Abstract
Background
To establish a normative database for macular vessel density (VD) measured by optical coherence tomography angiography (OCTA) and explore the parameters related to the VD.
Methods
An observational study in epidemiology. 5840 healthy elderly participants in Beichen district, Tianjin, China underwent detailed ophthalmic and systemic examinations. OCTA was performed in all subjects using a 6 × 6-mm line scan mode centered on the macula and the built-in software was used to quantify VD and stratify the retina.
Results
One thousand four hundred sixty-one healthy elderly citizens (30.4% men) were included, with a median age of 60.0 years (8.0 years) and an age range of 50 to 87 years.VDs in the different plexuses: superficial capillary plexus (SCP) 43.9% (3.2%), deep capillary plexus (DCP) 44.3% (2.8%), outer capillary plexus (OCP) 21.9% (5.9%), choriocapillaris (CC) 52.1% (1.4%). 90% medical reference range of the VDs at different plexuses was reported. Age was correlated with the VDs of each capillary plexus. Sex was correlated with the VDs of DCP and OCP, and the VDs of DCP (p < 0.001) and OCP (p = 0.015) in women were higher than that in men. After age and sex adjustment, choroid average thickness was positively correlated with VDs of SCP (R = 0.067, p = 0.010) and DCP (R = 0.108, p < 0.001), ganglion cell layer (GCL) average thickness (R = 0.072, p = 0.006) was positively correlated with the VD of OCP, best-corrected visual acuity (BCVA) (R = 0.082, p = 0.002) was positively correlated with the VD of CC.
Conclusions
In this study, the normative VD database of the Chinese urban healthy elderly population measured by the OCTA was established, and parameters related to the VD of each capillary plexus were analyzed, providing new ideas for the future study of the relationship between macular VD and disease.
Trial registration
The Beichen Eye Study had been registered on the Chinese Clinical Trial Registry website (registry number: ChiCTR2000032280) on April 25, 2020.
Similar content being viewed by others
Background
The macula is one of the most metabolic tissues in the human body [1]. The macula capillary network plays an important role in maintaining visual acuity and normal macular structure [2]. Spaide et al. speculated that some diseases, especially those changing macular perfusion, may have different effects on one layer of the vascular network from the other [3]. Therefore, quantifying macular vessel density (VD) and structural in vivo is helpful to understand its physiological state and monitor the progress of the disease, and also has important reference significance for the study of retinal disease. Optical coherence tomography angiography (OCTA) is a new technique to reconstruct the retinal capillary network by detecting changes in blood flow signals [4]. It can visualize the capillary network of each layer of the retina without the need to inject the dye [5, 6]. Compared with traditional retinal vessel imaging techniques such as fundus fluorescein angiography (FFA), OCTA is safer, simpler, and faster, and can quantify the density and perfusion of retinal capillaries. In recent years, it has been widely used for screening retinal diseases [7].
Previous studies have shown that OCTA has good repeatability and clinical applicability in the diagnosis of retinal diseases and choroidal diseases [8, 9]. The present research on OCTA mostly focuses on the relationship between OCTA parameters and retinal or choroidal diseases such as diabetic retinopathy (DR) [10] and age-related macular degeneration (AMD) [11]. However, studies on normative OCTA parameters in large-scale healthy populations are still rare. The normal values of many parameters of OCTA and their relationship with other ocular and systemic parameters are mostly unknown so far. However, if a parameter is used to diagnose a disease, the normal change of the parameter and its associated parameters must be known. At present, there are many limitations in those studies of the normative data of OCTA parameters in healthy people [12,13,14,15,16]. For example, the sample sizes of those studies were usually small, the subjects of those studies were not from the population, and most of the studies used 3 × 3-mm OCTA equipment, resulting in a narrow view field in those studies. In addition, the contents and values of the data reported in many studies are different due to different manufacturers of OCTA devices and different retinal stratification criteria [4].
In the present study, we established a normative database of VD measured by 6 × 6-mm OCTA in the superficial capillary plexus (SCP), deep capillary plexus (DCP), outer capillary plexus (OCP), and choriocapillaris (CC) plexuses of the macula in a large Chinese urban healthy elderly population, and its correlation with other ocular parameters and systemic parameters were also analyzed.
