Abstract
Background
To evaluate the efficacy of 1% and 2% rebamipide clear solution in the treatment of dry eye disease (DED).
Methods
Two hundred twenty patients with DED were randomly assigned to one of three groups: the 1% rebamipide, 2% rebamipide, or placebo (eye drops containing the same ingredients, except for the active components). Each eye drop was instilled four times daily for 12 weeks. Changes in tear film break-up time (TBUT), corneal and conjunctival staining score, Schirmer 1 test, and the Ocular Surface Disease Index (OSDI) from baseline to 12-week visit between the study groups were compared for efficacy assessment.
Results
The mean age of study patients was 43.8±14.2 years. The 1% and 2% rebamipide groups showed greater improvement in TBUT (1.99±1.87 and 2.02±2.21 s) at 12 weeks from baseline than the placebo group (1.25±2.93 s). The 2% rebamipide group showed greater improvement in the corneal staining score (− 3.15±2.00) at 12 weeks from baseline than the placebo group (− 2.85±1.80). The 1% and 2% rebamipide groups showed improvement in Schirmer 1 test (1.27±3.86 and 1.50±4.14 mm) at 12 weeks of treatment, but not the placebo group (0.55±2.99 mm). Both the rebamipide groups and the placebo group showed significantly improved OSDI after treatment for 12 weeks; however, there was no significant difference among the three groups.
Conclusions
1% and 2% rebamipide clear solutions are an effective therapeutic option for improving TBUT and tear volume, and stabilizing the corneal staining score in DED.
Similar content being viewed by others
Background
Dry eye disease (DED) occurs as a result of insufficient tear production or excessive evaporation of tears, and the symptoms include dry eye surface, discomfort, visual impairment, and ocular pain [1, 2]. Moreover, DED is characterized by increased osmolality of the tear film and inflammation of the ocular surface, and the prevalence is estimated at 5–30% in individuals aged 50 years or older [2,3,4]. DED is a serious condition that increases healthcare costs and decreases quality of life and work productivity, which leads to a substantial cost burden as well as an increased use of healthcare resources [5].
Currently, various treatment options are available for patients with DED depending on the severity of symptoms. Tear replacement products or punctal plugs are used to restore the homeostasis of the ocular surface and tear film [6,7,8]. Moreover, pharmacotherapeutic options have been recently developed to promote tear production and tear replacement combined with diverse types of lubricants aims to improve discomfort of the ocular surface [9, 10].
A novel quinolinone derivative, rebamipide, promoted wound healing in an experimental rat model of gastric ulcer [11, 12] Rebamipide has diverse biological effects in raising gastric endogenous prostaglandin E2 and I2, stimulating the secretion of gastric epithelial mucin, scavenging oxygen free radicals, and inhibiting inflammatory responses [13,14,15] Moreover, rebamipide is used to treat stomatitis, pulmonary, renal and liver damage, and colitis, [16, 17] and, in an in vivo model, protected the cornea [18].
Possible effects of rebamipide on mucin secretion on the ocular surface have been studied [19,20,21,22]. Non-clinical studies have shown that rebamipide stimulated the secretion of corneal and conjunctival mucin and actions of goblet cells in rabbits [20, 23,24,25]. Presumably, rebamipide’s therapeutic actions originate from an ability not only to stimulate the secretion of corneal and conjunctival mucin-like substances but also to improve corneal and conjunctival injury in vivo [18]. Furthermore, clinical studies have shown that rebamipide is effective in improving both damages to the corneal and conjunctival epithelium as well as patient symptoms [23]. Kukje Pharma (Gyeonggi-do, Korea) developed a novel transparent rebamipide eye drop (KSR-001) for the treatment of patients with DED. Therefore, this study was conducted to assess the efficacy and safety of this novel rebamipide formulation in Korean patients with DED.
Methods
Study design
This multi-center, randomized, double-blind, placebo-controlled, parallel group-comparison, phase IIb/III clinical trial, comprising a 2-week wash-out period followed by a 12-week double-blind treatment period, was conducted in 15 medical institutions in Korea between February 18, 2020 and February 8, 2021.
The study was approved by the Internal Institutional Review Board (IRB) of respective sites involved in it (2019AS0275) and then conducted in compliance with the relevant ethics guidelines. All the study treatments and procedures described herein were performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All participants provided written informed consent. The current study is registered with ClinicalTrials.gov (registration number: NCT05017844, first posted date: 24/08/2021).
