Abstract
Objective
The aim of this study was to determine, retrospectively, the serum 25OHD and calcium concentrations of screened neonates of mothers at high risk of 25OHD deficiency and examine whether their measurement contributes to the management of these neonates.
Methods
Serum 25OHD and calcium concentrations from 600 samples of umbilical cord blood or venous blood collected from neonates over a 12-month period were analysed.
Results
There was a high prevalence of vitamin D insufficiency (27.6%, 30–50 nmol/L) and deficiency (21.3%, < 30 nmol/L) in neonates from high-risk maternal groups. There was a statistically positive but weak correlation (ρ = 0.22, P < 0.0001) between 25OHD and serum calcium. Only 7 neonates out of 569 (1.2%) had calcium concentrations in the hypocalcaemic range; however, a significant number (47.6%) were reported to be in the hypercalcaemic range. Nearly all of these were venous samples collected in first 24 h after birth.
Conclusion
Vitamin D deficiency is prevalent in neonates of high-risk mothers but the risk of hypocalcaemia due to vitamin D deficiency at birth is low. Screening neonates entails blood testing which can cause distress to neonates and their parents, substantial imposition on staff and financial burden on the health care system. Vitamin D supplementation of these neonates from birth without routine screening appears more reasonable. Also, the data from this study suggest that the paediatric reference range for corrected calcium concentrations in neonates should be re-evaluated.
Similar content being viewed by others
Introduction
Vitamin D (25 hydroxy-vitamin D (25OHD)) deficiency is a global health problem and together with poor calcium intake is responsible for nutritional rickets and osteomalacia. When severe, it leads to fractures and skeletal deformities in growing infants and children as well as asymptomatic and symptomatic hypocalcaemia in the form of cardiomyopathy, tetany and seizures [1,2,3,4]. Although vitamin D is primarily required to maintain serum calcium homeostasis, there is increasing evidence that it may play a role in many other metabolic and physiological processes apart from maintaining bone health [5, 6]. Vitamin D deficiency is especially prevalent during pregnancy in women with dark skin pigmentation and/or reduced ultraviolet radiation exposure due to ethno-cultural factors such modest/concealed clothing, application of sunscreen or less outdoor activity due to chronic illness and obesity [7,8,9,10,11]. The re-emergence of nutritional rickets in countries like Australia is not surprising due to increased immigration and diversity of ethnic groups, and thus a high proportion of the population is in the high-risk category of vitamin D and calcium deficiency [3, 12, 13].
The risk of nutritional rickets is greatest when vitamin D deficiency and dietary calcium deficiency are combined. If a child is deficient in only vitamin D or calcium, adequate bone mineralisation can still be sustained [14]. An exception to this is neonates and infants, who are growing rapidly and need both adequate vitamin D and calcium intake for bone mineralisation [4, 14, 15]. Neonates of vitamin D-deficient mothers or those at risk of vitamin D deficiency can exhaust compensatory mechanisms quickly and become hypocalcaemic. The parathyroid hormone stimulates osteoclasts to increase bone resorption to maintain normocalcaemia and impaired renal phosphate absorption and low phosphate levels leading to nutritional rickets and osteomalacia [16, 17].
The management of neonates with maternal vitamin D deficiency or mothers at risk of vitamin D deficiency varies across regions in Australia. In some units, neonates are routinely started on cholecalciferol 400 IU daily, in others they are screened and/or tested, while in many others no screening or treatment protocol exists [18,19,20]. The policy in the paediatric unit at Liverpool Hospital (Sydney, Australia) [21] was to screen neonates for vitamin D deficiency by measuring their 25OHD concentrations or levels after birth if their mothers had 25OHD < 25 nmol/L detected during pregnancy or unknown 25OHD concentrations and risk factors for vitamin D deficiency (Table 1).
Mothers at high risk of vitamin D deficiency were identified by midwives or nursing staff at the time of admission and neonatal cord blood samples obtained at birth. If that opportunity was missed, high risk neonates were themselves tested in the post-natal ward and then managed as per the 2006 Australia and New Zealand consensus statement guidelines [7].
Aim of study
The objective of this study was to determine, retrospectively, the prevalence of vitamin D deficiency and hypocalcaemia in a cohort of ‘high risk neonates’, so as to examine contribution of measurements to the current screening protocol of these neonates.