Method
Study population
The study for eye diseases among Beichen Eye Study (BCES) was conducted in the Beichen District, Tianjin, China from June 2020 to February 2022. Beichen district is located in the north of the central district of Tianjin, China, which is an inner-suburb between the central districts and outer-suburb as well as rural areas. As a transition region, Beichen district experienced rapid urbanization during the past 30 year [17]. Five thousand eight hundred forty participants greater than 50 years were recruited by a multi-stage cluster random sampling method and underwent a single study visit nested within BCES, at which visual function, systemic parameters, and retinal imaging were captured. The study adhered to the Declaration of Helsinki and was approved by the institutional review board of Tianjin Medical University Eye Hospital (2019ky-22). Written informed consent was signed by all participants. The BCES had been registered on the Chinese Clinical Trial Registry website (registry number: ChiCTR2000032280).
Participants who had a best-corrected visual acuity (BCVA) of 0.10 (logMAR) or better and spherical equivalent (SE) between + 3D and -6D were included in this cross-sectional analysis. Exclusion criteria were: any history or clinical evidence of glaucoma, pigment epithelial detachment, macular edema, subretinal or intraretinal fluid, epiretinal membrane, uveitis or other retinal diseases, presence of diabetes or hypertension, previous ocular surgery or laser photocoagulation, intraocular pressure (IOP) outside the normal range, axial length (AL) > 26 mm, a systemic drug that affects vessels. Eyes with poor-quality images on OCTA (signal strength index lower than 40) were also excluded (Fig. 1).
Examination
All examinations were performed at the community hospital or residential committee to which the participants belonged. The compound tropicamide eye drops were used for pupil dilation of all participants before the examination. The protocol included a detailed assessment of general health status, questionnaires, and the provision of blood samples for laboratory testing, all of which were performed by physicians, nurses, and optometrists. The examination included anthropometry, ocular biometry, visual acuity, swept-source anterior segment optical coherence tomography (OCT), slit lamp examination, tonometry, fundus photography, nonmydriatic 200° ultra-wide-field scanning laser ophthalmoscopy (SLO), OCT, OCTA, gonioscopy, and visual field test.
OCTA and quantitative detection of VD
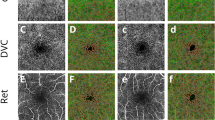
OCTA (Topcon Corporation, Tokyo, Japan) images of the bilateral macula were obtained after dilation. The device used a central wavelength of 1050 nm and acquired 100,000 A-scans per second scan. OCTA scans were performed in all subjects using a 6 × 6-mm line scan mode centered on the macula. By using the default automatic stratification function of Triton, the retina is divided into four layers: SCP, DCP, OCP, and CC (Fig. 2). SCP was defined as 2.6 μm below the internal limiting membrane (ILM) to 15.6 μm below the inner plexiform layer (IPL) / inner nuclear layer (INL). DCP was defined from 15.6 μm below the IPL / INL to 70.2 μm below the IPL. OCP was defined as 70 μm from IPL to 30 μm from the retinal pigment epithelium (RPE). The CC was defined as from 0 μm to 10.4 μm below the basement membrane (BM). Manually check the image quality. Manually check the image quality and adjust the center detection area to the macula if the macula is not in the center of the OCTA scan.Vessel density was defined as the percentage of the sample area occupied by the lumen of the vessel after image binarization reconstruction by the built-in software. According to the early treatment diabetic retinopathy study (ETDRS), the macular area was divided into five regions: central, superior, inferior, nasal, and temporal. The VDs were obtained and recorded by IMAGENET 6.0 automatic detection tool, and the VDs were entered into the database.
OCTA 6 × 6 mm scan showing macular vessel density in a healthy 60-year-old female with visual acuity of 0 (logMAR). A macular vessel density map of superficial capillary plexus (SCP). B macular vessel density map of deep capillary plexus (DCP). C macular vessel density map of outer capillary plexus (OCP). D macular vessel density map of choriocapillaris (CC). E coregistered OCT B-scan
Swept-source OCT (SS-OCT) imaging
The following scan patterns of SS-OCT (Topcon Corporation, Tokyo, Japan) were performed: linear B-scan (12 mm in length) centered on the fovea at 0°; 3D macula map covering a central area of a 7 mm × 7 mm scan mode to image the retina during a 1.3-s scan time, with a scan density of 512 A-scans × 512 B-scans. The built-in software of SS-OCT could automatically identify the boundary of each layer of the retina and report the retinal nerve fiber layer (RNFL) thickness, retina average thickness, macular ganglion cell layer (GCL) average thickness and choroid average thickness. The results of the automatic segmentation were evaluated by experts, manually adjusted if segmentation errors occur.
