Abstract
Purpose
This study aimed to verify that aerosolization ocular surface microorganisms (AOSMs) accumulated during non-contact tonometry (NCT) measurements.
Methods
A total of 508 participants (740 eyes) were enrolled in the study. In Experiment 1, before NCT was performed on each eye, the air was disinfected, and environment air control samples were collected via Air ideal® 3P (Bio Merieux). During NCT measurements, microbial aerosol samples were collected once from each eye. In Experiment 2, we collected initial blank control samples and then repeated Experiment 1. Finally, in Experiment 3, after the background microbial aerosol investigation, we cumulatively sampled AOSMs from each 10 participants then culture once, without any interventions to interrupt the accumulation. The collected samples were incubated and identified using matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry (MALDI-TOF-MS).
Results
Pathogenic Aerococcus viridans and other microorganisms from human eyes can spread and accumulate in the air during NCT measurements. The species and quantity of AOSMs produced by NCT measurements can demonstrate an accumulation effect.
Conclusion
AOSMs generated during NCT measurements are highly likely to spread and accumulate in the air, thereby may increase the risk of exposure to and transmission of bio-aerosols.
Precis
Pathogenic Aerococcus viridans and other species of aerosolization ocular surface microorganisms (AOSMs) can spread and accumulate with the increase of NCT measurement person times, demonstrating an accumulation effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Microorganisms, including viruses such as severe acute respiratory syndrome(SARS), influenza and other infectious microorganisms can spread through aerosol transmission; these microorganisms often combine with aerosols to form bio-aerosols, which are then transported through the air [1,2,3,4]. Unlike respiratory droplets, bio-aerosols can remain floating in the air for extended periods of time (up to hours), allowing them to repeatedly attack the human body and increase the risk of transmission, posing a threat to human and environmental health [5].
One common method used to measure intraocular pressure (IOP), is non-contact tonometry (NCT). NCT involves the use of an air puff to flatten the cornea, resulting in the dehiscence of the tear film and the generation of aerosols [6,7,8,9,10,11,12]. While there are new technologies available for measuring IOP [13]. Our previous study found that the aerosols produced during NCT measurements have a cumulative effect with increasing NCT spraying times [10]. There aerosols can carry microorganisms from the ocular surface, leading to the generation of aerosolized ocular surface microorganisms (AOSMs) that are dispersed in the air [14]. The presence of AOSMs in the air could be harmful to human and environmental health.
The aim of this study was to explore the cumulative effect of AOSMs produced during NCT measurements and provide a research foundation for assessing the risk of bio-aerosol transmission during NCT measurements, as well as its impact on human and environmental health.
Materials and methods
Ethical approval
This single-centre observational study was approved by the Institutional Review Board of the Eye Hospital of Wenzhou Medical University (2020-018-K-16) and adhered to the tenets of the Declaration of Helsinki.
Participants
The study comprised 508 participants (740 eyes) at The Eye Hospital, Wenzhou Medical University from December 2021 to February 2024. The inclusion criteria for participants were as follows: (a) patients requiring NCT measurement. The exclusion criteria were as follows: (a) those with a history of infectious diseases or from epidemic areas, and (b) those with contraindications for clinical NCT measurements.
Interventions and outcomes
Preparation prior to the experiment
Before conducting the experiments, the experimental room was ventilated and cleaned. Additionally, we used 75% alcohol to disinfect the object surfaces and the air in the room. To minimise interference from airflow, we avoided unnecessary and intense activities and limited the frequent opening and closing of doors. The room occupied an approximate area of 30 m2. We set up the medical terminal (M) and patient terminal (P). The medical staff and the participants were seated at the M and P terminals, respectively.
The experiments were carried out with a consistent indoor personnel density, and only one participant was seated at the patient terminal (P). The participant remained silent and wore a mask. The experimenter wore protective gear, including masks, goggles, a white gown, and sterile hats.
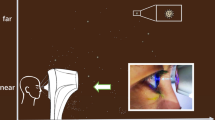
We used the TX-20P Automatic NCT (Canon Co., Tokyo, Japan) to measure IOP and generate air puffs for the study of background microbial aerosols investigation. The participants were instructed to sit on the P terminal and position their chin on the mandibular support. They were instructed to keep their forehead facing forward and fixed on the forehead support. They focused on the light source in the jet port, kept their eyes open naturally, and exposed their corneas. Subsequently, both eyes of participants were measured with the NCT and repeated three times measured for each eye.