Methods
This single-centre retrospective study was conducted at Liverpool Hospital in western Sydney and was approved by the South Western Sydney Local Health District Human Research Ethics Committee. The population in this area is quite diverse, with 37% born overseas in a non-English speaking country [22]. Babies born at Liverpool Hospital between January and December 2015 and identified as high risk who had cord blood or venous blood tested for 25OHD and calcium concentrations within the first week of life were included. The levels were obtained from the hospital laboratory records, and the neonatal medical record was used to determine the babies’ gestational age, sex and birth weight. 25 hydroxy-vitamin D and calcium concentrations were measured by automated immunoassay. 25OHD was assayed on the DiaSorin Liaison XL analyser. The laboratory participated in the Vitamin D External Quality Assurance Survey (DEQAS) for international standardization of 25OHD assay. Calcium and albumin were analysed on the Roche Cobas 702 analyser.
A total of 655 samples were collected over a 12-month period, of which 55 were reported as insufficient and were excluded from the analysis. Of the remaining 600 samples, 25OHD concentrations were reported for all while both the corrected calcium concentrations and 25OHD were available for 569 samples (Fig. 1).
The corrected calcium concentrations were reported from the laboratory based on reference intervals from the Clinical Guide to Laboratory Tests [23] as follows: Cord blood sample ref. range 2.32–2.99 mmol/L and Venous blood sample ref. range 0–1 day 2.25–2.65 mmol/L; > 1–2 days 1.75–3.00 mmol/L and > 2–7 days 2.25–2.73 mmol/L.
Statistical analyses
Clinical and patient characteristics were described by frequencies and percentages for categorical variables, while for continuous variables, median, mean (standard deviation) or range was used. Association between 25OHD and other variables was tested by chi-square test, t-tests, Pearson’s product-moment correlation and Spearman’s rank-order correlation. T-tests were used for gender (2 groups, male and female). For birth weight, gestational age and corrected calcium, cross-tabulation and chi-square tests were done with both variables categorised and correlation coefficient and scatter plots for both variables continuous. Differences were considered statistically significant when p- values were less than 0.05. There was no adjustment made for multiple statistical comparisons. SAS 9 statistical software was used for analysis and the reference interval of corrected calcium was calculated with statistics program Analyse It.
Results
The gender distribution was nearly equal in the sample of 600. Neonates were predominantly born at term gestation (≥ 37 weeks) with a mean age of 38.6 weeks and were predominantly of normal birth weight (≥ 2500 g) with mean birth weight of 3212 g (Tables 2 and 3). Cord blood made up 20.3% of the samples while the rest were venous samples. Most neonates had a cord blood or venous blood test done on the first day of life (81.1%) and nearly all samples were collected within 4 days of birth.
There was little or no evidence of association between neonatal 25OHD concentrations and the birth variables of gender, gestational age or birth weight. According to the classification of vitamin D deficiency from the International Global consensus guidelines 2016 [24] vitamin D levels were sufficient (25OHD > 50 nmol/L) in 51% of neonates, insufficient (25OHD 30–50 nmol/L) in 27.6% and deficient (25OHD < 30 nmol/L) in 21.3%. This indicated a high prevalence of vitamin D insufficiency and deficiency in high-risk maternal groups screened for vitamin D levels. (Fig. 2).
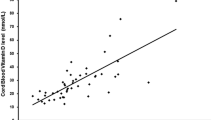
There was overall statistically positive correlation between vitamin D and corrected calcium concentrations (P < 0.0001). However, the strength of the correlation was weak (ρ = 0.22) (Fig. 3).
The corrected calcium concentrations were reported within the normal range in about half of the 569 samples available while levels were in the hypercalcaemic range in 47.6%. Nearly all the hypercalcaemic values were those of venous samples collected on first day of life. Only three venous samples collected after day 1 were in the hypercalcaemic range while none of the cord blood samples collected at birth were hypercalcaemic (Table 4).
The incidence of hypocalcaemia was incredibly low (1.2%). Out of the seven hypocalcaemic neonates, three were preterm, one was low birth weight and three had sufficient 25OHD concentrations (Table 5).