Study population
Patients aged 19 years or older, presenting with symptoms (e.g., foreign body sensation, dryness, glare, pain and blurred vision) suggestive of dry eye syndrome for ≥ 6 months before screening, and meeting the following criteria in one of both eyes at screening or randomization: corneal fluorescein staining score ≥ 4 points according to the National Eye Institute/Industry (NEI) scale and tear volume measured with Schirmer 1 test without anesthesia is ≤ 10 mm/5min (if Schirmer 1 test is 0 mm/5 min, then the result of Schirmer test with nasal stimulation should be ≥ 3 mm/5 min), and patients with best-corrected visual acuity (BCVA) of 20/100 or greater in the both eyes at both screening and randomization visits were included. Exclusion criteria for the current study are summarized in Table 1.
Study protocol
After submitting a written informed consent for study participation at Visit 1, the participants were assigned a screening number. If determined to be eligible for study participation based on inclusion/exclusion criteria in accordance with the study protocol at screening, participants were not allowed to use eye drops, including treatment agents for DED, during the 2-week pre-study washout period. Any participant who did not use other eye drops before randomization and met inclusion/exclusion criteria at Visit 2 was randomized to either of three treatment arms, such as the 1% rebamipide, 2% rebamipide, and placebo groups. Each of the eye drop formulations was instilled four times daily for 12 weeks. Packages of clinical trial drugs was established and the randomization scheme was generated using SAS Version 9.4 or higher (SAS Institute, Cary, NC, USA), and the list was delivered to the interactive web response system (IWRS) developer and personnel who were responsible for the packaging of the clinical trial drugs. The randomization number and the batch number of clinical trial drugs were confirmed by the IWRS.
All the randomized participants were allowed to receive study treatments for 12 weeks, and were instructed to visit each study center at 4, 8 and 12 weeks for assessment of efficacy, safety, and eye tolerability.
Study treatments
The study treatments were manufactured by Samil Co. Ltd. (Seoul, Korea) as requested by the Kukje Pharma and include the Study Treatment 1 (KSR-001-02; 1% rebamipide [rebamipide 10 mg/mL]), Study Treatment 2 (KSR-001-03; 2% rebamipide [rebamipide 20 mg/mL]), and Study Treatment 3 (KSR-001-04; 0% rebamipide [rebamipide 0 mg/mL]), all of which are colorless, transparent eyedrops dispensed in a translucent plastic container and stored in a tight container at room temperature (15–30 °C). The Study Treatment 3 (KSR-001-04) that contains the same ingredients except rebamipide was used as the placebo in the placebo group.
Efficacy assessments
Tear break-up time (TBUT)
To evaluate tear film stability, the TBUT was measured three times with fluorescein paper strips (Haag-Sterit, Bern, Switzerland) while using a stopwatch, and the mean value was recorded up to the second decimal place.
Corneal Fluorescein staining (0–15)
Corneal fluorescein staining grade with fluorescein sodium-impregnated paper strips (Haag-Sterit) was evaluated according to the NEI Scale that relies on a chart that divides the cornea into five sections and assigns a value from 0 (absent) to 3 (severe) to each section, based on the amount, size, and confluence of punctate keratitis, to obtain a maximum score of 15 points [26].
Conjunctival Lissamine Green Staining (0–18)
Conjunctival lissamine green staining grade with lissamine green–impregnated paper strips (Contacare Ophthalmics & Diagnostics, Padra, India) was evaluated according to the NEI Scale [grades 0 (absent) to 3 (severe)] for each of the six areas on each conjunctiva, for a maximum score of 18 points [26].
Schirmer 1 test
To measure tear volume, the Schirmer 1 test without topical anesthesia was performed. After placing a filter paper strip inside the inferior-temporal conjunctival sac for 5 min, the wetted length (in millimeters) is measured.
Ocular surface Disease Index (OSDI) questionnaire (0–100)
To assess dry eye symptoms, a 12-item OSDI questionnaire, which consists of 3 subscales (ocular symptoms, vision-related functions, and environmental triggers) during a 1 week recall period was completed [27].