Microbial aerosols were sampled using an Air Ideal® 3P (BioMérieux) sampler. The sampler allowed for an air volume of 100 L/min to pass through. The same microbial aerosol sampler was used for all the experiments. Before conducting the experiments, we sterilized the NCT and Air Ideal® 3P with 75% alcohol. We used blood agar plates with a diameter of 90 mm in the sampler (Bio-kont, China).
Experiment 1 (Video. 1)
Before measuring the right eye of the first participant with NCT, we used 75% alcohol to disinfect the air around the NCT. Additionally, we collected 30 L of environment air control sample, labeled as 17-P-1-O1. The sampler was placed horizontally next to the air jet of the NCT. Next, we measured the right eye of the first participant with the NCT.
The sampler was positioned perpendicular to the air flow and placed horizontally next to the air jet of the NCT outside of the participant’s eye for air sampling. It was fixed on the connecting line between the air jet and the participant’s eye, leaning towards the side of the participant’s eye. The 30 L of sample collected was labeled as 17-P-1-1. After measuring each eye with the NCT, we disinfected the air jet, mandibular support, forehead support, and facial seat using cotton dipped in alcohol. We repeated the same procedure for the left eye of the first participant, and the samples were labeled as 17-P-1-O2 and 17-P-1-2, respectively. The testing order for both eyes of each participant was as follows:
-
1.
Disinfect the environment air around the NCT, and collect an environmental air control sample before measuring the NCT of the right eye.
-
2.
The right eye accepted NCT measured, AOSMs spread during NCT measurements sampled.
-
3.
Disinfect the environment air around the NCT, and collect an environmental air control sample before measuring the NCT of the left eye.
-
4.
The left eye accepted NCT measured, AOSMs spread during NCT measurements sampled.
For testing, we repeated this process 20 times to test both eyes of 20 participants. After collecting the samples, we incubated the plates in an aerobic chamber at around 25˚C for 3–5 days. Then we compared the environmental air control samples with the samples collected from the participants’ eyes and analyzed the results. The analysis of the accumulation results in Experiment 1 and Experiment 2 were based on the original data from the work of Shen et al., which we had previously published [14].
Experiment 2 (Video. 1)
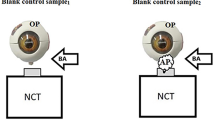
After initially disinfecting the environment air and object surfaces, we collected initial blank air control samples.
Initial blank air control sampling
We collected a 30 L of air sample labeled as blank air control sample1 to establish the baseline level of microbial aerosols in the environment. Then, we used the NCT to produce air puffs to impact the surface of the eye model. At the same time, we sampled another 30 L of air marked as blank air control sample2. The sampler was placed so that it was perpendicular to the airflow at the air jet port when the initial blank control samples were collected.
NCT measurement experimental sampling
Before measuring the NCT of the first participant’s right eye, we disinfected the surrounding air and the NCT using 75% alcohol. We then collected a 30 L air sample labeled as 21-1-PO1. After sampling 21-1-PO1, we measured the IOP of the right eye using NCT and collected a 30 L sample during NCT measurement. The samples were marked as 21-1-P1. We repeated the same procedure for the left eye of the first participant with the samples labeled as 21-1-PO2 and 21-1-P2, respectively. The sampler was placed in the same position as in Experiment 1 during the NCT measurement.
We followed the same testing order as in Experiment 1. We repeated 41 times to test 41 participants. After sample collection, we incubated the plates at 37˚C in an aerobic chamber for 24–48 h.
Experiment 3 (Video. 1)
The background microbial aerosols investigation
Baseline level sampling
Before conducting the experiments, the room was cleaned, and ventilated overnight. Ultraviolet disinfection was performed, and the object surfaces and air environment were disinfected with a 75% alcohol solution at least three times to minimize the concentration of microbial aerosols in the air. Human movements were limited and kept as consistent as possible. The experimental position was kept away from the air vent. For each participant, the air jet, mandibular support, forehead support, and facial seat were disinfected using cotton dipped in alcohol. For the overall assessment of the microbial aerosols during NCT measurements, samples were taken from the M and P terminals.