Statistical analysis
Data were analyzed using SPSS software version 26 (SPSS Inc, Chicago Illinois). Only participants ' right eye data were included in the study. All quantitative variables were reported as median (interquartile range) after confirming the non-normality with the Kolmogorov–Smirnov test. The percentile method was used to determine the 90% medical reference range of macular VDs at different sexes, retinal plexuses and age groups. Mann–Whitney test was used to compare parameters between different sexes. Kruskal–Wallis H test was used to compare the VDs of different parafoveal subfields in the same retinal capillary plexus and the VDs of different age groups. Post hoc test was used for pairwise comparison and the Bonferroni was used for adjusting the p-value. The Spearman correlation test was used in the correlation analysis. Correlations between macular vascular density and age, sex, diastolic pressure, systolic pressure, Body mass index (BMI), AL, SE, BCVA, IOP, RNFL thickness, retina average thickness, GCL average thickness and choroid average thickness were analyzed. Partial correlations with the sex and age adjustments were used to study the relationship among those parameters. A P value less than 0.05 was considered statistically significant.
Result
A total of 5840 individuals ranged from 50 to 87 years old were initially included in the BCES. Lastly, after the inclusion and exclusion criteria screening mentioned previously, 1461 eyes of 1461 participants were included in this study (444 eyes of men, 30.4%) and the median age was 60.0 years (8.0 years). The median VD of SCP was 43.9% (3.2%), the median VD of DCP was 44.3% (2.8%), the median VD of OCP was 21.9% (5.9%), and the median VD of CC was 52.1% (1.4%). Comparing the main characteristics between different sexes, it was found that there were significant differences in age, diastolic blood pressure, systolic blood pressure, BMI, AL, mean retinal thickness, and the VD of DCP and OCP. The main characteristics of the study participants were presented in Table 1.
90% medical reference ranges of the VDs were 39.4–47.6% at the level of SCP, 40.1–47.7% at the level of DCP, 15.6–29.1% at the level of OCP, and 49.9–54.3% at the level of CC (Table 2). Participants were grouped by age, 50–59 years old as group 1, 60–79 years old as group 2, and 80–90 years old as group 3. The 90% medical reference ranges of different age groups and different sexes were reported respectively (Table 2). The VDs of SCP(p < 0.001), DCP(p < 0.001) and OCP(p = 0.001) were statistically significant different in different age groups. The post hoc test showed that except for age group 1 and group 2 of OCP (p = 1.000), the VDs of SCP, DCP and OCP in different age groups were statistically significant different.
The VDs of different macular regions in each capillary plexus were reported respectively. At the level of SCP, there was no significant difference in VD between superior and inferior (p = 0.535), but there were significant differences in other regions when compared pairwise (all p < 0.001). At the level of DCP, the VD in the central region was significantly different from that in other regions (all p < 0.001), and the VD in the temporal region was significantly different from that in the inferior (p = 0.005) and nasal (p = 0.021) regions. At the level of OCP, there were significant differences between the VD of the central region and the other regions (all p < 0.001) and there was a significant difference in VD between the inferior and nasal (p = 0.001), and between the superior and nasal (p = 0.004). At the level of CC, there was no significant difference in VD between superior and nasal (p = 1.000), nor between central and inferior (p = 1.000). However, there were significant differences between the other regions when compared pairwise (all p < 0.001) (Fig. 3).
The results of correlation analysis are shown in Table 3. Age (R = -0.165, p < 0.001) was negatively correlated with VD of SCP. GCL average thickness (R = 0.077, p = 0.003) and choroid average thickness (R = 0.104, p < 0.001) were positively correlated with VD of SCP, but after age and sex adjustment, only choroid average thickness (R = 0.067, p = 0.010) was still positively correlated with VD of SCP. Sex (R = 0.123, p < 0.001) was correlated with VD of DCP, age (R = -0.201, p < 0.001) was negatively correlated with VD of DCP. Retina average thickness (R = 0.071, p = 0.006), GCL average thickness (R = 0.063, p = 0.016) and choroid average thickness (R = 0.144, p < 0.001) were positively correlated with VD of DCP. After adjusting for age and sex, there was only choroid average thickness (R = 0.108, p < 0.001) still positively correlated with VD of DCP. At the level of OCP, sex was correlated with the VD (R = 0.058, p = 0.027), age (R = 0.099, p < 0.001) was positively correlated with the VD, choroid average thickness (R = -0.056, p = 0.010) was negatively correlated with the VD. But, after adjusting for age and sex, choroid average thickness (R = -0.028, p = 0.293) was no longer correlated with the VD and GCL + average thickness (R = 0.072, p = 0.006) was positively correlated with the VD. At the level of CC, age (R = -0.088, p < 0.001) was negatively correlated with the VD, BCVA (R = 0.063, p = 0.017) and choroid average thickness (R = 0.068, p = 0.010) were positively correlated with the VD. However, after adjusting for age and sex, only BCVA (R = 0.082, p = 0.002) was positively correlated with the VD. After adjusting for age, it was found that sex was still correlated with the VD of DCP (R = 0.086, p < 0.001) and OCP (R = 0.080, p = 0.002). After adjusting for sex, age was still negatively correlated with the VDs of SCP, DCP and CC, and positively correlated with the VD (Table 4).