Safety assessments
Differences in the BCVA and intraocular pressure (IOP) at baseline and the 12-week visit between the rebamipide treatment arms and the placebo group was the safety outcome measure. IOP was measured twice using non-contact tonometry, and if the difference between the two values was 2 mmHg or less, the average value was recorded. If the difference between the two values exceeded 2 mmHg, and additional measurement was obtained and the average value of the three measurements was recorded.
Eye tolerability assessments
To evaluate eye tolerability of study treatments, seven symptoms (stinging/burning, itching, blurred vision, sandiness/grittiness, dryness, light sensitivity, and pain or soreness score) were evaluated at a grade from 0 to 3 (0, no symptom; 1, mild; 2, moderate; and 3, severe) after the instillation of study treatments.
Participant assessment and criteria
For efficacy assessment, both the full-analysis and the per-protocol analysis were performed. The full-analysis set (FAS) comprised participants with available efficacy outcome data who received study treatments at least once after randomization. The per-protocol set (PPS) comprised participants of the FAS who had completed the current study without serious violation of the study protocol.
Safety analysis was performed for safety assessment, from the safety set which comprised participants who received study treatments at least once after randomization.
Statistical analysis
The sample size was estimated based on previously published studies (Appendix A) [28, 29]. All data are expressed as mean ± standard deviation (SD) or the number of the participants with percentage, where appropriate. Difference in baseline characteristics of the participants between the rebamipide treatment arms and the placebo group were compared using one-way analysis of variance (ANOVA) and the chi-square test. Intragroup differences in efficacy outcome measures between baseline and the 12-week visit were compared using the paired t-test and Wilcoxon signed rank test in each group. Changes in efficacy outcome measured scores from baseline to the 12-week visit between the rebamipide treatment arms and the placebo group were compared using Wilcoxon rank sum test. Comparison of efficacy outcome measures between the baseline visit and each follow-up visit in each group were compared using the repeated-measures ANOVA with Tukey’s post hoc test. Differences in safety outcome measures at baseline and the 12-week visit between the rebamipide treatment arms and the placebo group were compared using one-way ANOVA. Eye tolerability symptom scores of both eyes at the 4-, 8-, and 12-week visits between the rebamipide treatment arms and the placebo group were compared using repeated-measures ANOVA. All statistical analyses were performed using SPSS ver. 23 (IBM corp., Armonk, NY, USA). A p-value < 0.05 was considered statistically significant.
Results
Patient demography
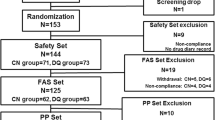
Among the 259 patients who were screened for this study, 222 participants were randomized to one of three treatment groups. Two patients were excluded due to non-administration of investigational drug and a total of 220 eyes of 220 participants (of 222 enrolled participants) – 74 in the 1% rebamipide group, 72 in the 2% rebamipide group, and 74 in the placebo group – were included in the FAS. In total, 189 (85.9%) participants completed the study as per protocol and were included in the PPS (Fig. 1). Of the 220 participants, 184 were female (83.6%) and the mean age ± SD was 43.8 ± 14.2 (range, 19–76) years. There were no significant intergroup differences in age, sex, height, and weight among the three study groups (Table 2).
Efficacy outcomes
The full analysis showed that, compared with the baseline, all the 1% and 2% rebamipide groups and the placebo group showed significantly improved TBUT, corneal fluorescein staining scores, conjunctival lissamine green staining scores, and OSDI after 12 weeks of treatment (all p < 0.0001). All three groups showed significantly improved corneal fluorescein staining scores, conjunctival lissamine green staining scores, and OSDI after 4 and 8 weeks of treatment, as compared with the baseline (all p < 0.05). However, at 8 weeks after treatment, the 1% and 2% rebamipide groups showed significantly improved TBUT compared with the baseline, whereas the placebo group did not (Fig. 2). Moreover, the 1% and 2% rebamipide groups showed significant differences in changes in TBUT at 12 weeks from baseline from the placebo group (p = 0.0148 and 0.0190, respectively). The 1% and 2% rebamipide groups showed improvement in Schirmer 1 test after 12 weeks of treatment, but not the placebo group. In addition, the 2% rebamipide group showed significant differences in changes in corneal fluorescein staining scores at 12 weeks from baseline from the placebo group (p = 0.0444). However, there were no significant differences in changes in conjunctival lissamine green staining scores and OSDI at 12 weeks from baseline between the rebamipide treatment arms and the placebo group (p = 0.6560, p = 0.4545, p = 0.1346, and p = 0.0908, respectively; Table 3).
