Abstract
Background
Many clinicians have concerns about the safety of atopic dermatitis (AD) treatments, particularly in children requiring long-term daily maintenance therapy. Topical corticosteroids (TCS) have been widely used for >5 decades. Long-term TCS monotherapy has been associated with adverse cutaneous effects including atrophy, rebound flares, and increased percutaneous absorption with potential for adverse systemic effects. Topical calcineurin inhibitors (TCIs), tacrolimus and pimecrolimus, available for 1–2 decades, are not associated with atrophy or increased percutaneous absorption after prolonged use and have much lower potential for systemic effects. However, since 2006 TCIs have carried a controversial Boxed Warning based on a theoretical risk of malignancy (eg, skin and lymphoma) that has limited TCI use for standard-of-care maintenance therapy.
Methods
A comparative systematic search of PubMed was done for long-term (≥12 week) clinical trials of TCS or TCI treatment in patients <12 years with AD. Citations were reviewed for inclusion based on MeSH terms, abstracts, and relevant article text. Studies were excluded if they did not encompass subjects <12 years, or were <12 weeks’ duration, retrospective, meta-analyses, or limited to anecdotal case reports.
Results
Of 27 trials meeting criteria, 21 included 5825 pediatric patients treated with TCIs, and 6 included 1999 patients treated with TCS. TCS studies were limited to low- to mid-potency products, and all but one study lacked a vehicle control. Eight TCI studies were vehicle-controlled, and safety data were well reported, with ≤5 % of patients reporting discontinuation due to adverse effects (DAEs). Cutaneous and systemic adverse events (AEs) were similar in TCI and vehicle groups, with no reports of lymphoma. Safety data in TCS trials were less well reported. DAE incidence was addressed in just 2 trials, and systemic and cutaneous AEs were mostly unreported.
Conclusions
Data supporting long-term use of TCIs are robust, documenting safety and efficacy, while data supporting long-term TCS use are limited to low- to mid-potency products. Our review identifies a lack of information on the safety of commonly prescribed, long-term monotherapy with mid- to high-potency TCS in pediatric AD, and supports standard-of-care maintenance therapy with TCIs and intermittent use of low- to mid-potency TCS for flares.
Similar content being viewed by others
Background
Atopic Dermatitis (AD) is a chronic, pruritic inflammatory skin disease that occurs most frequently in children. It is the most common chronic pediatric inflammatory skin disease, affecting 12.5 % of US children (aged 0–17 years) from 2009 to 2011, an increase of 5.1 % from 1997 to 1999 [1]. More than half of pediatric patients with AD have mild disease [2], yet the majority of pediatricians refer even their mild patients to dermatologists after providing initial, limited care [3, 4]. This pattern of referrals is due at least in part to questions about the safety of using topical corticosteroids (TCS) and topical calcineurin inhibitors (TCI) to treat AD, particularly in pediatric patients. However, given the shortage of specialists and an increased emphasis on the accountable care model, primary care physicians and pediatricians will continue to play an important role in the management of AD, both as a first-line contact and in regular maintenance following consultation with a specialist.
TCS, which are considered to be first-line treatment for AD flares, have been FDA-approved for a variety of grandfathered indications since 1955. Their mechanism of action, though not well understood, is multifaceted and includes broad-spectrum impact on immune and skin barrier function. Despite their demonstrated efficacy in AD, TCS are associated with a known potential for cutaneous atrophy-related adverse effects such as telangiectasia, striae and purpura, as well as focal hypertrichosis, hypopigmentation and perioral dermatitis [5–9]. In addition, long-term and/or more than once-daily use is associated with subclinical barrier disruption that can result in rebound flares following discontinuation. Long-term subclinical barrier disruption may also cause cumulative increases in percutaneous absorption, with the possibility of rare but insidious and difficult-to-quantify systemic adverse events such as adrenal suppression, poor growth, hypertension, hyperglycemia, insulin resistance, and cataracts [10–14]. These safety concerns are increased in pediatric patients, whose greater body surface area-to-weight ratio is thought to cause increased percutaneous absorption. This risk may be compounded by concomitant use of corticosteroids for other atopic comorbidities (asthma, allergic rhinitis).
In 2000–2001, TCIs were approved in the US for “short-term and noncontinuous chronic treatment of AD in nonimmunocompromised individuals who have failed to respond adequately to other topical prescription AD treatments” [15, 16]. Tacrolimus ointment is available in 2 concentrations: 0.03 %, approved for patients over age 2, and 0.1 %, approved only for patients over age 16 due to theoretical concerns. Pimecrolimus 1 % cream is approved for patients over age 2. In 2006, the FDA instituted a Boxed Warning for both TCIs based on a theoretical risk of malignancy (including lymphomas) that sparked a debate over their safety and appropriate use [17].
Since then, no clear link has been demonstrated between TCI use and lymphoma risk, despite almost a decade of clinical and epidemiological studies, post-marketing surveillance, and monitoring of reports to the FDA Adverse Event Reporting System (AERS). Recent published reviews and/or meta-analyses that assess lymphoma risk of TCIs based on the last decade of clinical experience conclude that there is no evidence that TCI use is associated with increased risk of lymphoma (Table 1) [18–39]. Yet the Boxed Warning remains, leaving many clinicians hesitant to prescribe TCIs despite their potential benefit to some patients. Unlike TCS, TCIs do not carry the risks of skin atrophy, percutaneous absorption, or rebound flares, and have been also been demonstrated to reduce TCS use in long-term studies [40–48]. Therefore TCIs are potentially useful as steroid-sparing agents, and as first-line topical anti-inflammatory treatment on the face and in skin folds.
To assess the safety of TCS and TCI use in children, we performed a comparative systematic literature search for published, long-term clinical trials of TCS or TCI treatment in pediatric patients with AD.