Discussion
OCTA has recently become a useful imaging technique for providing robust, highly reproducible and high-resolution cross-sectional images and valuable anatomic information on the macular vessel. Before the large-scale application of OCTA technology in clinics, it is difficult to obtain standardized data of macular VDs. OCTA has been successfully proven to be able to qualitatively study the microvascular changes of diabetic retinopathy, retinal vein occlusion, arterial occlusion, glaucoma and other retinal and choroidal diseases [18,19,20]. So far, there are few references to macular VD values measured by OCTA in the literature, and the main determinants of VD values have not been determined [14, 16, 21, 22]. Our study filled the gap in the study of VD measured by OCTA in the Chinese elderly healthy population, established a normative database of VD in Chinese healthy elderly population, and analyzed the relationship between VD of different capillary plexus and other ocular parameters or systemic parameters.
Our data revealed VDs were 43.9% (3.2%), 44.3% (2.8%), 21.9% (5.9%), 52.1% (1.4%) in the SCP, DCP, OCP and CC. Because our study participants were the elderly population, the reported VDs are lower than those reported in the previous literature. Anna Dastiridou et al. reported that the mean VDs of SCP, DCP, CC in a population with an average age of 48.2 was 51.0% ± 3.1%, 54.0% ± 5.9%, 67.8% ± 2.6%, respectively [23]. Similarly, in a study with participants' average age of 48.3, Florence Coscas et al. reported that mean VDs of SCP and DCP were 52.58% ± 3.22% and 57.87% ± 2.82%, respectively [16]. A study in Turkey reported that mean VDs of SCP and DCP in participants older than 60 were 41.25% ± 2.51% and 36.01% ± 5.07% [12]. Yanan Dong et al. [24]reported that the mean VD values of SCP ( 44.3% ± 3.5% and 43.5% ± 3.8%) and DCP ( 44.7% ± 4.9% and 43.7% ± 4.8%) in participants under 80 years old were higher than those in participants over 80 years old.We speculate that this difference in the senior participants may be due to the measurements made with different OCTA devices.
Our study also found a significant negative relation between age and VD in the SCP, DCP and CC that is in line with some of the findings reported in previous studies [1, 15, 23, 25, 26]. The discovery of OCTA is consistent with the anatomical trend of human retinal structure with age that is the retinal capillary density of the elderly decreases significantly [27]. With the increase of age, the metabolic activity of human brain tissue begins to weaken. As a continuation of the central nervous system, the metabolic activity of the eye also begins to weaken [28]. The decrease of VD in different capillary plexus of macular area may reflect this metabolic change of aging. According to Coscas et al., after the age of 60 years, VD is higher in women than men [16], similar results were found in our study. However, this difference was not shown in some studies with younger participants [13, 14], probably due to late vascular aging in females.
Our study found that there were differences in VD in different regions of the same macular capillary plexus. However, the differences we found were different from the results reported by Florence Coscas et al. Their study found that the superior and inferior regions mean VDs were higher (P < 0.001) than those in the temporal and nasal regions in both SCP and DCP plexuses [16], but there was no such obvious trend in our study results. These results are also inconsistent with the result of Hayreh [29] on the watershed zones: Blood flow to the temporal retina was approximately three times larger than that to the nasal retina, with no difference between the superior and inferior retina. We think that the differences between different regions could be related to the difference in metabolism in different regions of the macula.