We used the natural sedimentation method and opened the agar plate to sample the baseline microbial aerosols in the air when no participant was present. The plate was immediately closed, and this sample was designated as the Baseline (initial state) 1. Then, 30 L of air was sampled during six air puffs impinging at the eye model and this sample was designated as the Baseline (air puff )2. The sampler was used to collect a 30 L of air sample during the first participant entered the room and sat at P terminal prior to the NCT measurements, marked as Baseline (human movements)3.
Microbial aerosols sampling during air puffs
The NCT device generated six air puffs without aiming at the participants’ eyes. They also took care to avoid touching the participants’ hair and other body parts. The sampler was positioned in the same position as in Experiments 1 and 2 during NCT measurements of participants’ eyes. Approximately 30 L of air was collected from each individual.
We cumulatively sampled microbial aerosols from 10 participants, which were then cultured once. The culture were performed after the 10th, 20th, 30th, 40th, 50th, and 60th participants from the M and P terminals, respectively. No interventions were performed to interrupt the accumulation of microbial aerosols.
After overnight air stewing, the baseline level sampling was repeated [30 L of Baseline (initial state) 1 was collected using an Air Ideal® 3P, with other procedures same as above]. Then we cumulatively sampled microbial aerosols from each 10 participants without NCT measurements, which were then cultured once. The culture were performed from the M and P terminal, respectively, this part was same as above.
AOSMs sampling during NCT measurements
Baseline level sampling repeated. Then NCT produced air pulses lead to the dehiscence of the tear film during IOP measurements for both eyes of each participant. Approximately 30 L of air was collected from each individual using the sampler.
We cumulatively sampled from 10 participants, which were then cultured once. The culture were performed after the 10th, 20th, 30th, 40th participants and so on from the M and P terminal, respectively. No interventions were performed to interrupt the accumulation during the experiment.
A total of 310 participants (618 eyes) were enrolled to repeat the accumulation experiment. Plates in rounds 4 and round 5 were kept in aerobic conditions at about 35 °C for 24–48 h. Blood agar plates in other rounds were kept in aerobic conditions at approximately 25 °C for 3–5 days after sample collection.
Identification
The species of colonies were identified using the matrix-assisted laser desorption/ ionization time-of-flight mass spectrometry (MALDI TOF MS). We performed cluster analysis for Rounds 3–5 using the Autof Analyzer software included in the MALDI-TOF MS (Autof MS 600, Autobio, China). A double-blind study was conducted, with the experimental sampling personnel distinct from those responsible for identification.
Statistical analysis
Microsoft Office 4.3.4.14 (Microsoft, Redmond, Wash, USA) and Epidata 3.1 (The EpiData Association, Denmark) was used to establish a database for parallel double input. Statistical analyses were performed by IBM SPSS Statistics (version 25.0; IBM Corp., Armonk, NY, USA).
Results
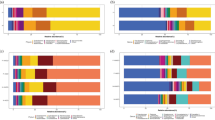
In Experiment 1, the following microorganisms were found to spread and accumulate in the air after NCT measurements were performed on 20 participants (40 eyes): Psychrobacter faecalis, Kocuria palustris, Staphylococcus epidermidis, Chryseobacterium, Stenotrophomonas acidaminiphila, Brevundimonas, Pseudomonas otitidis, Chryseobacterium arachidiradicis, Chryseobacterium indologenes (Video 2).
In Experiment 2, the following microorgansims were found to accumulate after NCT measurements were performed on 41 participants (82 eyes): Kocuria palustris, Bacillus megaterium, Bacillus subterraneus, Aerococcus viridans, Enterobacter hormaechei, Sporosarcina (Video 2).
The number of microbial aerosols quantitatively accumulated and increased with an increase in the number of participants NCT measured in Experiments 1–2 (see Supplementary Table S1).
In Experiment 3, after cluster analysis, the species of AOSMs may accumulate with an increase in the number of NCT measurements performed (Video 2). Compared to the baseline (background), the number of microbial aerosols quantitatively accumulated and increased with an increase in the number of participants NCT measured (see Supplementary Table S1).