Methods
Systematic searches for published, long-term clinical trials of TCI and TCS trials in pediatric patients
The search strategies we used to identify published, long-term (≥ 12 week) clinical trials of TCI and TCS in pediatric patients (<12 yrs of age) with AD are shown in Figs. 1 and 2. For TCI trials, PubMed was queried with the following terms: (topical calcineurin inhibitor OR TCI OR pimecrolimus OR tacrolimus) AND (atopic dermatitis OR eczema). Results were filtered for clinical trials to eliminate other types of publications (eg, review articles, meta-analyses, case reports) and minimize risk of bias. For TCS trials, PubMed was queried with the terms: topical AND (glucocorticoid OR glucocorticosteroid OR corticosteroid OR steroid OR hydrocortisone OR fluocinolone OR triamcinolone OR desonide OR prednicarbate OR fluticasone OR mometasone) AND (atopic dermatitis OR eczema). Results were filtered for clinical trials to eliminate other types of publications (eg, review articles, meta-analyses, case reports) and minimize risk of bias. For all citations obtained, MeSH terms, abstracts, and when necessary the article text, were reviewed. Citations were excluded if they were not English-language or were duplicate PubMed entries; if they reported trials in animals or in healthy subjects without AD; if they included subjects with psoriasis, asthma, or hand eczema; or if TCI, or TCS, were not the active treatment being assessed. Meta-analyses/review articles that did not present new, previously unpublished data were excluded, as well as retrospective studies, case reports, and studies of 10 or fewer patients.
Systematic search strategy for published long-term (≥12 weeks) TCI trials in pediatric patients (<12 years) with AD. a Exclusions were based on review of MeSH terms, abstract, or (when necessary) the article text. b Meta-analyses and reviews were excluded if no new (previously unpublished) data were presented
Systematic search strategy for published long-term (≥12 weeks) TCS trials in pediatric patients (<12 years) with AD. a Exclusions were based on review of MeSH terms, abstract, or (when necessary) the article text. b Meta-analyses and reviews were excluded if no new (previously unpublished) data were presented
Finally, we excluded studies that did not include safety data, were less than 12 weeks’ duration, or did not include pediatric patients (<12 yrs of age).
Summary of safety data from TCI and TCS trials
Long-term safety data from the TCI and TCS trials that met inclusion criteria were summarized descriptively; statistical analyses were not performed due to the inconsistency in reporting of these data. We limited the focus to safety data that we judged to be most relevant to long-term AD treatment: discontinuations due to AEs, cutaneous AEs (skin infection, atrophy), and systemic AEs (infection, lymphoma, gastrointestinal [GI] events, respiratory tract infections [RTIs]). We did not present the incidence of minor application site reactions (burning, pruritus) that typically occur early in treatment and resolve, potentially atopic or allergic events (allergies, asthma, conjunctivitis) that are common in patients with AD and are generally not related to treatment, or events with unclear origin that are unlikely to be treatment-related (cough, fever, headache, nasopharyngitis, rhinitis).
Results
Published trials included in safety summary
The PubMed queries resulted in 221 TCI citations and 487 TCS citations (Figs. 1 and 2). Of these, 21 TCI trials (27 citations [41, 44–69]) met the inclusion criteria for our safety summary and are presented in order of duration in Tables 2 and 3. All 21 TCI trials were published after 2000, and more than half were published after the Boxed Warning was issued in 2006. Six trials (6 citations [50, 61, 70–73]) met inclusion criteria and are included in the TCS safety summary (Table 4), 2 of which were actually TCI trials with a TCS treatment arm as an active comparator (both trials are also included in the TCI safety summary [50, 61]). One long-term TCS trial in pediatric patients was excluded from our summary due to its retrospective design (n = 756) [74]. In this study, patients using TCS of any potency were included in the study, and no systemic safety data were reported. All but 1 of the TCS trials included in our summary were published after 2000, but only 1 was published after 2006.
Study designs
Treatment varied according to individual study designs, but typically a brief twice-daily regimen to control the initial flare was followed by a maintenance period of intermittent, as-needed treatment for flares (study treatments during the maintenance phase are indicated in bold in Tables 2, 3 and 4).
TCI trials
Three tacrolimus trials were double-blind, vehicle-controlled (2 tacrolimus 0.03 % vs vehicle [51–54], 1 tacrolimus 0.1 % vs tacrolimus 0.03 % vs vehicle [49]), 1 was double-blind, active- and no treatment-controlled (tacrolimus 0.03 % vs hydrocortisone vs no treatment [50]), and 5 were uncontrolled, open-label [55–59]. Five pimecrolimus studies were double-blind, vehicle-controlled [41, 44–48], and 1 was double-blind, active-controlled (pimecrolimus BID vs QD [60]). One study was open-label, active-controlled (the Petite Study, pimecrolimus vs low- or mid-potency TCS [61]), and 5 were uncontrolled, open-label trials [62–69].
TCS trials
Two TCS studies were double-blind, active-controlled: 1 low-potency TCS (hydrocortisone) vs mid-potency TCS (betamethasone valerate) [70], and 1 low-potency TCS (desonide) vs low-potency TCS (hydrocortisone) [71]. One was an open-label, vehicle-controlled trial of mid-potency TCS (fluticasone) vs vehicle [72], and another was a meta-analysis of 2 previously unpublished, double-blind, active-controlled trials of mid- or low-potency TCS (fluticasone or hydrocortisone) [73].
Two additional studies that met inclusion criteria for TCS safety summary were actually TCI studies in which TCS treatment was an active comparator: one was a double-blind trial of tacrolimus vs low-potency TCS (hydrocortisone) vs no treatment [50], and the other was the Petite Study, an open-label trial of pimecrolimus vs low- or mid-potency TCS (hydrocortisone or hydrocortisone butyrate) [61].
We did not identify any published long-term pediatric trials of high-potency TCS.
TCS use in TCI studies
All except 3 of the tacrolimus studies prohibited TCS use; 1 study permitted TCS use per protocol during the first 4 weeks [55], 1 permitted TCS use for flares not controlled by study medication [58], and 1 prohibited TCS use but did not exclude an unspecified number of patients that deviated from study protocol and used TCS [59]. In 10 of the 12 pimecrolimus studies, TCS use was permitted for flares not controlled by study medication; in these studies, 28–72 % of patients in any treatment group reported using a TCS (2 trials did not report the incidence of TCS use).