In this study, we found that the choroid average thickness was positively correlated with VDs of SCP and DCP. This result is consistent with the conclusions of Al-Sheikh et al. [30] The capillary network of the retina is the basis of maintaining retinal nutrition. The density or morphological change of the capillary network of the retina will affect the homeostasis of the retina [31]. We speculate that the relationship between VD and choroid average thickness may be related to the nutritional function of capillaries. We also found that VD in CC was positively correlated with BCVA (logMAR), which means the greater the VD in CC was, the worse the visual acuity was. The choroid is responsible for supplying nutrients to the outer retina, and the layer of rods and cones is located in the outer retina [32]. Therefore, the relationship between BCVA and VD in CC may be caused by the compensatory increase of choroidal capillaries caused by insufficient blood supply in the layer of rods and cones.
Our study had several strengths. Firstly, it was one of the largest studies to date of OCTA retinal measures among Chinese urban healthy elderly participants. Secondly, we used OCTA 6 × 6-mm line scan mode to obtain a wider view field of macula, and all included OCTA images had a high quality (signal strength index ≥ 40). Thirdly, we divided macular area into different regions according to ETDRS and then we analyzed the differences in different regions of different capillary layers. Finally, the measurement of all parameters in this study follows a strict, prespecified principle, and all processes are operated by professionals to ensure the reliability of the data. However, our findings are subject to some limitations. Firstly, this study is a cross-sectional study and cannot assess the causal relationship between those parameters. Secondly, this study only included elderly participants and could not obtain the standard value of VD measured by OCTA in a longer age range which limits the transferability of the present results. Thirdly, there is a significant difference in sex proportion among the included research subjects, with men accounting for only 30.4% of the study population. Finally, the segmentation of OCTA images in this experiment depends on the built-in software of Triton, but at present, the correct segmentation of images by this software is only possible for normal macula. This method may lead to wrong image segmentation [4].
In conclusion, this study analyzed VD measured by OCTA in a large Chinese urban healthy elderly population, providing an example for the establishment of a normative database of VD in the future. In this study, we also explored the related factors of different capillary plexus, and provided new ideas for the future study of the relationship between macular VD and disease.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, Juping Liu, upon reasonable request.
Abbreviations
- VD:
-
Vessel density
- OCTA:
-
Optical coherence tomography angiography
- FFA:
-
Fundus fluorescein angiography
- DR:
-
Diabetic retinopathy
- AMD:
-
Age-related macular degeneration
- SCP:
-
Superficial capillary plexus
- DCP:
-
Deep capillary plexus
- OCP:
-
Outer capillary plexus
- CC:
-
Choriocapillaris
- BCVA:
-
Best-corrected visual acuity
- logMAR:
-
Logarithmic minimum angle of resolution
- SE:
-
Spherical equivalent
- IOP:
-
Intraocular pressure
- AL:
-
Axial length
- SLO:
-
Nonmydriatic 200° ultra-wide-field scanning laser ophthalmoscopy
- ILM:
-
Internal limiting membrane
- IPL:
-
Inner plexiform layer
- INL:
-
Inner nuclear layer
- RPE:
-
Retinal pigment epithelium
- BM:
-
Basement membrane
- ETDRS:
-
Early treatment diabetic retinopathy study
- SS-OCT:
-
Swept-source optical coherence tomography
- RNFL:
-
Retinal nerve fiber layer
- GCL:
-
Ganglion cell layer
- BMI:
-
Body mass index
References
Yu D-Y, Cringle SJ, Su E-N. Intraretinal oxygen distribution in the monkey retina and the response to systemic hyperoxia. Invest Ophthalmol Vis Sci. 2005;46(12):4728–33.
Crawford TN, et al. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8–13.
Spaide RF, Klancnik JM, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50.
Spaide RF, et al. Optical coherence tomography angiography. Prog Retin Eye Res. 2018;64:1–55.
Jia Y, et al. Quantitative optical coherence tomography angiography of vascular abnormalities in the living human eye. Proc Natl Acad Sci U S A. 2015;112(18):E2395–402.
Manalastas PIC, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma. 2017;26(10):851–9.
Kashani AH, et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog Retin Eye Res. 2017;60:66–100.
Lee M-W, et al. Long-term repeatability of optical coherence tomography angiography parameters in healthy eyes. Acta Ophthalmol. 2020;98(1):e36–42.
Fernández-Vigo JI, et al. Repeatability of choriocapillaris flow voids by optical coherence tomography angiography in central serous chorioretinopathy. PLoS ONE. 2022;17(12): e0279243.
Sun Z, et al. Optical coherence tomography angiography in diabetic retinopathy: an updated review. Eye (Lond). 2021;35(1):149–61.