In this study, using the corrected calcium concentrations measured in venous blood in the first 24 h of life, we calculated a normal reference range of 2.38–3.04 mmol/L for corrected calcium. The upper limit of this calculated reference range is significantly higher than the standard reference range used in the laboratory at Liverpool Hospital (2.25–2.65 mmol/L).
Discussion
This is the first study, to our knowledge, to critically examine the practice of screening neonates of high maternal risk for vitamin D deficiency. The absence of a correlation between birth variables and neonatal 25OHD concentrations from our data is consistent with other studies [25,26,27]. However, our study found a higher prevalence of vitamin D deficiency and insufficiency than previously reported by Bowyer et al. in Australia [8] and is comparable to prevalence recorded in mixed ethnic populations of other Western nations [28, 29]. The high prevalence of vitamin D deficiency in predominantly non-white regions like Africa and India is well known [30]; nonetheless, high prevalence of vitamin D deficiency is documented in regions at high latitude with a majority of fair skinned people and in other studies of mainly white ethnic populations [26, 31,32,33]. It seems then measuring 25OHD in high-risk neonates is unnecessary, given that an increased prevalence of vitamin D deficiency has been well established in these groups. One could argue that neonates may need screening to treat them according to the severity of their vitamin D deficiency to prevent complications. We found a very low incidence of hypocalcaemia and no relationship between severity of vitamin D deficiency and hypocalcaemia at birth as well as no reports of clinical seizures in those neonates. Also, there is evidence that even significantly low 25OHD concentrations in term neonates are readily corrected after birth with oral vitamin D supplementation as early as 6 weeks after treatment [34, 35]. Moreover, a systematic review by Mimouni et al. of randomised controlled trials involving vitamin D supplementation from birth to 23 months of age concluded no benefit of doses more than 400 IU for bone mineralisation. There was no effect on long-term outcomes with increased doses; rather, higher doses were potentially associated with adverse effects [36].
There are additional disadvantages of routine testing: cord blood samples are not available in the majority of cases and venepuncture causes undesirable effects of inflicting pain to babies and stress to parents [37]. It takes considerable staff time in organising for the tests, follow up of test results, communicating results to parents, arranging further follow up and thus significant financial costs to the health services [37, 38]. Besides, over half of the neonates were vitamin D sufficient on testing and were not supplemented. Nevertheless, they are at risk of developing vitamin D deficiency if they were exclusively breast fed or until sufficient feed volume is reached in formula fed infants [2, 7]. Hence, most international guidelines recommend oral supplementation with vitamin D for all infants [24, 39]. The major challenge to daily infant cholecalciferol supplementation remains poor adherence [40, 41] which is substantially improved with education and emphasis on cholecalciferol supplementation from health care providers or paediatricians in early post-partum period [42, 43].
We found an overall positive correlation between 25OHD and corrected calcium; however, the strength of the correlation was weak. This is in agreement with the study of Hillman et al. where they documented serial measurements of total calcium and 25OHD levels in term and premature neonates [44]. The correlation between neonatal vitamin D levels and neonatal hypocalcaemia at birth nevertheless is not clear in the literature [45]. Our study indicated a very low incidence of hypocalcaemia at birth, even with severe neonatal vitamin D deficiency. This is corroborated by case reports of symptomatic hypocalcaemia due to vitamin D deficiency usually presenting after first week of life [46,47,48,49]. Thus, testing for hypocalcaemia due to vitamin D deficiency early at birth is not reasonable.
Nearly half of the corrected calcium levels in our study were in the hypercalcaemic range and nearly all of them were venous samples in the first 24 h after birth. Although neonates have higher calcium levels at birth and cord blood calcium levels correlate well with maternal calcium levels [50], the calcium levels drop after birth over the first 12–48 h in neonates [51]. We postulate that the reason for the very high number of hypercalcaemic values may be due to the low upper limit of the reference interval used in the laboratory for venous samples collected in first 24 h [23]. We calculated the reference interval for corrected calcium of venous samples in first 24 h from our data and the upper limit was significantly higher. Many laboratories still use reference intervals for paediatric populations derived from old studies using obsolete equipment, adult populations or unwell children in hospital, all of which are inaccurate [52]. There are initiatives to establish more accurate reference intervals for paediatric populations [53,54,55] however further studies are required to establish correct reference intervals for corrected calcium in neonates.