Number of patients analyzed and duration of treatment
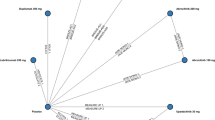
The TCI studies ranged in duration from 12 weeks to 5 years (260 weeks) and included 5825 pediatric patients with mild to severe AD (tacrolimus studies = 1370, pimecrolimus studies = 4455) (Fig. 3). Six TCS trials (including 2 in which TCS treatment was an active comparator) included 1999 pediatric patients with mild to severe AD. The only TCS study longer than 48 weeks was the Petite study, in which TCS was an active comparator for pimecrolimus [61]; the 1213 TCS patients in this study accounted for more than half of the total number of TCS subjects in the analysis. In contrast, there were 8 TCI trials of >2700 patients with durations of more than 48 weeks [41, 46, 53, 54, 57–59, 61, 68, 69], including a 2-year study (n = 91), 4-year study (n = 391), and the 5-year Petite study (n = 1205).
Total subjects included in summary of long-term (≥12 weeks) pediatric trials by therapeutic agent and study duration. a Lighter shading indicates the proportion of patients that received TCS treatment as an active comparator in studies of a TCI. b One of the trials was a 1-yr OL extension of a 1-yr DB study. N = Number of studies. n = Number of subjects in respective treatment group
Reporting of safety data in the published trials
Reporting of safety data was highly variable from study to study, especially in the TCS studies. While TCI trials usually reported the cutaneous and systemic AEs that occurred most frequently during the study, many TCS studies only reported application-site events, and did not report the incidence of systemic AEs. Further, instead of reporting any AE that occurred frequently, regardless of severity or relation to study treatment, some TCS studies reported only AEs that were classified as serious/severe and/or thought to be treatment-related. Terminology and classification of AEs was inconsistent across all the studies, with some studies reporting incidences of specific AEs, and others reporting incidences of general AE categories (eg, bacterial, viral, or fungal infection). In the studies that reported the incidence of specific AEs, the terminology used for the AEs was not consistent between studies.
Summary of safety data
Safety data are summarized in Table 5. Studies are listed from shortest to longest duration, and divided by treatment received: tacrolimus (N = 1370; 0.03 %, n = 524; 0.1 %, n = 846), pimecrolimus (N = 4455), low-potency TCS (N = 400), mid-potency TCS (N = 257), low- or mid-potency TCS (N = 1342), vehicle + TCS rescue (TCS was permitted to treat flares not controlled by study medication, N = 729), vehicle (N = 355), and no treatment (N = 50; these subjects did not have AD).
To increase clarity and interpretability, AEs were categorized as either cutaneous events (bacterial infection, viral, infection, fungal infection, or atrophy) or systemic events (bacterial infection, viral infection, RTIs, GI events, or lymphoma). A list of the AE terms that were included in each category is provided in the footer of Table 5. When multiple AEs in the same category were reported, incidences are shown as a range. If the incidence of an AE category was not reported in the published study, it could not be assumed to be 0 and is therefore shown as “●”. AE incidences of >1 % were rounded to the nearest whole number.
Discontinuations due to adverse events
Fourteen of 21 TCI studies reported the incidence of discontinuation due to AEs (DAEs, Table 5); in those studies it was ≤5 %. About half of the DAEs were caused by application-site events (with the exception of 1 tacrolimus study in which all 9 DAEs in the vehicle group were application-site events). In contrast, only 2 TCS trials reported the incidence of DAEs; in those 2 studies, the incidence was <5 %.
Cutaneous adverse events
The incidence of skin infections in TCI-treated patients was generally low (Table 5), and similar to those reported by vehicle-treated patients. In all but one trial of <1 year duration, ≤5 % of patients reported skin infections (in a 24-week, uncontrolled, open-label tacrolimus study, 13 % of patients reported impetigo [56]). In most longer-term studies (≥1 year duration), skin infection occurred in <10 % of patients. Varicella was reported by 18 % of patients in a 2-year tacrolimus study and 20 and 16 % of pimecrolimus and vehicle patients, respectively in a 1-year study [41, 58]), and impetigo was reported by 27 % of vehicle patients (but just 8 % of pimecrolimus patients) in another 1-year trial [46]. In the 5-year Petite study, rates of skin infection were similar in the pimecrolimus and TCS groups (impetigo was reported in 12 % of pimecrolimus and 10 % of low- or mid-potency TCS patients, and varicella was reported in 25 and 23 %, respectively [61]). There were no reports of skin atrophy in the TCI studies.
Besides the Petite study, the incidence of skin infection in TCS patients was reported in just 2 studies, a 16-week trial and a 28-week trial; the incidence was ≤2 % in those studies. Three TCS studies used various methods to assess skin for signs of atrophy. An 18-week study measured skin thickness with an ultrasound scanner [70] and found that 8 and 12 % of patients using low- and mid-potency TCS, respectively, had >25 % reduction in skin thickness. In a 25-week study of low-potency TCS [71], investigators found no signs of atrophy using an 8x magnifying lamp. In a meta-analysis of 2 16-week studies of low- or mid-potency TCS [72], investigators visually assessed skin and found no evidence of atrophy.
Systemic adverse events
The most frequently reported systemic AEs in the TCI studies were common childhood infections that were considered unrelated to treatment. Systemic infections were reported in up to 20 % of patients in TCI studies of <1 year duration; higher incidences were reported in some longer studies. In one 1-year study, 27 and 25 % of patients in the pimecrolimus and vehicle groups, respectively, reported an RTI, and 28 and 26 % reported a gastrointestinal (GI) event [41]. Almost half of patients in a 4-year, open-label tacrolimus study reported a viral infection [59], and in a 2-year, open-label tacrolimus study up to 90 % of patients reported an RTI, and 38 % reported a GI event [58].