Comparison of tear break-up time (A), corneal fluorescein staining score (B), conjunctival lisammine green staining score (C), Schirmer 1 test (D), and Ocular Surface Disease Index questionnaire (E) at each visit among the three groups. The asterisks indicate significant changes in parameters from baseline to each follow-up visit in the 1% rebamipide group. The daggers indicate statistically significant changes in parameters from baseline to each follow-up visit in the 2% rebamipide group. The double daggers indicate statistically significant changes in parameters from baseline to each follow-up visit in the placebo group. Asterisks, daggers, and double daggers indicate p < 0.05 per repeated-measures ANOVA with Tukey’s post hoc test
The per protocol analysis showed that all participants in the 1% and 2% rebamipide groups and the placebo group showed significantly improved TBUT, corneal fluorescein staining scores, conjunctival lissamine green staining scores, and OSDI after 12 weeks of treatment (p < 0.0001). 12 weeks of treatment of 1% and 2% rebamipide significantly improved the Schirmer 1 test, but placebo did not. The 1% and 2% rebamipide treatment arms showed significant differences in changes in TBUT at 12 weeks from baseline from the placebo group (p = 0.0279 and 0.0055, respectively; Table 4).
Safety outcomes
There was no significant difference in the BCVA and IOP of both eyes at baseline and the 12-week visit between the rebamipide treatment arms and the placebo group (all p > 0.05; Table 5).
Eye tolerability assessments
All symptom scores of stinging/burning, itching, blurred vision, sandiness/grittiness, dryness, light sensitivity, and pain or soreness after the instillation of study treatments of both eyes at the 4-, 8-, and 12-week visits of all three groups were less than 0.4. There were no significant differences in the seven symptom scores after the instillation of study treatments of both eyes at each visit among three groups (all p > 0.05; Fig. 3A and B).
Comparison of the eye tolerability symptom score of the right eye (A) and the left eye (B) at each visit between the three groups. There were no significant intergroup differences in symptom score at each visit between the rebamipide treatment arms and the placebo group (all P > 0.05; repeated-measures ANOVA). One participant in the placebo group only reported symptom scores in their left eye at post-treatment 12 weeks
Discussion
This randomized controlled trial evaluated the efficacy and safety of 1% and 2% rebamipide clear solution in the treatment of Korean patients with DED. The results of this study showed that the 1% and 2% rebamipide clear solution resulted in greater TBUT improvement effect at 12 weeks of treatment compared to the placebo group. In addition, the 2% rebamipide clear solution showed significantly greater improvement in the corneal fluorescein staining scores at 12 weeks of treatment compared to the placebo group. The efficacy of rebamipide in improving TBUT and corneal fluorescein staining has been advocated previously in the literature [28]. Based on previous studies which showed that rebamipide was effective in stimulating the secretion of mucin-like substances on the cornea/conjunctiva, rebamipide is expected to stabilize the tear film and to improve the damage caused to the cornea/conjunctiva in patients with DED [18, 21].This is further supported by a large-scale, dose–response phase II study which showed that 1% and 2% rebamipide ophthalmic suspension significantly improved the TBUT and corneal fluorescein staining scores as compared with the control [28].
In this study, 12 weeks treatment of 1% and 2% rebamipide significantly improved tear volume represented as the Schirmer 1 test, but placebo did not. Unlike this study, previous randomized multicenter phase II and III studies evaluating the efficacy of 2% rebamipide solution haven’t show the improvement of Schirmer 1 test in dry eye patients [28, 29]. This difference is attributable to the difference in treatment period because rebamipide was used for 4 weeks in previous studies whereas rebamipide was used for 12 weeks in this study. There was no significant improvement in the Schirmer 1 test results at 4 and 8 weeks after treatment in this study. Previous study which evaluated the effect of rebamipide ophthalmic solution on dry eye disease mice showed that the treatment of rebamipide increased not only the conjunctival goblet cell density but also the tear volume represented as the phenol red test [30]. Therefore, according to the results of this study, if rebamipide is used continuously for more than 12 weeks, it is thought that DED can be improved by increasing tear volume as well as mucin layer of tear film.