Discussion
The results suggested that AOSMs generated during NCT measurements are highly likely to spread and accumulate in the air. This may be due to the cumulative effect of aerosols produced during NCT measurements [10]. Supporting this, our previous study demonstrated that microorganisms from the ocular surface can attach to the aerosols produced during NCT measurements, leading to the generation and dispersion of AOSMs in the air [14, 15]. As the number of NCT measurements increased, the number of microbial aerosols also accumulated, indicating that these microbial aerosols may persist in the air and pose a risk to human health over time. In light of these findings, it is essential to implement measures to mitigate pollution risks.
Although basic disinfection measures, such as ultraviolet light and alcohol, were found to have some effectiveness in reducing AOSMs in final samples, we recommend enhancing these practices. Specifically, using ultraviolet rays for 30 min and 75% alcohol to disinfect the environment and object surfaces at least three times may help decrease the basic of aerosols and bio-aerosols in the air to a certain level; and during the following aerosol generation procedure (AGP), the bio-aerosols level in the air can’t increase quickly and highly. These recommendations align with the findings of Shang et al., who identified ultravioletray for 30 min as the optimal inactivation time and timely inactivation is therefore recommended [16].
However, during NCT measurements, there is still an accumulation of AOSMs in the air. This accumulation poses an increased risk of microbial aerosols to human health. Therefore, it is urgent to propose a novel method to continuously and stably decrease the bio-aerosols produced during NCT measurements. This will help maintain a stable and safe level of bio-aerosols in the air. One possible solution is to use layer-by-layer self-assembly technology to synthesize polymer nanocomposites that can inactivate microorganisms and reduce the risk of microbial aerosols to protect human health [17]. Further more, we can formulate dopamine in situ reduction of nano silver and other anti-microorganism solution by making device. Poly(acrylic acid)(PAA)(4 g, 35 wt%), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)(0.2 g), dopamine hydrochloride (0.35 g), and N-Hydroxysuccinimide(NHS) (0.05 g) were dissolved in 50 mL of deionized water; stirring then continued for 8 h; the product was obtained after dialyzing for 72 h and freeze-drying at -20 °C [17]. Poly (ethyleneimine) (branched polyethylenimine [PEI] combined with silver nitrate. Finally, PAA-dopa (0.4 mg/mL, pH = 3.2) and PEI-Ag+ (0.4 mg/mL, pH = 10.8, AgNO3 0.05 mg/mL) can be prepared as two solutions and their buffer solutions with the same pH value [17]. They can be layer-by-layer modified the baffles on NCT, other medical equipment and any other AGP conditions to decrease microbial aerosols. Notably, the formulation of dopamine for in situ reduction of nano silver is portable and can be used worldwide. And other Nano Silver formula also have excellent or even better effect of anti-microbial aerosols and aerosols [18].
When NCT measurements involve more than 20 participants, it is recommended to disinfect at least tree times using anti-microorgansims solutions after every ten participants, or to disinfect more frequently, in order to inactivate and prevent airborne microorganisms and eliminate cumulative effects during AGP. Moreover, while Next-Generation Sequencing (NGS) metagenomics is a new technology that can achieve extremely accurate and efficient detection of microorganisms, it was not necessary for our experiment [19].
The limitation of our study is that in the future, more new and harmless materials should be developed to reduce the accumulation of aerosols and microbial aerosols in various fields. Additionally, future studies should utilize models such as Quantitative Microbial Risk Assessment (QMRA) to further estimate the risk of aerosol-borne illnesses caused by pathogenic microorganisms such as SARS as well as to evaluate the impact of risk mitigation efforts, in order to provide references for decreasing the risk of pathogenic microorganism aerosol transmission [20,21,22].
Conclusion
There is a high likelihood that AOSMs produced during NCT measurements can accumulate with an increase in the number of NCT measurement person times, thereby may increase the risk of microbial aerosol transmission to human health.
Data availability
No datasets were generated or analysed during the current study.
References
Liu Z, Liu H, Zhang M, He J, Cao G. Experimental study on the generation of aerosol particles and microorganisms from surgical staff in an operating room. Build Environ. 2023;236:110266.
Leung NHL. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 2021;19(8):528–45. https://doi.org/10.1038/s41579-021-00535-6. Epub 2021 Mar 22. PMID: 33753932; PMCID: PMC7982882.