In the 5-year Petite study, up to 17 % of pimecrolimus and TCS patients reported some type of systemic bacterial infection, and 17 % reported influenza-like illness [61]. Up to 35 and 32 % of pimecrolimus and TCS patients, respectively, reported RTIs, and up to 32 and 31 % had GI events. Besides the Petite study, just 2 TCS studies of 16- and 18-week durations reported incidences of systemic AEs of ≤6 % [70, 73].
Effects on adrenal and immune system function
The Petite study investigated the effects of up to 5 years of AD treatment on growth rate and immune system function in a large population of infants, and found no difference in growth rate between the pimecrolimus and TCS treatment groups [61]. Similarly, immunologic investigation showed that infants in the pimecrolimus and TCS groups developed antibody titers to common vaccine antigens that were similar to each other and to what would be expected in the general population.
Prior to the Petite study, there were few published studies that evaluated the effects of long-term TCS treatment on immune system or HPA-axis function in pediatric patients. In a 44-week study of mid-potency TCS, 2 patients from a small cohort that were administered a cosyntropin stimulation test (CST) had reduced serum cortisol responses [72]. In a 28-week study, neither tacrolimus nor low-potency TCS interfered with immune response following vaccination [50], and a third study found that long-term (up to 2 years) treatment with pimecrolimus did not interfere with the development of systemic immune responses in young children [68].
Ocular risk
None of the TCI and TCS trials we identified in our systematic literature search assessed glaucoma or cataracts.
Lymphoma risk
There were no reports of lymphoma in any of the TCI and TCS trials, including the 5-year Petite Study (Table 5).
Discussion
Given the increasing frequency of AD, primary care physicians will continue to play an important role in its management. Some clinicians, patients, and/or parents have concerns about the safety of topical AD treatments, especially in children who have an increased risk of percutaneous absorption and systemic exposure. These safety concerns are also increased with long-term daily maintenance therapy.
We conducted a systematic search for safety data from published pediatric TCI and TCS clinical trials of at least 12 weeks duration, and identified 27 clinical trials that met our inclusion criteria. Twenty-one TCI studies included 5825 pediatric patients, >2700 of whom were followed for 48 weeks to 5 years. Six TCS studies were limited to low- to mid-potency products and included 1999 pediatric patients, more than half of whom received TCS as an active comparator in a study designed to evaluate the safety of pimecrolimus. Most TCI studies included a vehicle control group, while all but one TCS study lacked a vehicle control.
Safety data were generally well reported in the TCI studies, including the incidences of cutaneous and systemic infections. Less than 5 % of patients in the TCI studies discontinued due to AEs. Incidences of cutaneous AEs were similar in TCI and vehicle treatment groups, and were within expected range given the predisposition of the AD patient to cutaneous infections [75]. Systemic AEs that occurred were common childhood infections and not considered to be related to treatment; incidences were similar in treatment and vehicle groups. Extracutaneous viral infections were reported in 47 and 90 % of patients in 2 uncontrolled tacrolimus studies; these incidences are not unexpected given that children under age 5 are expected to have 3 to 6 respiratory infections per year [76, 77]. In contrast, safety data in TCS trials were not as well reported: systemic and cutaneous AEs were mostly unreported, and DAE incidence was addressed in just 2 trials. None of the TCS studies assessed the incidence of glaucoma or cataracts.
The inconsistency of safety data reporting in the trials may be due to timing, as more than half of the TCI studies were published after 2006, when the Boxed Warning for TCIs increased the focus on their safety and appropriate use. In contrast, only 2 TCS studies (including the Petite Study) were published in or after 2006. More recent trials tend to have improved protocols with standardized and validated methods for assessing safety data. This is especially true following the establishment by the NIH in 2000 of a clinical trial registry (ClinicalTrials.gov), and FDA-mandated expansions in 2007 of registry requirements, including disclosure of study design. Further, journal criteria for publication continue to become more rigorous, requiring more clear and complete reporting of data as well as clinical trial registration prior to publication.
According to current treatment guidelines, low-to-mid potency TCS should be used as first-line, short-term treatment of flares [3, 8, 78]. Choice of potency depends on the severity, extent, and site of the flare; the least potent TCS that can control the symptoms should be used. No TCS is indicated for >4 consecutive weeks of use, and few are approved for patients younger than 2 years of age because of risk of atrophy and rebound effects. For mild AD, long-term control is often possible with intermittent TCS for flares and trigger avoidance, bleach baths, and daily moisturizers. Moderate-to-severe AD usually requires long-term daily maintenance therapy with intermittent TCS or TCIs, which are most useful as steroid-sparing agents. The regimen and choice of product for maintenance therapy depends on factors including patient preference, access to medications, cost, and a careful evaluation of benefits versus risks.
Our review supports the long-term safety of TCIs and low-to-mid potency TCS in pediatric patients with AD. Long-term treatment with TCIs and intermittent use of low-to-mid potency TCS was generally well tolerated in 27 trials of >5800 and >1900 pediatric patients, respectively, with no evidence of cutaneous atrophy or cumulative systemic exposure and no reports of lymphoma. This reflects preclinical animal studies that detected malignancy signals only after systemic TCI exposure was high enough to cause immune suppression. This exposure was much higher than the negligible systemic exposure detected after twice daily topical administration (the highest blood concentrations reported for infants with pimecrolimus 1 % range from 1.8 to 4.14 mg/ml, and the average maximum concentration with tacrolimus 0.03 % was 3 % of that observed in pediatric liver-transplant patients receiving oral tacrolimus) [17, 79–84].
Many recent meta-analyses and reviews have also contributed to the body of evidence that has filed to detect increased lymphoma risk with TCIs [18–39]. One study compared the incidence of TCI-associated malignancies reported to multiple TCI AE databases and found a rate similar to or lower than the expected rate of malignancy in the general population [17], and another compared incidences of lymphoma in health insurance claims databases and did not find an increased risk among patients treated with TCIs versus TCS [85]. And no increased risk of malignancy was detected in 7457 children enrolled as of May 2014 in the ongoing prospective 10-year observational cohort study of children with a history of AD and pimecrolimus use (Pediatric Eczema Elective Registry, PEER) [86].