According to the Korean Corneal Disease Study Group (KCDSG) guidelines for the diagnosis and treatment of DED, patients presenting with at least ocular or visual symptoms as well as one of three objective signs, such as corneal fluorescein staining scores, TBUT, and Schirmer test, can be diagnosed with DED. This study intended to enroll dry eye patients with dry eye Levels I, II, or, III, as defined by the KCDSG guidelines. Thus, patients who presented with symptoms suggestive of dry eye syndrome for ≥ 6 months and with increased ocular surface staining and decreased Schirmer 1 test were enrolled. However, TBUT was not included in the inclusion criteria as most dry eye patients had short TBUT in South Korea and Japan in previous studies [31,32,33,34]. Although there was no inclusion criterion related to TBUT, the mean TBUT of the three groups in this study were 3.69 ± 1.78, 3.68 ± 1.84, and 3.67 ± 2.96 s.
The treatment recommended for patients with DED includes inoculation of artificial tears or anti-inflammatory agents (e.g., steroid or cyclosporine eye drops) [35]. However, these treatments have limited efficacy because artificial tears may produce transient effects or the frequent use of eye drops containing preservatives may irritate the epithelial cells or cause epithelial damages [36]. Steroid eye drops are effective in treating inflammation of the eyelid and cellular injuries, but they may increase the risk of infections, increased IOP, and cataract [37]. Moreover, patients with DED commonly stop using treatment agents after they achieve a partial recovery from their symptoms [38]. It is therefore imperative that a novel treatment agent be developed to stimulate continuous secretion of mucin on the ocular surface and thereby stabilize the tear film and to improve the damage to the ocular surface.
Diquafosol tetrasodium and rebamipide are well-known mucin secretagogues, and both are approved for the treatment of DED in Japan [39, 40]. Diquafosol, a purinergic P2Y2 receptor agonist, not only enhances tear fluid production from conjunctival epithelial cells but also promotes mucin secretion from conjunctival goblet cells because purinergic P2Y2 receptors have been identified in conjunctival epithelial and goblet cells [40,41,42]. The main mechanisms of rebamipide are to stimulate prostaglandin and mucus glycoprotein synthesis while inhibiting inflammatory cytokines, reactive oxygen species, and neutrophil activation [15]. In addition, rebamipide promotes the growth of conjunctival goblet cells through the activation of the EGFR-signaling pathway, which is linked to goblet cells,[25] and increases MUC5 mRNA expression on the ocular surface [43] However, 2% diquafosol and 2% rebamipide did not meet the data requirements for regulatory approval and have not been approved by the FDA [39]. Thus, although this study showed that the newly developed transparent 2% rebamipide are effective in the treatment of DED, the 2% rebamipide formulation that has been used in this study may not satisfy the FDA’s requirements, and additional research on the effect in DED is needed.
Patients with DED are vulnerable to ocular discomfort and irritation, burning sensation, itching, and blurred vision [44, 45]. Moreover, these patients are at a risk of decreased functional visual acuity,[46] which may greatly affect social and physical functioning, workplace productivity, and quality of life in patients with DED [47, 48]. In the current study, rebamipide treatment arms showed no significant differences in BCVA and IOP at 12 weeks compared with the placebo group. In addition, there were no differences in the eye tolerability symptom scores after the instillation of study treatments at each visit among three groups. Taken together, these results attest to the safety of 1% and 2% rebamipide treatments in patients with DED.
In this study, both 1% and 2% rebamipide clear solutions and placebo significantly improved all signs and symptoms except Schirmer 1 test in the efficacy assessment after 12 weeks of treatment. When studying the effect of topical eye drops, a vehicle that resembles tear substitutes could improve signs and symptoms of patients. Nevertheless, in this study, rebamipide clear solution showed the effect of significantly improving TBUT, Schirmer 1 test, and corneal fluorescein staining compared to the placebo group.
Conclusions
In conclusion, although there was no significant difference between the rebamipide treatment arms and the placebo groups in the improvement of OSDI after 12-week treatment, this study demonstrated that 1% and 2% rebamipide clear solutions are an effective, safe therapeutic option for improving TBUT and tear volume, and can stabilize the ocular surface in patients with DED.
