Quantifying this variation within a country is important for the regional policymakers to allocate resources, and for policymakers in other countries to compare and benchmark their results and variation. This variation is sometimes reflective of local policies and costing mechanisms. Our data differ because of the consistency in the costing and outcome methods. We found that the variation persisted regardless of the gestational age. Indeed, the variation in median total costs was striking even after rigorous adjustments. For example, median costs for infants born at 28 weeks gestation, a more stable population in this cohort, varied 2.9-fold between the regions. These differences persisted in the multivariate model, supporting the notion that regional variation contributed significantly to the cost of care. Examining the costs for 28-week infants is highly illustrative because their survival rate is close to 100%, and they would complete their stay to discharge. Indeed, their course is typically less complicated [2, 45] and expected to be less expensive. Therefore, regional practices and their inherent costs are more explanatory of the variation in their cost of care.
There are several potential causes for cost variation. Previously listed [46] drivers of healthcare cost are population complexity, physician billing, inflation, pharmaceuticals, materials, remunerations and administrative costs. Some have noted [35] that acuity and complexity can drive these cost differences. However, less is known about cost differences between jurisdictions when comparing the same condition with similar acuity. While there are demonstrable variations in specific cost components between regions, we currently cannot determine the specific causes, or subcategories, of the differences in our data [47]. This is well demonstrated in the fact that one province (SK) had higher median cost while having another had a relatively shorter length of stay (NFL). The differences may stem from local hospital costs, medication and procedural practices, and expensive interventions such as ventilation and parenteral nutrition. The variation in these practices are reflected in national level reports [2] but have not been translated to costs.
Our study has several limitations. First, we excluded some jurisdictions from the analysis due to availability of or quality of data. Nevertheless, we include over 70% of the national population. Additional data may only add to the observed variation. Second, as in many studies, our findings rely on coding accuracy and consistency of administrative data. However, the standardized approach to cost calculation that has been applied to acute care hospitals across Canada in CIHI methodologies [27, 28] was demonstrated to be highly accurate. This enables the calculation of accumulated cost of hospital stay of a preterm infant from birth, through hospital units or transfers, to discharge or demise. Third, our analyses considered only hospital costs from the birth to discharge home or death. It did not include health services in later life that many of these infants, who suffer from complications related to preterm birth, will require. While this may lead to an underestimate of costs, our focus was on the costing of entire hospital stay, thereby better reflecting the local policies. Fourth, the cost of care did not adjust for clinical outcomes or adverse events. These important aspects need to be included within an in-depth comparison of programs, which should be considered in future work. Fifth, we were unable to adjust for clinical practice differences (such as particular procedures, ventilation modes, staffing, or nutrition). This could assist in calculation of cost avoidance due to local systemic contributors to costing. Confidentiality agreements or data limitations prevented us from performing this type of analysis. Sixth, physician compensations are not included in this analysis since this is not reported to CIHI as part of the cost of care calculation. Although this puts an underestimation to the societal cost, this emphasizes even more the high cost in preterm care. Finally, we report cost of hospital stay without ethical consideration regarding quality of life, and without performing a formal cost-effectiveness or a cost-utility analysis. Indeed, ethics in the costs of medical care have been considered in other policy relevant work [14, 18,19,20,21,22,23, 48,49,50].
Consclusions
We found extensive regional cost variation for extremely preterm infants. The findings persisted after adjusting for several predictive factors. These results demonstrate that there is much room for cost reduction and standardization in support of cost reduction, one of the quadruple aims of healthcare quality improvement [51]. Reducing large cost variation through standardization can lead to cost savings [52, 53]. Our findings may be useful to policymakers for planning and resource allocation decisions. Moreover, small cost differences can be amplified over large patient cohorts. In our study, even a small cost variation of 3% translated to large total differences of $2786 per patient and $19,315,117 in total. These were further magnified when potentially achievable amounts for lower cost regions were applied broadly and over several years [54]. Decreasing such variation can help centres and regions decrease their cost while maintaining excellent care. In time, this will allow for channelling the savings towards further investments and innovations to improve the care of these fragile infants.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to data sharing agreements but are available from the corresponding author on reasonable request.