A decade’s worth of clinical experience, epidemiological data, postmarketing surveillance, and adverse event database monitoring have failed to demonstrate a causal relationship between TCI use and malignancy, yet TCI labelling continues to include a Boxed Warning. The biggest impact of the warning is to limit patient access to the most well-studied medications for long-term maintenance AD treatment, especially in children.
Conclusions
This comprehensive literature review supports the long-term safety of TCI and low- to mid-potency TCS therapy in children with AD, with no evidence of cutaneous atrophy or cumulative systemic exposure and no reports of lymphoma. We found comparatively limited data on the long-term safety of mid- to high-potency TCS. Our findings are not reflected by the current TCI labelling and Boxed Warning; therefore we hope our review facilitates the rescindment of the Boxed Warning.
Abbreviations
- AD:
-
atopic dermatitis
- AE:
-
adverse event
- BID:
-
twice daily
- BMV:
-
betamethasone valerate
- d:
-
days
- DAE:
-
discontinuation due to adverse effect
- DB:
-
double-blind
- DES:
-
desonide
- EASI:
-
eczema area and severity index
- FTC:
-
fluticasone propionate
- GI:
-
gastrointestinal
- HB:
-
hydrocortisone butyrate
- HYD:
-
hydrocortisone
- IGA:
-
investigator’s global assessment
- Mo:
-
months
- NR:
-
not reported
- OL:
-
open-label
- PM:
-
pimecrolimus
- PSGA:
-
physician’s static global assessment
- Pts:
-
patients
- QD:
-
once daily
- R&L:
-
Rajka and Langeland
- RTI:
-
respiratory tract infection
- SASSAD:
-
six area, six sign atopic dermatitis
- TC:
-
tacrolimus
- TCI:
-
topical calcineurin inhibitor
- TCS:
-
topical corticosteroids
- Tx:
-
treatment
- URTI:
-
upper respiratory tract infection
- VEH:
-
vehicle
- Wk:
-
week(s)
- Yr:
-
year(s)
References
Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. Hyattsville: National Center for Health Statistics; 2013.
Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013;24(5):476–86. doi:10.1111/pai.12095.
Eichenfield LF, Boguniewicz M, Simpson EL, Russell JJ, Block JK, Feldman SR et al. Translating atopic dermatitis management guidelines into practice for primary care providers. Pediatrics. 2015. [In Progress]
Saavedra JM, Boguniewicz M, Chamlin S, Lake A, Nedorost S, Czerkies LA, et al. Patterns of clinical management of atopic dermatitis in infants and toddlers: a survey of three physician specialties in the United States. J Pediatr. 2013;163(6):1747–53. doi:10.1016/j.jpeds.2013.06.073.
Eichenfield LF, Totri C. Optimizing outcomes for paediatric atopic dermatitis. Br J Dermatol. 2014;170 Suppl 1:31–7. doi:10.1111/bjd.12976.
Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15. doi:10.1016/j.jaad.2005.01.010.
Charman C, Williams H. The use of corticosteroids and corticosteroid phobia in atopic dermatitis. Clin Dermatol. 2003;21(3):193–200.
Leung AK, Barber KA. Managing childhood atopic dermatitis. Adv Ther. 2003;20(3):129–37.
Stoppoloni G, Prisco F, Santinelli R, Sicuranza G, Giordano C. Potential hazards of topical steroid therapy. Am J Dis Child. 1983;137(11):1130–1.
Ellison JA, Patel L, Ray DW, David TJ, Clayton PE. Hypothalamic-pituitary-adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics. 2000;105(4 Pt 1):794–9.
Keipert JA, Kelly R. Temporary Cushing’s syndrome from percutaneous absorption of betamethasone 17-valerate. Med J Aust. 1971;1(10):542–4.
May P, Stein EJ, Ryter RJ, Hirsh FS, Michel B, Levy RP. Cushing syndrome from percutaneous absorption of triamcinolone cream. Arch Intern Med. 1976;136(5):612–3.
Munro DD. Topical corticosteroid therapy and its effect on the hypothalamic-pituitary-adrenal axis. Dermatologica. 1976;152 Suppl 1:173–80.
Patel L, Clayton PE, Jenney ME, Ferguson JE, David TJ. Adult height in patients with childhood onset atopic dermatitis. Arch Dis Child. 1997;76(6):505–8.
Astellas Pharma US, Inc. Protopic. ® (tacrolimus) ointment 0.03% and ointment 0.1%. US ed. Deerfield2011.
Valeant Pharmaceuticals North America LLC. Elidel. ® (pimecrolimus) cream 1%. US ed. Bridgewater2014.
Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14(3):163–78. doi:10.1007/s40257-013-0020-1.
Berger TG, Duvic M, Van Voorhees AS, VanBeek MJ, Frieden IJ. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54(5):818–23. doi:10.1016/j.jaad.2006.01.054.
Deleuran M, Zachariae C, Thestrup-Pedersen K. Topical immune modulation and risk of cancer. Ugeskr Laeger. 2009;171(35):2468–71.
Ehrchen J, Sunderkotter C, Luger T, Steinhoff M. Calcineurin inhibitors for the treatment of atopic dermatitis. Expert Opin Pharmacother. 2008;9(17):3009–23. doi:10.1517/14656560802498040.
Fonacier L, Spergel J, Charlesworth EN, Weldon D, Beltrani V, Bernhisel-Broadbent J, et al. Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2005;115(6):1249–53. doi:10.1016/j.jaci.2005.04.006.
Langley RG, Luger TA, Cork MJ, Schneider D, Paul C. An update on the safety and tolerability of pimecrolimus cream 1%: evidence from clinical trials and post-marketing surveillance. Dermatology. 2007;215 Suppl 1:27–44. doi:10.1159/000102118.
Lebwohl M, Gower T. A safety assessment of topical calcineurin inhibitors in the treatment of atopic dermatitis. MedGenMed. 2006;8(4):8.
Legendre L, Barnetche T, Mazereeuw-Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: A systematic review and meta-analysis. J Am Acad Dermatol. 2015. doi:10.1016/j.jaad.2015.02.1116.