Data Availability
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- DED:
-
dry eye disease
- IRB:
-
institutional review board
- NEI:
-
National Eye Institute/Industry
- BCVA:
-
best-corrected visual acuity
- IWRS:
-
interactive web response system
- TBUT:
-
tear film break-up time
- OSDI:
-
Ocular Surface Disease Index
- IOP:
-
intraocular pressure
- FAS:
-
full-analysis set
- PPS:
-
per-protocol set
- SD:
-
standard deviation
- ANOVA:
-
analysis of variance
References
Javadi MA, Feizi S. Dry eye syndrome. J Ophthalmic Vis Res. 2011;6(3):192–8.
Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71–81. quiz 82.
Yamaguchi T. Inflammatory response in Dry Eye. Invest Ophthalmol Vis Sci. 2018;59(14):Des192–des199.
The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. (2007). Ocul Surf 2007, 5(2):75–92.
Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, de Paiva CS, Gomes JAP, Hammitt KM, Jones L, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802–12.
Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, Boland TS, Buznego C, Gira JP, Goldberg DF, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. 2017;27(Suppl 1Suppl 1):3–47.
Zhang X, Qu MVJ, He Y, Ou X, Bu S, Jia J, Wang C, Wu J, Liu H. Z : Dry Eye Management: targeting the Ocular Surface Microenvironment. Int J Mol Sci 2017, 18(7).
Song JS, Woo IH, Eom Y, Kim HM. Five Misconceptions related to Punctal Plugs in Dry Eye Management. Cornea. 2018;37(Suppl 1):S58–s61.
Kojima T, Dogru M, Kawashima M, Nakamura S, Tsubota K. Advances in the diagnosis and treatment of dry eye. Prog Retin Eye Res 2020:100842.
Thulasi P, Djalilian AR. Update in Current Diagnostics and therapeutics of Dry Eye Disease. Ophthalmology. 2017;124(11s):27–s33.
Yamasaki K, Kanbe T, Chijiwa T, Ishiyama H, Morita S. Gastric mucosal protection by OPC-12759, a novel antiulcer compound, in the rat. Eur J Pharmacol. 1987;142(1):23–9.
Yamasaki K, Ishiyama H, Imaizumi T, Kanbe T, Yabuuchi Y. Effect of OPC-12759, a novel antiulcer agent, on chronic and acute experimental gastric ulcer, and gastric secretion in rats. Jpn J Pharmacol. 1989;49(4):441–8.
Kleine A, Kluge S, Peskar BM. Stimulation of prostaglandin biosynthesis mediates gastroprotective effect of rebamipide in rats. Dig Dis Sci. 1993;38(8):1441–9.
Iinuma S, Naito Y, Yoshikawa T, Takahashi S, Takemura T, Yoshida N, Kondo M. In vitro studies indicating antioxidative properties of rebamipide. Dig Dis Sci. 1998;43(9 Suppl):35s–9.
Arakawa T, Higuchi K, Fujiwara Y, Watanabe T, Tominaga K, Sasaki E, Oshitani N, Yoshikawa T, Tarnawski AS. 15th anniversary of rebamipide: looking ahead to the new mechanisms and new applications. Dig Dis Sci. 2005;50(Suppl 1):3–s11.
Takagi T, Naito Y, Uchiyama K, Okuda T, Mizushima K, Suzuki T, Handa O, Ishikawa T, Yagi N, Kokura S, et al. Rebamipide promotes healing of colonic ulceration through enhanced epithelial restitution. World J Gastroenterol. 2011;17(33):3802–9.
Kishimoto S, Haruma K, Tari A, Sakurai K, Nakano M, Nakagawa Y. Rebamipide, an antiulcer drug, prevents DSS-induced colitis formation in rats. Dig Dis Sci. 2000;45(8):1608–16.
Urashima H, Okamoto T, Takeji Y, Shinohara H, Fujisawa S. Rebamipide increases the amount of mucin-like substances on the conjunctiva and cornea in the N-acetylcysteine-treated in vivo model. Cornea. 2004;23(6):613–9.
Uchino Y, Woodward AM, Argüeso P. Differential effect of rebamipide on transmembrane mucin biosynthesis in stratified ocular surface epithelial cells. Exp Eye Res. 2016;153:1–7.
Takeji Y, Urashima H, Aoki A, Shinohara H. Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. J Ocul Pharmacol Ther. 2012;28(3):259–63.