Chen PZ, Bobrovitz N, Premji Z, Koopmans M, Fisman DN, Gu FX. Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. Elife. 2021;10:e65774. https://doi.org/10.7554/eLife.65774. PMID: 33861198; PMCID: PMC8139838.
Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8(9):914–24. https://doi.org/10.1016/S2213-2600(20)30323-4. Epub 2020 Jul 24. PMID: 32717211; PMCID: PMC7380927.
Zuo YY, Uspal WE, Wei T. Airborne transmission of COVID-19: aerosol dispersion, lung deposition, and virus-receptor interactions. ACS Nano. 2020;14(12):16502–24. https://doi.org/10.1021/acsnano.0c08484. Epub 2020 Nov 25. PMID: 33236896.
Britt JM, Clifton BC, Barnebey HS, Mills RP. Microaerosol formation in noncontact ‘air-puff’ tonometry. Arch Ophthalmol. 1991;109(2):225-8. https://doi.org/10.1001/archopht.1991.01080020071046. PMID: 1993032.
Yousefi A, Ma Y, Roberts CJ, Moroi SE, Reilly MA. Hydrodynamic interaction between tear film and air puff from noncontact tonometry. Transl Vis Sci Technol. 2022;11(2):2. PMID: 35103798; PMCID: PMC8819281.
Chen X, Li B, Zhang C. Assessment of temporal and spatial distribution patterns of aerosol produced by air-puff non-contact tonometer. Eur J Ophthalmol. 2022;32(5):3012–3018. doi: 10.1177/11206721211054727. Epub 2021 Dec 3. PMID: 34859700.
Guo H, Li W, Huang Y, Li X, Li Z, Zhou H, Sun E, Li L, Li J. Increased microbial loading in aerosols produced by non-contact air-puff tonometer and relative suggestions for the prevention of coronavirus disease 2019 (COVID-19). PLoS ONE. 2020;15(10):e0240421. https://doi.org/10.1371/journal.pone.0240421. PMID: 33031477; PMCID: PMC7544126.
Li C, Tang Y, Chen Z, Wang A, Huang X, Chen Y, et al. Aerosol formation during non-contact air-puff tonometry and its significance for prevention of COVID-19. Chin J Exp Ophthalmol. 2020;38(3):212–6.
Shetty R, Balakrishnan N, Shroff S, Shetty N, Kabi P, Roy D, Joseph SM, Khamar P, Basu S, Sinha Roy A. Quantitative high-speed assessment of droplet and aerosol from an eye after impact with an air-puff amid COVID-19 scenario. J Glaucoma. 2020;29(11):1006–1016. https://doi.org/10.1097/IJG.0000000000001672. PMID: 32947358.
Tang Y, Li C, Chen Y, Chen Z, Zhang P, Wang A, Huang X, Qu J, Li M, Ma S, Vasudevan B. Effect of intraocular pressure on aerosol density generated by noncontact tonometer measurement. J Glaucoma. 2020;29(11):1001–1005. https://doi.org/10.1097/IJG.0000000000001669. PMID: 32941321.
Borroni D, Gadhvi KA, Hristova R, McLean K, Rocha de Lossada C, Romano V, Kaye S. Influence of corneal visualization Scheimpflug technology tonometry on intraocular pressure. Ophthalmol Sci. 2021;1(1):100003. https://doi.org/10.1016/j.xops.2021.100003. PMID: 36246003; PMCID: PMC9562332.
Shen X, Xu Y, Huang J, Wu P, Zhou W, Chen Y. A comparative study on two methods of ocular surface microbial sampling. BMC Ophthalmol. 2023;23(1):228. https://doi.org/10.1186/s12886-023-02979-1. PMID: 37217905; PMCID: PMC10201025.
Shen X. 2024. URL: https://twitter.com/sxy19980826/status/1790655248253800843?s=19. Accessed 15 May 2024.
Shang M, Kong Y, Yang Z, Cheng R, Zheng X, Liu Y, Chen T. Removal of virus aerosols by the combination of filtration and UV-C irradiation. Front Environ Sci Eng. 2023;17(3):27. https://doi.org/10.1007/s11783-023-1627-y. Epub 2022 Sep 7. PMID: 36118139; PMCID: PMC9470504.