McNeill AM, Koo JY. “Unknown Risks” of non-steroid topical medications for atopic dermatitis. Int J Dermatol. 2007;46(6):656–8. doi:10.1111/j.1365-4632.2007.02306.x.
Munzenberger PJ, Montejo JM. Safety of topical calcineurin inhibitors for the treatment of atopic dermatitis. Pharmacotherapy. 2007;27(7):1020–8. doi:10.1592/phco.27.7.1020.
Orlow SJ. Topical calcineurin inhibitors in pediatric atopic dermatitis: a critical analysis of current issues. Paediatr Drugs. 2007;9(5):289–99.
Ormerod AD. Topical tacrolimus and pimecrolimus and the risk of cancer: how much cause for concern? Br J Dermatol. 2005;153(4):701–5. doi:10.1111/j.1365-2133.2005.06899.x.
Ortiz De Frutos FJ. Atopic dermatitis and tacrolimus in adults. Actas Dermosifiliogr. 2008;99 Suppl 2:8–13.
Patel TS, Greer SC, Skinner Jr RB. Cancer concerns with topical immunomodulators in atopic dermatitis: overview of data and recommendations to clinicians. Am J Clin Dermatol. 2007;8(4):189–94.
Ring J, Mohrenschlager M, Henkel V. The US FDA ‘black box’ warning for topical calcineurin inhibitors: an ongoing controversy. Drug Saf. 2008;31(3):185–98.
Rustin MH. The safety of tacrolimus ointment for the treatment of atopic dermatitis: a review. Br J Dermatol. 2007;157(5):861–73. doi:10.1111/j.1365-2133.2007.08177.x.
Sanchez Perez J. Safety information for tacrolimus: present and future. Actas Dermosifiliogr. 2008;99 Suppl 2:19–25.
Spergel JM, Leung DY. Safety of topical calcineurin inhibitors in atopic dermatitis: evaluation of the evidence. Curr Allergy Asthma Rep. 2006;6(4):270–4.
Tennis P, Gelfand JM, Rothman KJ. Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol. 2011;165(3):465–73. doi:10.1111/j.1365-2133.2011.10363.x.
Thaçi D, Salgo R. The topical calcineurin inhibitor pimecrolimus in atopic dermatitis: a safety update. Acta Dermatovenerol Alp Panonica Adriat. 2007;16(2):58. 60-2.
Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28(1):52–6. doi:10.1016/j.clindermatol.2009.04.001.
Weischer M, Rocken M, Berneburg M. Calcineurin inhibitors and rapamycin: cancer protection or promotion? Exp Dermatol. 2007;16(5):385–93. doi:10.1111/j.1600-0625.2007.00555.x.
Werfel T. Topical use of pimecrolimus in atopic dermatitis: update on the safety and efficacy. J Dtsch Dermatol Ges. 2009;7(9):739–42. doi:10.1111/j.1610-0387.2009.07141.x.
Gollnick H, Kaufmann R, Stough D, Heikkila H, Andriano K, Grinienko A, et al. Pimecrolimus cream 1% in the long-term management of adult atopic dermatitis: prevention of flare progression. A randomized controlled trial. Br J Dermatol. 2008;158(5):1083–93. doi:10.1111/j.1365-2133.2008.08484.x.
Kapp A, Papp K, Bingham A, Folster-Holst R, Ortonne JP, Potter PC, et al. Long-term management of atopic dermatitis in infants with topical pimecrolimus, a nonsteroid anti-inflammatory drug. J Allergy Clin Immunol. 2002;110(2):277–84.
Meurer M, Folster Holst R, Wozel G, Weidinger G, Junger M, Brautigam M. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205(3):271–7. doi:65863.
Meurer M, Fartasch M, Albrecht G, Vogt T, Worm M, Ruzicka T, et al. Long-term efficacy and safety of pimecrolimus cream 1% in adults with moderate atopic dermatitis. Dermatology. 2004;208(4):365–72. doi:10.1159/000078462.
Siegfried E, Korman N, Molina C, Kianifard F, Abrams K. Safety and efficacy of early intervention with pimecrolimus cream 1% combined with corticosteroids for major flares in infants and children with atopic dermatitis. J Dermatolog Treat. 2006;17(3):143–50. doi:10.1080/09546630600647297.
Sigurgeirsson B, Ho V, Ferrandiz C, Andriano K, Grinienko A, Jimenez P. Effectiveness and safety of a prevention-of-flare-progression strategy with pimecrolimus cream 1% in the management of paediatric atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22(11):1290–301. doi:10.1111/j.1468-3083.2008.02785.x.
Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics. 2002;110(1 Pt 1):e2.
Zuberbier T, Heinzerling L, Bieber T, Schauer U, Klebs S, Brautigam M. Steroid-sparing effect of pimecrolimus cream 1% in children with severe atopic dermatitis. Dermatology. 2007;215(4):325–30. doi:10.1159/000107627.
Zuberbier T, Brautigam M. Long-term management of facial atopic eczema with pimecrolimus cream 1% in paediatric patients with mild to moderate disease. J Eur Acad Dermatol Venereol. 2008;22(6):718–21. doi:10.1111/j.1468-3083.2008.02586.x.
Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol. 2001;44(1 Suppl):S47–57.
Hofman T, Cranswick N, Kuna P, Boznanski A, Latos T, Gold M, et al. Tacrolimus ointment does not affect the immediate response to vaccination, the generation of immune memory, or humoral and cell-mediated immunity in children. Arch Dis Child. 2006;91(11):905–10. doi:10.1136/adc.2006.094276.
Paller AS, Eichenfield LF, Kirsner RS, Shull T, Jaracz E, Simpson EL. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics. 2008;122(6):e1210–8. doi:10.1542/peds.2008-1343.
Breneman D, Fleischer AB Jr, Abramovits W, Zeichner J, Gold MH, Kirsner RS, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol. 2008;58(6):990–9. doi:10.1016/j.jaad.2008.02.008.
Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol. 2008;159(6):1348–56. doi:10.1111/j.1365-2133.2008.08813.x.