Simsek C, Kojima T, Nakamura S, Dogru M, Tsubota K. The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal corneal nerves after environmental Dry Eye stress. Int J Mol Sci 2019, 20(16).
Ueda K, Matsumiya W, Otsuka K, Maeda Y, Nagai T, Nakamura M. Effectiveness and relevant factors of 2% rebamipide ophthalmic suspension treatment in dry eye. BMC Ophthalmol. 2015;15:58.
Kashima T, Itakura H, Akiyama H, Kishi S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol. 2014;8:1003–10.
Ríos JD, Shatos M, Urashima H, Tran H, Dartt DA. OPC-12759 increases proliferation of cultured rat conjunctival goblet cells. Cornea. 2006;25(5):573–81.
Ríos JD, Shatos MA, Urashima H, Dartt DA. Effect of OPC-12759 on EGF receptor activation, p44/p42 MAPK activity, and secretion in conjunctival goblet cells. Exp Eye Res. 2008;86(4):629–36.
Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. Clao j. 1995;21(4):221–32.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface Disease Index. Arch Ophthalmol. 2000;118(5):615–21.
Kinoshita S, Awamura S, Oshiden K, Nakamichi N, Suzuki H, Yokoi N. Rebamipide (OPC-12759) in the treatment of dry eye: a randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology. 2012;119(12):2471–8.
Kinoshita S, Oshiden K, Awamura S, Suzuki H, Nakamichi N, Yokoi N. A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology. 2013;120(6):1158–65.
Fu R, Jiang Y, Zhou J, Zhang J. Rebamipide ophthalmic solution modulates the ratio of T helper cell 17/regulatory T cells in dry eye disease mice. Mol Med Rep. 2019;19(5):4011–8.
Uchino M, Yokoi N, Uchino Y, Dogru M, Kawashima M, Komuro A, Sonomura Y, Kato H, Kinoshita S, Schaumberg DA, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156(4):759–66.
Eom Y, Hyon JY, Lee HK, Song JS, Kim HM. A multicenter cross-sectional survey of dry eye clinical characteristics and practice patterns in Korea: the DECS-K study. Jpn J Ophthalmol. 2021;65(2):261–70.
Eom Y, Song JS, Kim HM. Effectiveness of topical cyclosporin a 0.1%, Diquafosol Tetrasodium 3%, and their combination, in Dry Eye Disease. J Ocul Pharmacol Ther. 2022;38(10):682–94.
Eom Y, Yoon KC, Kim HK, Song JS, Hyon JY, Kim HM. A Multicenter, Randomized, double-blind evaluation of the efficacy of TJO-087 Versus 0.05% cyclosporine A in moderate to severe Dry Eye. J Ocul Pharmacol Ther. 2023;39(1):27–35.
Hyon JY, Kim HM, Lee D, Chung ES, Song JS, Choi CY, Lee J. Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014;28(3):197–206.
Asbell PA. Increasing importance of dry eye syndrome and the ideal artificial tear: consensus views from a roundtable discussion. Curr Med Res Opin. 2006;22(11):2149–57.
Barber LD, Pflugfelder SC, Tauber J, Foulks GN. Phase III safety evaluation of cyclosporine 0.1% ophthalmic emulsion administered twice daily to dry eye disease patients for up to 3 years. Ophthalmology. 2005;112(10):1790–4.
Hwang H. The pathogenesis of dry eye disease and trends in treatment. J Korean Med Association. 2016;59(9):713–8.
Jones L, Downie LE, Korb D, Benitez-Del-Castillo JM, Dana R, Deng SX, Dong PN, Geerling G, Hida RY, Liu Y, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575–628.
Hori Y. Secreted mucins on the ocular surface. Invest Ophthalmol Vis Sci. 2018;59(14):Des151–des156.
Cowlen MS, Zhang VZ, Warnock L, Moyer CF, Peterson WM, Yerxa BR. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003;77(1):77–84.
Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012;29(7):579–89.
Ohguchi T, Kojima T, Ibrahim OM, Nagata T, Shimizu T, Shirasawa T, Kawakita T, Satake Y, Tsubota K, Shimazaki J, et al. The effects of 2% rebamipide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (sod1) knockout mice. Invest Ophthalmol Vis Sci. 2013;54(12):7793–802.