Scharf J, et al. Optical Coherence Tomography Angiography of the Choriocapillaris in Age-Related Macular Degeneration. J Clin Med. 2021;10(4):751.
Yilmaz H, et al. Normative Data Assessment of Vessel Density and Foveal Avascular Zone Metrics Using AngioScan Software. Curr Eye Res. 2019;44(12):1345–52.
Shahlaee A, et al. In Vivo Assessment of Macular Vascular Density in Healthy Human Eyes Using Optical Coherence Tomography Angiography. Am J Ophthalmol. 2016;165:39–46.
Fernández-Vigo JI, et al. Normative database and determinants of macular vessel density measured by optical coherence tomography angiography. Clin Exp Ophthalmol. 2020;48(1):44–52.
Falavarjani KG, et al. Foveal Avascular Zone and Vessel Density in Healthy Subjects: An Optical Coherence Tomography Angiography Study. J Ophthalmic Vis Res. 2018;13(3):260–5.
Coscas F, et al. Normative Data for Vascular Density in Superficial and Deep Capillary Plexuses of Healthy Adults Assessed by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT211–23.
TBDBo S. Beichen District National Economic and Social Development Statistical Bulletin. 2019. http://www.tjbc.gov.cn/zwgk/sjfb/202012/t20201229_5231511.html.
Seknazi D, et al. Optical coherence tomography angiography in retinal vein occlusion: Correlations Between Macular Vascular Density, Visual Acuity, and Peripheral Nonperfusion Area on Fluorescein Angiography. Retina. 2018;38(8):1562–70.
Couturier A, et al. Capillary plexus anomalies in diabetic retinopathy on optical coherence tomography angiography. Retina. 2015;35(11):2384–91.
Wang X, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol. 2015;253(9):1557–64.
Iafe NA, et al. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2016;57(13):5780–7.
Yu J, et al. Macular perfusion in healthy Chinese: an optical coherence tomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56(5):3212–7.
Dastiridou A, et al. Age and signal strength-related changes in vessel density in the choroid and the retina: an OCT angiography study of the macula and optic disc. Acta Ophthalmol. 2022;100(5):e1095–102.
Dong Y, et al. Association of Optical Coherence Tomography and Optical Coherence Tomography Angiography Retinal Features With Visual Function in Older Adults. JAMA Ophthalmol. 2022;140(8):809–17.
Chen R, et al. Normative Data and Determinants of Macular, Disc, and Peripapillary Vascular Density in Healthy Myopic Children Using Optical Coherence Tomography Angiography. Front Med (Lausanne). 2022;9: 890294.
Ghassemi F, et al. The Quantitative Measurements of Vascular Density and Flow Areas of Macula Using Optical Coherence Tomography Angiography in Normal Volunteers. Ophthalmic Surg Lasers Imaging Retina. 2017;48(6):478–86.
Cavallotti C, et al. Age-related changes in the human retina. Can J Ophthalmol. 2004;39(1):61–8.
Ivanisevic J, et al. Metabolic drift in the aging brain. Aging (Albany NY). 2016;8(5):1000–20.
Hayreh SS. In vivo choroidal circulation and its watershed zones. Eye (Lond). 1990;4(Pt 2):273–89.
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–68.
Tan PEZ, et al. Quantitative confocal imaging of the retinal microvasculature in the human retina. Invest Ophthalmol Vis Sci. 2012;53(9):5728–36.
Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81.
Acknowledgements
We would like to thank all memberships of the BCES Group,
Funding
This study was sponsored by Tianjin Health Research Project (Grant No. TJWJ2021QN044) and Tianjin Municipal Science and Technology Bureau (Grant No. 18ZXRHSY00210).
Author information
Authors and Affiliations
Consortia
Contributions
XSZ and GF analyzed and interpreted the data. XSZ, GF and LR prepared the manuscript. LYQ and LJP aided in revising the manuscript. LXR and LJP designed this study. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study adhered to the Declaration of Helsinki and was approved by the institutional review board of Tianjin Medical University Eye Hospital (2019ky-22). Written informed consent was signed by all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, S., Gao, F., Luan, R. et al. Normative data and correlation parameters for vessel density measured by 6 × 6-mm optical coherence tomography angiography in a large chinese urban healthy elderly population: date from the Beichen eye study. BMC Ophthalmol 24, 298 (2024). https://doi.org/10.1186/s12886-024-03561-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03561-z