Abbreviations
- AB:
-
Alberta
- BC:
-
British Columbia
- MB:
-
Manitoba
- NB:
-
New Brunswick
- NL:
-
Newfoundland and Labrador
- NS:
-
Nova Scotia
- ON:
-
Ontario
- SK:
-
Saskatchewan
- CAD:
-
Canadian Dollar
- GA:
-
Gestational age
- NICU:
-
Neonatal intensive care unit
References
Irvine B, Dzakpasu S, León JA. Perinatal health indicators 2013: a surveillance report by the Public Health Agency of Canada’s perinatal surveillance system. Health Promot Chronic Dis Prev Can. 2015;35(1):23–4. https://doi.org/10.24095/hpcdp.35.1.05.
The Canadian Neonatal Network™. Accessed June 20, 2019. http://www.canadianneonatalnetwork.org/portal/
Shah PS, McDonald SD, Barrett J, et al. The Canadian preterm birth network: a study protocol for improving outcomes for preterm infants and their families. CMAJ Open. 2018;6(1):E44–9. https://doi.org/10.9778/cmajo.20170128.
Petrou S, Khan K. Economic costs associated with moderate and late preterm birth: primary and secondary evidence. Semin Fetal Neonatal Med. 2012;17(3):170–8. https://doi.org/10.1016/j.siny.2012.02.001.
Barradas DT, Wasserman MP, Daniel-Robinson L, Bruce MA, DiSantis KI, Navarro FH, Jones WA, Manzi NM, Smith MW, Goodness BM. Hospital utilization and costs among preterm infants by payer: Nationwide inpatient sample, 2009. Matern Child Health J. 2016;20(4):808–18. https://doi.org/10.1007/s10995-015-1911-y.
Clements KM, Barfield WD, Ayadi MF, Wilber N. Preterm birth-associated cost of early intervention services: an analysis by gestational age. Pediatrics. 2007;119(4):e866–74. https://doi.org/10.1542/peds.2006-1729.
Guilcher SJT, Bronskill SE, Guan J, Wodchis WP. Who are the high-cost users? A method for person-Centred attribution of health care spending. PLoS One. 2016;11(3):e0149179. https://doi.org/10.1371/journal.pone.0149179.
Conway P, Goodrich K, Machlin S, Sasse B, Cohen J. Patient-centered care categorization of U.S. health care expenditures. Health Serv Res. 2011;46(2):479–90. https://doi.org/10.1111/j.1475-6773.2010.01212.x.
Jefferies AL, Kirpalani HM, Society CP, Committee F. And N. Counselling and management for anticipated extremely preterm birth. Paediatr Child Health. 2012;17(8):443–6. https://doi.org/10.1093/pch/17.8.443.
Ladhani NNN, Chari RS, Dunn MS, Jones G, Shah P, Barrett JFR. No. 347-obstetric Management at Borderline Viability. J Obstet Gynaecol Can. 2017;39(9):781–91. https://doi.org/10.1016/j.jogc.2017.03.108.
Schwartz RM. What price prematurity? Fam Plan Perspect. 1989;21(4):170–4. https://doi.org/10.2307/2135808.
Singer P. A report from Australia: which babies are too expensive to treat? Bioethics. 1987;1(3):275–83. https://doi.org/10.1111/j.1467-8519.1987.tb00013.x.
Yu V. Extremely premature infants: to treat or not to treat? Bioeth News. 1984;3(4):6–12. https://doi.org/10.1007/BF03351131.
Meadow W, Cohen-Cutler S, Spelke B, Kim A, Plesac M, Weis K, Lagatta J. The prediction and cost of futility in the NICU. Acta Paediatr. 2012;101(4):397–402. https://doi.org/10.1111/j.1651-2227.2011.02555.x.
DeRienzo C, Kohler JA, Lada E, Meanor P, Tanaka D. Demonstrating the relationships of length of stay, cost and clinical outcomes in a simulated NICU. J Perinatol. 2016;36(12):1128–31. https://doi.org/10.1038/jp.2016.128.
Diehl-Svrjcek BC, Richardson R. Decreasing NICU costs in the managed care arena: the positive impact of collaborative high-risk OB and NICU disease management programs. Lippincotts Case Manag. 2005;10(3):159–66. https://doi.org/10.1097/00129234-200505000-00007.
Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, And Cost. Health Affairs. 2008;27(3):759–69. https://doi.org/10.1377/hlthaff.27.3.759.