Thaçi D, Chambers C, Sidhu M, Dorsch B, Ehlken B, Fuchs S. Twice-weekly treatment with tacrolimus 0.03% ointment in children with atopic dermatitis: clinical efficacy and economic impact over 12 months. J Eur Acad Dermatol Venereol. 2010;24(9):1040–6. doi:10.1111/j.1468-3083.2010.03577.x.
Kubota Y, Yoneda K, Nakai K, Katsuura J, Moriue T, Matsuoka Y, et al. Effect of sequential applications of topical tacrolimus and topical corticosteroids in the treatment of pediatric atopic dermatitis: an open-label pilot study. J Am Acad Dermatol. 2009;60(2):212–7. doi:10.1016/j.jaad.2008.09.034.
Tan J, Langley R. Safety and efficacy of tacrolimus ointment 0.1% (Protopic) in atopic dermatitis: a Canadian open-label multicenter study. J Cutan Med Surg. 2004;8(4):213–9. doi:10.1007/s10227-003-0115-z.
Kang S, Lucky AW, Pariser D, Lawrence I, Hanifin JM. Long-term safety and efficacy of tacrolimus ointment for the treatment of atopic dermatitis in children. J Am Acad Dermatol. 2001;44(1 Suppl):S58–64.
Mandelin JM, Rubins A, Remitz A, Cirule K, Dickinson J, Ho V, et al. Long-term efficacy and tolerability of tacrolimus 0.03% ointment in infants:* a two-year open-label study. Int J Dermatol. 2012;51(1):104–10. doi:10.1111/j.1365-4632.2011.05015.x.
Hanifin JM, Paller AS, Eichenfield L, Clark RA, Korman N, Weinstein G, et al. Efficacy and safety of tacrolimus ointment treatment for up to 4 years in patients with atopic dermatitis. J Am Acad Dermatol. 2005;53(2 Suppl 2):S186–94. doi:10.1016/j.jaad.2005.04.062.
Ruer-Mulard M, Aberer W, Gunstone A, Kekki OM, Lopez Estebaranz JL, Vertruyen A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol. 2009;26(5):551–8. doi:10.1111/j.1525-1470.2009.00981.x.
Sigurgeirsson B, Boznanski A, Todd G, Vertruyen A, Schuttelaar ML, Zhu X, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. 2015;135(4):597–606. doi:10.1542/peds.2014-1990.
Kaufmann R, Folster-Holst R, Hoger P, Thaci D, Loffler H, Staab D, et al. Onset of action of pimecrolimus cream 1% in the treatment of atopic eczema in infants. J Allergy Clin Immunol. 2004;114(5):1183–8. doi:10.1016/j.jaci.2004.08.015.
Staab D, Kaufmann R, Brautigam M, Wahn U. Treatment of infants with atopic eczema with pimecrolimus cream 1% improves parents’ quality of life: a multicenter, randomized trial. Pediatr Allergy Immunol. 2005;16(6):527–33. doi:10.1111/j.1399-3038.2005.00306.x.
Lübbe J, Friedlander SF, Cribier B, Morren MA, Garcia-Diez A, Gelmetti C, et al. Safety, efficacy, and dosage of 1% pimecrolimus cream for the treatment of atopic dermatitis in daily practice. Am J Clin Dermatol. 2006;7(2):121–31.
Simon D, Lübbe J, Wuthrich B, Wiesner A, Weber MM, Laffitte E, et al. Benefits from the use of a pimecrolimus-based treatment in the management of atopic dermatitis in clinical practice. Analysis of a Swiss cohort. Dermatology. 2006;213(4):313–8. doi:10.1159/000096195.
Whalley D, Huels J, McKenna SP, Van Assche D. The benefit of pimecrolimus (Elidel, SDZ ASM 981) on parents’ quality of life in the treatment of pediatric atopic dermatitis. Pediatrics. 2002;110(6):1133–6.
Langley RG, Eichenfield LF, Lucky AW, Boguniewicz M, Barbier N, Cherill R. Sustained efficacy and safety of pimecrolimus cream 1% when used long-term (up to 26 weeks) to treat children with atopic dermatitis. Pediatr Dermatol. 2008;25(3):301–7. doi:10.1111/j.1525-1470.2008.00671.x.
Papp KA, Breuer K, Meurer M, Ortonne JP, Potter PC, de Prost Y, et al. Long-term treatment of atopic dermatitis with pimecrolimus cream 1% in infants does not interfere with the development of protective antibodies after vaccination. J Am Acad Dermatol. 2005;52(2):247–53. doi:10.1016/j.jaad.2004.08.046.
Papp KA, Werfel T, Folster-Holst R, Ortonne JP, Potter PC, de Prost Y, et al. Long-term control of atopic dermatitis with pimecrolimus cream 1% in infants and young children: a two-year study. J Am Acad Dermatol. 2005;52(2):240–6. doi:10.1016/j.jaad.2004.09.016.
Thomas KS, Armstrong S, Avery A, Po AL, O’Neill C, Young S, et al. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ. 2002;324(7340):768.
Jorizzo J, Levy M, Lucky A, Shavin J, Goldberg G, Dunlap F, et al. Multicenter trial for long-term safety and efficacy comparison of 0.05% desonide and 1% hydrocortisone ointments in the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol. 1995;33(1):74–7.
Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol. 2002;147(3):528–37.
Kirkup ME, Birchall NM, Weinberg EG, Helm K, Kennedy CT. Acute and maintenance treatment of atopic dermatitis in children - two comparative studies with fluticasone propionate (0.05%) cream. J Dermatolog Treat. 2003;14(3):141–8.
Furue M, Terao H, Rikihisa W, Urabe K, Kinukawa N, Nose Y, et al. Clinical dose and adverse effects of topical steroids in daily management of atopic dermatitis. Br J Dermatol. 2003;148(1):128–33.
Hayashida S, Furusho N, Uchi H, Miyazaki S, Eiraku K, Gondo C, et al. Are lifetime prevalence of impetigo, molluscum and herpes infection really increased in children having atopic dermatitis? J Dermatol Sci. 2010;60(3):173–8. doi:10.1016/j.jdermsci.2010.09.003.