The study was limited by the fact that we did not have full maternal data to pair mother–infant groups and compare maternal vitamin D status during pregnancy with neonatal 25OHD and calcium levels. A chemiluminescent immunoassay was used for 25OHD measurements rather than the gold standard liquid chromatography-tandem mass spectrometry. We did not have calcium levels for 31 out of 600 samples; however, this is unlikely to have influenced the results. Although no seizures were reported in the hypocalcaemic neonates in our study, we cannot rule out other symptoms of hypocalcaemia.
Conclusion
Vitamin D deficiency is highly prevalent in our mixed-ethnicity population, and neonatal screening of vitamin D levels affirms what is largely known. Neonates must undergo an invasive procedure if cord blood is not available which causes pain to neonates, provokes anxiety in parents and stretches hospital resources. In addition, we found a very low incidence of hypocalcaemia in these healthy neonates with vitamin D deficiency at birth. It also appears that vitamin D deficiency is corrected relatively easily in neonates with supplementation. An alternative model of care of supplementing these babies with cholecalciferol without routine testing appears to offer better value of care.
We found an unusually high incidence of hypercalcaemia in neonates in the first 24 h of life likely due to unsubstantiated normative serum calcium range being used. We calculated a higher upper limit of reference range for corrected calcium. The data from this study suggests there is a need for ratification of reference ranges for corrected calcium levels in neonates.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- 25OHD:
-
25 hydroxy-vitamin D
- IU:
-
International Units
- ref. range:
-
Reference range
References
Paxton GA, Teale GR, Nowson CA, Mason RS, McGrath JJ, Thompson MJ, Siafarikas A, Rodda CP, Munns CF, Australian, B. New Zealand, S. Mineral, A. Osteoporosis. Vitamin D and health in pregnancy, infants, children and adolescents in Australia and New Zealand: a position statement. Med J Aust. 2013;198(3):142–3.
Wagner CL, Greer FR, B. American Academy of Pediatrics Section on, N. American Academy of Pediatrics Committee on. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008;122(5):1142–52.
Robinson PD, Hogler W, Craig ME, Verge CF, Walker JL, Piper AC, Woodhead HJ, Cowell CT, Ambler GR. The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child. 2006;91:564–8.
Hogler W. Complications of vitamin D deficiency from the foetus to the infant: one casue, one prevention but who's responsibility? Best Pract Res Clin Endocrinol Metab. 2015;25:385–98.
Grober U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: update 2013: from rickets prophylaxis to general preventive healthcare. Dermatoendocrinol. 2013;5(3):331–47.
Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25(4):531–41.
Munns C, Zacharin M, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. MJA. 2006;185(5):272.
Bowyer L, Catling-Paull C, Diamond T, Homer C, Davis G, Craig ME. Vitamin D, PTH and calcium levels in pregnant women and their neonates. Clin Endocrinol. 2009;70(3):372–7.
Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child. 2007;92(9):750–3.
Nowson C, Margerison C. Vitamin D intake and vitamin D status of Australians. MJA. 2002;177:149–52.
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Thomsen J, Charles P, Eriksen EF. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited. J Intern Med. 2000;247:260–8.
Welch TR, Bergstrom WH, Tsang RC. Vitamin D–deficient rickets: the reemergence of a once-conquered disease. J Pediatr. 2000;137:143–5.
Di Marco N, Kaufman J, Rodda CP. Shedding light on Vitamin D status and its complexities during pregnancy, infancy and childhood: an Australian perspective. Int J Environ Res Public Health. 2019;16(4):538.
Aggarwal V, Seth A, Aneja S, Sharma B, Sonkar P, Singh S, Marwaha RK. Role of calcium deficiency in development of nutritional rickets in Indian children: a case control study. J Clin Endocrinol Metab. 2012;97(10):3461–6.
Thatcher TD, et al. A comparison of calcium, Vitamin D, or both for nutritional rickets in Nigerian Children. N Engl J Med. 1999;341(8):563–8.
Pettifor JMP, Prentice A. The role of vitamin D in paediatric bone health. Best Pract Res Clin Endocrinol Metab. 2011;25:573–84.