Rushing S, Ment LR. Preterm birth: a cost benefit analysis. Semin Perinatol. 2004;28(6):444–50. https://doi.org/10.1053/j.semperi.2004.10.007.
Partridge JC, Robertson KR, Rogers EE, Landman GO, Allen AJ, Caughey AB. Resuscitation of neonates at 23 weeks’ gestational age: a cost-effectiveness analysis. J Matern Fetal Neonatal Med. 2015;28(2):121–30. https://doi.org/10.3109/14767058.2014.909803.
Nair PM. Survival of extreme pre-term infants in an intensive care set up. Saudi Med J. 2000;21(9):891–2.
Siegel LS. The long-term prognosis of pre-term infants: conceptual, methodological, and ethical issues. Hum Nat. 1994;5(1):103–26. https://doi.org/10.1007/BF02692194.
Hernandez JA, Offutt J, Butterfield LJ. The cost of care of the less-than-1000-gram infant. Clin Perinatol. 1986;13(2):461–76. https://doi.org/10.1016/S0095-5108(18)30832-7.
Walker D-JB, Feldman A, Vohr BR, Oh W. Cost-benefit analysis of neonatal intensive care for infants weighing less than 1,000 grams at birth. Pediatrics. 1984;74(1):20–5.
Cheah IGS. Economic assessment of neonatal intensive care. Transl Pediatr. 2019;8(3):246–56. https://doi.org/10.21037/tp.2019.07.03.
Profit J, Lee D, Zupancic JA, Papile LA, Gutierrez C, Goldie SJ, Gonzalez-Pier E, Salomon JA. Clinical benefits, costs, and cost-effectiveness of neonatal intensive care in Mexico. PLoS Med. 2010;7(12):e1000379. https://doi.org/10.1371/journal.pmed.1000379.
Zainal H, Dahlui M, Soelar SA, Su TT. Cost of preterm birth during initial hospitalization: A care provider’s perspective. PLoS One. 2019;14(6). https://doi.org/10.1371/journal.pone.0211997.
Scales DC, Guan J, Martin CM, Redelmeier DA. Administrative data accurately identified intensive care unit admissions in Ontario. J Clin Epidemiol. 2006;59(8):802–7. https://doi.org/10.1016/j.jclinepi.2005.11.015.
Joseph KS, Fahey J. Canadian perinatal surveillance system. Validation of perinatal data in the discharge Abstract database of the Canadian institute for health information. Chronic Dis Can. 2009;29(3):96–100. https://doi.org/10.24095/hpcdp.29.3.01.
Canadian Institute for Health Information. Cost of a Standard Hospital Stay: Appendices to Indicator Library — Methodology Notes, May 2020. CIHI; 2020.
Benoit D, Skea W, Mitchell S. DEVELOPING COST WEIGHTS WITH LIMITED COST DATA - EXPERIENCES USING CANADIAN COST DATA 2000;2(3):9.
Poole B, Robinson S, MacKinnon M. Resource intensity Weights™ and Canadian hospital costs: some preliminary data. Healthcare Management Forum. 1998;11(1):22–6. https://doi.org/10.1016/S0840-4704(10)61000-9.
CIHI’s Information Quality Framework. :25.
Richards J, Brown A, Homan C. THE DATA QUALITY STUDY OF THE CANADIAN DISCHARGE ABSTRACT DATABASE. :10.
Government of Canada SC. Add/Remove data - Consumer Price Index by product group, monthly, percentage change, not seasonally adjusted, Canada, provinces, Whitehorse, Yellowknife and Iqaluit. Published March 22, 2019. Accessed September 11, 2019. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1810000413
Baker DW, Yendro S. Setting achievable benchmarks for value-based payments: no perfect solution. JAMA. 2018;319(18):1857–8. https://doi.org/10.1001/jama.2018.2360.
Rios JD, Shah PS, Beltempo M, et al. Costs of Neonatal Intensive Care for Canadian Infants with Preterm Birth. J Pediatr Published online September 23. 2020:161–167.e12. https://doi.org/10.1016/j.jpeds.2020.09.045.
Marchildon G. Canada: health system review. Health Syst Transit. 2013;15(1):1–179.