Wang B, Ye Z, Xu Q, et al. In situ construction of Ag NPs in bio-inspired multilayer films for long-term bactericidal and biofilm inhibition properties. Polym Test. 2017;62(2017):162–70. https://doi.org/10.1016/j.polymertesting.2017.06.023
Ju Y, Han T, Yin J, Li Q, Chen Z, Wei Z, Zhang Y, Dong L. Bumpy structured nanofibrous membrane as a highly efficient air filter with antibacterial and antiviral property. Sci Total Environ. 2021;777:145768. https://doi.org/10.1016/j.scitotenv.2021.145768. Epub 2021 Feb 15. PMID: 33684755; PMCID: PMC7954306.
Parekh M, Borroni D, Romano V, Kaye SB, Camposampiero D, Ponzin D, Ferrari S. Next-generation sequencing for the detection of microorganisms present in human donor corneal preservation medium. BMJ Open Ophthalmol. 2019;4(1):e000246. https://doi.org/10.1136/bmjophth-2018-000246. PMID: 31179394; PMCID: PMC6528759.
Clements N, Arvelo I, Arnold P, Heredia NJ, Hodges UW, Deresinski S, Cook PW, Hamilton KA. Informing building strategies to reduce infectious aerosol transmission risk by integrating dna aerosol tracers with quantitative microbial risk assessment. Environ Sci Technol. 2023;57(14):5771–81. https://doi.org/10.1021/acs.est.2c08131. Epub 2023 Mar 31. PMID: 37000413.
Schijven J, Vermeulen LC, Swart A, Meijer A, Duizer E, de Roda Husman AM. Quantitative microbial risk assessment for airborne transmission of SARS-CoV-2 via breathing, speaking, singing, coughing, and sneezing. environ health perspect. 2021;129(4):47002. https://doi.org/10.1289/EHP7886. Epub 2021 Apr 1. Erratum in: Environ Health Perspect. 2021;129(9):99001. https://doi.org/10.1289/EHP10105. PMID: 33793301; PMCID: PMC8016178.
Henriques A, Mounet N, Aleixo L, Elson P, Devine J, Azzopardi G, Andreini M, Rognlien M, Tarocco N, Tang J. Modelling airborne transmission of SARS-CoV-2 using CARA: risk assessment for enclosed spaces. Interface Focus. 2022;12(2):20210076. https://doi.org/10.1098/rsfs.2021.0076. PMID: 35261732; PMCID: PMC8831086.
Acknowledgements
We thank Yangyang Shen and Bailiang Wang for their contributions to the statistical analysis and valuable suggestions. We also acknowledge the healthcare workers who have contributed to this study. We would like to thank Editage (www.editage.com) for the English language editing.
Funding
The project did not receive any funding.
Author information
Authors and Affiliations
Contributions
XS: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YX: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Writing – review & editing. YY: Investigation, Project administration, Resources, Supervision, Writing – review & editing. SH、JH: Data curation, Investigation, Methodology, Validation, Writing – review & editing. PW: Project administration, Resources, Writing –review & editing. WZ: Data curation, Formal Analysis, Methodology, Software, Supervision, Validation, Writing – review & editing. CL: Methodology, Conceptualization, Writing –review & editing. YC: Conceptualization, Formal Analysis, Funding acquisition, Projectadministration, Resources, Writing – review & editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and received the approval from the Ethics Committee of the Eye Hospital of Wenzhou Medical University(2020-018-K-16). Written informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Video 1: The video of the experiments procedure in the study
Supplementary Material 2: Video 2: The video of the experiments results
Supplementary Material 3: Supplementary Table S1
: The numbers and properties of microbial aerosols produced during NCT measurements. Supplementary Table S1 sequentially displayed the number and properties of microbial aerosols produced in Experiment 1-2; the number of microbial aerosols produced in Experiment 3 background and Rounds 1-5; the number of microbial aerosols produced during non-contact tonometer measurements in our prior experiments
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, X., Xu, Y., Ye, Y. et al. Aerosolization ocular surface microorganisms accumulation effect during non-contact tonometer measurements. BMC Ophthalmol 24, 392 (2024). https://doi.org/10.1186/s12886-024-03664-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03664-7