Tiosano D, Hochberg Z. Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27(4):392–401.
South Australia Child Health Clinical Network, Policy clinical guideline - Management of Vitamin D deficiency in children, (2013).
Southern Health, Vitamin D in pregnancy and the term newborn guideline, (2009).
SESLHD NSW policy, Vitamin D deficiency- Management in pregnancy and neonatal period, (2016).
Paediatric Postnatal Ward Handbook. Vitamin D screening algorithm. NSW: Department of Paediatrics, Liverpool Hospital; 2017.
Liverpool City Council, 2011 Census results Liverpool City, (2011).
C. A. Bell, Clinical Guide to Laboratory Tests. 3rd edition. Norbert W. Tietz, ed, Transfusion 35(11) (1995) 972.
Munns CF, et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016;101(2):394–415.
Dalgard C, et al. Umbilical cord serum 25-Hydroxyvitamin D concentrations and relation to Birthweight, head circumference and infant length at age 14 days. Paediatr Perinat Epidemiol. 2016;30:238–45.
Lykkedegn S, Beck-Nielsen SS, Sorensen GL, Andersen LB, Fruekilde PBN, Nielsen J, Kyhl HB, Joergensen JS, Husby S, Christesen HT. Vitamin D supplementation, cord 25-hydroxyvitamin D and birth weight: findings from the Odense child cohort. Clin Nutr. 2017;36(6):1621–7.
Marshall I, Mehta R, Ayers C, Dhumal S, Petrova A. Prevalence and risk factors for vitamin D insufficiency and deficiency at birth and associated outcome. BMC Pediatr. 2016;16(1):208.
Y. JacquemYn, et al, Vitamin D levels in maternal serum and umbilical cord blood in a multi-ethnic population in Antwerp, Belgium, FVV in obGYn 5 (2013).
Vinkhuyzen AAE, Eyles DW, Burne TH, Blanken LME, Kruithof CJ, Verhulst F, Jaddoe VW, Tiemeier H, McGrath JJ. Prevalence and predictors of vitamin D deficiency based on maternal mid-gestation and neonatal cord bloods: the generation R study. J Steroid Biochem Mol Biol. 2016;164:161–7.
Sachan A, et al. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81:1060–4.
Vieth Streym S, Kristine Moller U, Rejnmark L, Heickendorff L, Mosekilde L, Vestergaard P. Maternal and infant vitamin D status during the first 9 months of infant life-a cohort study. Eur J Clin Nutr. 2013;67(10):1022–8.
Weisse K, Winkler S, Hirche F, Herberth G, Hinz D, Bauer M, Roder S, RolleKampczyk U, von Bergen M, Olek S, Sack U, Richter T, Diez U, Borte M, Stangl GI, Lehmann I. Maternal and newborn vitamin D status and its impact on food allergy development in the German LINA cohort study. Allergy. 2013;68(2):220–8.
Haggarty P, Campbell DM, Knox S, Horgan GW, Hoad G, Boulton E, McNeill G, Wallace AM. Vitamin D in pregnancy at high latitude in Scotland. Br J Nutr. 2013;109(5):898–905.
Onwuneme C, Diya B, Uduma O, McCarthy RA, Murphy N, Kilbane MT, McKenna MJ, Molloy EJ. Correction of vitamin D deficiency in a cohort of newborn infants using daily 200 IU vitamin D supplementation. Ir J Med Sci. 2016;185(3):683–7.
Huynh J, Lu T, Liew D, Doery JC, Tudball R, Jona M, Bhamjee R, Rodda CP. Vitamin D in newborns. A randomised controlled trial comparing daily and single oral bolus vitamin D in infants. J Paediatr Child Health. 2017;53(2):163–9.
Mimouni FB, et al. Vitamin D requirements in infancy: a systematic review. Curr Opin Clin Nutr Metab Care. 2017;20(3):222–36.
Anand K, et al. Pain and its effects in the human neonate and fetus. N Engl J Med. 1987;317(21):1321–9.
Boyages SC. Vitamin D testing: new targeted guidelines stem the overtesting tide. Med J Aust. 2016;204(1):18.