De Oliveira C, Pataky R, Bremner KE, et al. Estimating the cost of Cancer Care in British Columbia and Ontario: a Canadian inter-provincial comparison. Healthc Policy. 2017;12(3):95–108.
How does health spending differ across provinces and territories? | CIHI. Published January 10, 2018. Accessed 10 June 2019. https://www.cihi.ca/en/how-does-health-spending-differ-across-provinces-and-territories-2017
Information CI for H. National Health Expenditure Trends, 1975 to 2015. Canadian institute for health information North York, ON; 2015.
Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, Hopman WM, Lam M, Sears MR, Szpiro K, To T, Paterson NA, Ontario Respiratory Outcomes Research Network. The Ontario asthma regional variation study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129(4):909–17. https://doi.org/10.1378/chest.129.4.909.
Crowson MG, Chen JM, Tucci D. Provincial variation of Cochlear implantation surgical volumes and cost in Canada. Otolaryngol Head Neck Surg. 2017;156(1):137–43. https://doi.org/10.1177/0194599816668325.
Menzies D, Lewis M, Oxlade O. Costs for tuberculosis Care in Canada. Can J Public Health. 2008;99(5):391–6. https://doi.org/10.1007/BF03405248.
Fernandes N, Spencer BG. The private cost of long-term Care in Canada: where you live matters. Can J Aging. 2010;29(3):307–16. https://doi.org/10.1017/S0714980810000346.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, van Meurs KP, Faix RG, Phelps DL, Frantz ID, Watterberg KL, Saha S, Das A, Higgins RD, for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126(3):443–56. https://doi.org/10.1542/peds.2009-2959.
Marchildon GP. Health care cost drivers: the facts. Canadian Institute for Health Information; 2011.
Understanding Variability in the Cost of a Standard Hospital Stay.; 2017. Accessed 1 Apr 2020. https://apps.uqo.ca/LoginSigparb/LoginPourRessources.aspx?url=http://www.deslibris.ca/ID/10096045
Culyer AJ. Economics and ethics in health care. J Med Ethics. 2001;27(4):217–22. https://doi.org/10.1136/jme.27.4.217.
Scheunemann LP, White DB. The ethics and reality of rationing in medicine. Chest. 2011;140(6):1625–32. https://doi.org/10.1378/chest.11-0622.
Meadow W. Epidemiology, economics, and ethics in the NICU: reflections from 30 years of neonatology practice. J Pediatr Gastroenterol Nutr. 2007;45(Suppl 3):S215–7. https://doi.org/10.1097/01.mpg.0000302975.98491.7f.
Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573–6. https://doi.org/10.1370/afm.1713.
Friedman KG, Fulton DR. Reducing cost through standardization. Curr Treat Options Peds. 2016;2(4):296–310. https://doi.org/10.1007/s40746-016-0068-2.
Guzman MJ, Gitelis ME, Linn JG, Ujiki MB, Waskerwitz M, Umanskiy K, Muldoon JP. A model of cost reduction and standardization: improved cost savings while maintaining the quality of care. Dis Colon Rectum. 2015;58(11):1104–7. https://doi.org/10.1097/DCR.0000000000000463.
Weissman NW, Allison JJ, Kiefe CI, Farmer RM, Weaver MT, Williams OD, Child IG, Pemberton JH, Brown KC, Baker CS. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5(3):269–81. https://doi.org/10.1046/j.1365-2753.1999.00203.x.
Acknowledgements
Not Applicable.
Funding
We received no funding or financial support for this study.
Author information
Authors and Affiliations
Contributions
AR conceptualized and designed the study, collected the data, performed the analyses, drafted, and revised the manuscript. SU critically reviewed the design, reviewed the data analysis, reviewed the draft, and revised the manuscript. DU critically reviewed the design, revised the analysis, reviewed the draft, and revised the manuscript. CB conceptualized the design, collected the data, revised the analysis, critically reviewed, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study was approved by Sunnybrook Health Sciences Centre REB, #485–2016 and Canadian Institute for Health Information approved and released the data.
Consent for publication
Not Applicable.
Competing interests
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Rolnitsky, A., Urbach, D., Unger, S. et al. Regional variation in cost of neonatal intensive care for extremely preterm infants. BMC Pediatr 21, 134 (2021). https://doi.org/10.1186/s12887-021-02600-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-021-02600-8