Monto AS, Ullman BM. Acute respiratory illness in an American community. Tecumseh Stud Jama. 1974;227(2):164–9.
Rudan I, Tomaskovic L, Boschi Pinto C, Campbell H, Group WHOCHER. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ. 2004;82(12):895–903. doi:/S0042-96862004001200005.
Lewis-Jones S. Atopic dermatitis in childhood. Hospital Med. 2001;62(3):136–43.
Van Leent EJ, Ebelin ME, Burtin P, Dorobek B, Spuls PI, Bos JD. Low systemic exposure after repeated topical application of Pimecrolimus (Elidel), SD Z ASM 981) in patients with atopic dermatitis. Dermatology. 2002;204(1):63–8.
Rubins A, Gutmane R, Valdmane N, Stevenson P, Foster C, Undre N. Pharmacokinetics of 0.1% tacrolimus ointment after first and repeated application to adults with moderate to severe atopic dermatitis. J Invest Dermatol. 2005;125(1):68–71. doi:10.1111/j.0022-202X.2005.23754.x.
Ling M, Gottlieb A, Pariser D, Caro I, Stewart D, Scott G, et al. A randomized study of the safety, absorption and efficacy of pimecrolimus cream 1% applied twice or four times daily in patients with atopic dermatitis. J Dermatolog Treat. 2005;16(3):142–8. doi:10.1080/09546630510033159.
Van Leent EJ, De Vries HJ, Ebelin ME, Burtin P, Scott G, Bos JD. Blood concentrations of pimecrolimus in adult patients with atopic dermatitis following intermittent administration of pimecrolimus cream 1% (Elidel) for up to 1 year. J Dermatolog Treat. 2007;18(1):19–22. doi:10.1080/09546630601121037.
Undre NA, Moloney FJ, Ahmadi S, Stevenson P, Murphy GM. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160(3):665–9. doi:10.1111/j.1365-2133.2008.08974.x.
Lakhanpaul M, Davies T, Allen BR, Schneider D. Low systemic exposure in infants with atopic dermatitis in a 1-year pharmacokinetic study with pimecrolimus cream 1%*. Exp Dermatol. 2006;15(2):138–41. doi:10.1111/j.1600-0625.2006.00398.x.
Schneeweiss S, Doherty M, Zhu S, Funch D, Schlienger RG, Fernandez-Vidaurre C, et al. Topical treatments with pimecrolimus, tacrolimus and medium- to high-potency corticosteroids, and risk of lymphoma. Dermatology. 2009;219(1):7–21. doi:10.1159/000209289.
Margolis DJ, Abuabara K, Hoffstad OJ, Wan J, Raimondo D, Bilker WB. Association between malignancy and topical use of pimecrolimus. JAMA Dermatology. 2015. doi:10.1001/jamadermatol.2014.4305.
Paller AS, Mancini AJ. Chapter 3: Eczematous eruptions in childhood. In: Paller AS, Mancini AJ, editors. Hurwitz Clinical Pediatric Dermatology. 4th ed. St. Louis: Elsevier Inc; 2011. p. 49.
Acknowledgments
There were no contributors to this paper beyond the individuals that are listed as authors.
Funding
Financial support for the writing of this manuscript was provided by Valeant Pharmaceuticals NorthAmerica LLC. Valeant Pharmaceuticals had no role in the design of the literature searches, or analysis andpresentation of results.
Availability of data and materials
All data supporting our findings are contained within the manuscript; therefore this published report serves as theaccessible record of the protocol and findings of our systematic review. No identifying/confidential patient data wereshared in this paper.
Authors’ contributions
ES contributed to the concept of this paper, analysis of search results, provided clinical perspective, and criticallyreviewed each draft. JCJ contributed to the concept and preparation of this paper, design of the literature searches,and analysis and presentation of results. JK was involved in the design of the literature searches, backgroundresearch, analysis and presentation of results, and drafting and coordinating the manuscript. AH contributed to theconcept of this paper, analysis of search results, provided clinical perspective, and critically reviewed each draft. Allauthors read and approved the final manuscript as submitted.
Authors’ information
TAG conceived of the study, participated in its design and coordination and helped to draft the manuscript. PYuP carried out the investigation of possible effects of NGF mimetics on the body weight of rats, performed the corresponding statistical analysis, participated in the sequence alignment and drafted the manuscript. TAA carried out all in vitro studies using cultures and performed the corresponding statistical analysis. YuNF performed the synthesis of dipeptide NGF mimetics. MAK carried out the investigation of possible effects of NGF mimetics on pain sensitivity in rats and performed the corresponding statistical analysis. SBS participated in the study design and coordination. All authors read and approved the final manuscript.
Competing interests
Elaine C. Siegfried has participated in contract research with Astellas Pharma (prior to 2001) and Novartis Pharmaceuticals Corporation (prior to 2004) for which financial compensation was paid to her employer. She received travel expenses for presentation of some of this contract research from Valeant Pharmaceuticals North America LLC (in 2012). She has received consulting fees from Novartis Pharmaceuticals (prior to 2006).
Jennifer Jaworski and Jennifer Kaiser are employees of Prescott Medical Communications Group and participated in this manuscript with financial support by Valeant Pharmaceuticals North America LLC.
Adelaide A. Hebert has received consulting fees, been a member of speakers’ bureaus, and/or served on Advisory Boards for Astellas Pharma US, Novartis Pharmaceutical Corporation (prior to 2008), and Valeant Pharmaceuticals North America LLC. She has also served as a member of data safety monitoring boards for Valeant Pharmaceuticals North America LLC and Novartis Pharmaceuticals Corporation. In addition, she has participated in contract research with Astellas Pharma US and Novartis Pharmaceuticals Corporation for which all research funds were paid to heremployer.
Consent to publish
Not applicable.
Ethics and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Siegfried, E.C., Jaworski, J.C., Kaiser, J.D. et al. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 16, 75 (2016). https://doi.org/10.1186/s12887-016-0607-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-016-0607-9