Randev S, Kumar P, Guglani V. Vitamin D supplementation in childhood – a review of guidelines. Indian J Pediatr. 2018;85(3):194–201.
Perrine CG, Sharma AJ, Jefferds MED, Serdula MK, Scanlon KS. Adherence to Vitamin D recommendations among US infants. Pediatrics. 2010;125(4):627–32.
Taylor JA, Geyer LJ, Feldman KW. Use of supplemental Vitamin D among infants breastfed for prolonged periods. Pediatrics. 2010;125(1):105–11.
Bennett AE, Kearney JM. Predictors of vitamin D supplementation amongst infants in Ireland throughout the first year of life. J Public Health. 2018;26(5):577–83.
Le B, Vitamin D. Patient Education with a Provided Prescription Prior to Newborn discharge Improves Adherence to Vitamin D recommendation in Infants Returning to Clinic for Follow-up. Pediatrics. 2019;144(2 MeetingAbstract):162.
Hillman LS, et al. Serial measurements of Sr calcium, magnesium, PTH, calcitonin adn 25 OH Vit D in premature and term infants during the first week of life. Pediatr Res. 1977;11:739–44.
Camadoo L, Tibbott R, Isaza F. Maternal vitamin D deficiency associated with neonatal hypocalcaemic convulsions. Nutr J. 2007;6(1):23.
Thomas TC, Smith JM, White PC, Adhikari S. Transient neonatal hypocalcemia: presentation and outcomes. Pediatrics. 2012;129(6):e1461–7.
Ashraf A, Mick G, Atchison J, Petrey B, Abdullatif H, McCormick K. Prevalence of Hypovitaminosis D in Early Infantile Hypocalcemia. J Pediatr Endocrinol Metab. 2016;19:1025–31.
Toaima FH, Al AK. Nineteen cases of symptomatic neonatal HypocalcemiaSecondary to Vitamin D deficiency: a 2-year study. J Trop Pediatr. 2010;56(2):108–10.
Kovacs CS. Maternal vitamin D deficiency: Fetal and neonatal implications. Semin Fetal Neonatal Med. 2013;18:129–35.
Deshpande N, Patil L, Deshpande S, Chavan S. Study of ionic calcium in maternal and cord blood and baby's blood at 48-h age. Med J. 2014;7(2):152–5 Dr. D.Y. Patil Vidyapeeth.
Kovacs C. Bone development and mineral homeostasis in the fetus and neonate: role of the calciotropic and phosphotropic hormones. Physiol Rev. 2014;94:1143–218.
Tahmaseb H, et al. Pediatric reference intervals for biochemical markersgaps and challenges, recent national initiatives and future perspectives. J Int Fed Clin Chem Lab Med. 2017;28(1):43–63.
Tate JR, et al. Opinion paper- deriving harmonised reference intervals– global activities. J Int Fed Clin Chem Lab Med. 2016;27(1):48–65.
Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, Adeli K. Canadian laboratory initiative on pediatric reference interval database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem. 2009;42(9):885–91.
Roizen JD, Shah V, Levine MA, Carlow DC. Determination of reference intervals for serum total calcium in the Vitamin D-replete pediatric population. J Clin Endocrinol Metab. 2013;98(12):E1946–50.
Acknowledgements
Elizabeth Barnes (Biostatistician, Kids Research Institute, the Children’s Hospital at Westmead) and Frank Alvaro (Clinical Chemistry Laboratory Manager, Liverpool Hospital) provided assistance with statistical analysis and their help is much appreciated.
Funding
Funding for data extraction from the laboratory was provided by Liverpool Hospital.
Author information
Authors and Affiliations
Contributions
SAK analysed and interpreted the data and was a major contributor in writing the manuscript. PC and CM supervised the study and manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the South Western Sydney Local Health District Human Research Ethics Committee, New South Wales, Australia (HREC Reference: LNR/16/LPOOL/48). The ethics committee classified this retrospective project as low risk and consent was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kozgar, S.A.M., Chay, P. & Munns, C.F. Screening of vitamin D and calcium concentrations in neonates of mothers at high risk of vitamin D deficiency. BMC Pediatr 20, 332 (2020). https://doi.org/10.1186/s12887-020-02204-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-020-02204-8