Begley CG, Caffery B, Nichols K, Mitchell GL, Chalmers R. Results of a dry eye questionnaire from optometric practices in North America. Adv Exp Med Biol. 2002;506(Pt B):1009–16.
O’Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep. 2004;4(4):314–9.
Goto E, Yagi Y, Matsumoto Y, Tsubota K. Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. 2002;133(2):181–6.
Mertzanis P, Abetz L, Rajagopalan K, Espindle D, Chalmers R, Snyder C, Caffery B, Edrington T, Simpson T, Nelson JD, et al. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46(1):46–50.
Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–15.
Acknowledgements
The authors thank the following professors for assistance with data collection: So Hyang Chung, College of Medicine, The Catholic University of Korea, Republic of Korea; Chul Young Choi, Sungkyunkwan University School of Medicine, Republic of Korea; Byung Yi Ko, Konyang University College of Medicine, Republic of Korea; Hong Kyun Kim, School of Medicine, Kyungpook National University, Republic of Korea; Jong Suk Song, Korea University College of Medicine, Republic of Korea; Youngsub Eom, Korea University College of Medicine, Republic of Korea; Hyo Myung Kim, Korea University College of Medicine, Republic of Korea; Jong Soo Lee, Pusan National University College of Medicine, Republic of Korea; Joon Young Hyon, Seoul National University College of Medicine, Republic of Korea; Tae-Young Chung, Sungkyunkwan University School of Medicine, Republic of Korea; Mee Kum Kim, Seoul National University College of Medicine, Republic of Korea; Jae Yong Kim, University of Ulsan College of Medicine, Republic of Korea; Hyung Keun Lee, Yonsei University College of Medicine, Republic of Korea; Kyoung Yul Seo, Yonsei University College of Medicine, Republic of Korea; and Kyung Chul Yoon, Chonnam National University Medical School, Republic of Korea.
Funding
This research was funded by Kukje Pharma (Gyeonggi-do, Republic of Korea) and Samil Co. Ltd. (Seoul, Republic of Korea).
Author information
Authors and Affiliations
Contributions
Youngsub Eom participated in acquisition of data, analysis and interpretation of data, and drafting the manuscript. Jong Suk Song participated in conception and design of study, acquisition of data, and analysis and interpretation of data. Hyo Myung Kim participated in conception and design of study, acquisition of data, analysis and interpretation of data, and the final design of the study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was a part of the Health Insurance Portability and Accountability Act compliant, adhered to the tenets of the Declaration of Helsinki, and was approved by the Institutional Review Board (IRB) of Catholic University of Korea Seoul St. Mary’s Hospital (IRB approval code: KC19MDDS0905), Kangbuk Samsung Hospital (IRB approval code: KBSMC 2019-11-002), Konyang University Hospital (IRB approval code: KYUH 2019-11-009-001), Kyungpook National University Hospital (IRB approval code: KNUH 2019-11-009), Korea University Guro Hospital (IRB approval code: 2020GR0017), Korea University Ansan Hospital (IRB approval code: 2019AS0275), Korea University Anam Hospital (IRB approval code: 2019AN0493), Pusan National University Hospital (IRB approval code: D-1912-001-096), Seoul National University Bundang Hospital (IRB approval code: B-2001/588 − 406), Samsung Medical Center (IRB approval code: SMC 2019-12-041-002), Seoul National University Hospital (IRB approval code: H-1911-101-1081), Asan Medical Center (IRB approval code: 2019 − 1559), Gangnam Severance Hospital (IRB approval code: 3-2019-0373), Severance Hospital (IRB approval code: 4-2019-1079), and Chungnam National University Hospital (IRB approval code: CNUH-2019-354). The protocol of this study was registered with ClinicalTrials.gov (Trial registration: ClinicalTrials, registration number: NCT05017844, first posted date: 24/08/2021, https://clinicaltrials.gov/ct2/show/NCT05017844?term=NCT05017844&draw=2&rank=1). All participants provided written informed consent.
Consent for publication
Not Applicable.
Competing interests
The authors have no financial or proprietary interest in any product, method, or material described herein.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eom, Y., Chung, S.H., Chung, TY. et al. Efficacy and safety of 1% and 2% rebamipide clear solution in dry eye disease: a multicenter randomized trial. BMC Ophthalmol 23, 343 (2023). https://doi.org/10.1186/s12886-023-03004-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-023-03004-1