Abstract
Background
Pigmented paravenous retinochoroidal atrophy (PPRCA) is a rare fundus disease characterized by the presence of osteoblast-like pigment, atrophy of retinal pigment epithelium (RPE), and choroid deposition along the large retinal veins.
Case presentation
A 55-year-old Chinese female presented with right eye distention and bilateral vision loss. Osteocyte-like pigmentation and retinal choroidal atrophy distributed along the large retinal veins were seen in the fundus of bilateral eyes. The atrophy in the left eye was more severe compared to the right eye. The patient also presented with bilateral acute angle-closure glaucoma (AACG) and posterior subcapsular cataract (PSC) accompanied with anterior segmental manifestations, similar to the complications of retinitis pigmentosa (RP). The patient underwent ultrasound biomicroscopy (UBM), Humphrey field analyser (HFA), optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein fundus angiography (FFA), electroretinogram (ERG), and electrooculography (EOG), all of which confirmed the aforementioned diagnose.
Conclusion
PPRCA is a rare disease of unknown etiology. The patient in this case presented with complications similar to those of RP, and the two conditions may share a genetic basis. Further studies are needed to confirm this relationship.
Similar content being viewed by others
Background
PPRCA is characterized by osteoblast-like pigmentation along the large retinal veins accompanied with atrophy of the outer retina and choroid. This condition is prevalent in men, and it is symmetrical in bilateral eyes [1]. The cause of PPRCA remains unknown, and there is no effective treatment. PPRCA can be diagnosed by visual field examination, fundus photography, optical coherence tomography (OCT), fundus autofluorescence (FAF), fluorescein fundus angiography (FFA), electroretinogram (ERG), and electrooculography (EOG). Since both RP and PPRCA have mutations in the CRB1 locus [2], some researchers have suggested that PPRCA is another form of RP [1]. Here, the patient was a 55-year-old Chinese woman who had asymmetrical fundus manifestations. She presented with AACG and PSC accompanied with PPRCA, which was consistent with the complications of RP. This case report might suggest a genetic link between PPRCA and RP.
Case presentation
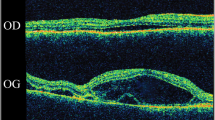
A 55-year-old Chinese woman was first seen at our hospital due to distention and vision loss in her right eye (oculus dexter (OD)) for one month. She was diagnosed with PPRCA twenty years ago and had no history of night blindness, ocular or systemic diseases or any other inflammatory and infection diseases. Best corrected visual acuity (BCVA) at the first visit was 0.2 in bilateral eyes, and the anterior segmental examination of the bilateral eyes revealed characteristic symptoms of AACG and PSC such as shallow anterior chamber and posterior subcapsular cataract. Intraocular pressure (IOP) in the OD was 37 mmHg and 18 mmHg in the left eye (oculus sinister (OS)). In bilateral eyes, HFA demonstrated that the visual field was extremely poor. (Fig. 1 a to b) UBM results showed chamber angle closure in the OD and narrowing in the OS. (Fig. 2 a to b) Wide-angle fundus photography confirmed retinal choroidal atrophy and osteocyte-like pigmentation along the large veins in bilateral eyes, and large choroidal vessels could be seen in the area of atrophy near the optic disc. Large foci of atrophy was seen in the inferior temporal area of the OS. Further, FAF showed significant hypoautofluorescence in bilateral eyes corresponding to the area of atrophy, with some borders surrounding the jagged hyperautofluorescence, suggesting progressive RPE atrophy. In addition, there was a weak background fluorescence in the early atrophic area and transmitted fluorescence at the edge of atrophy that was affected by pigmentation. The pigmentation resulted in obscured fluorescence seen next to the veins. A small patch of obscured fluorescence was found in the posterior pole affected by posterior subcapsular cataract. In the late stage, fluorescence staining was seen in the atrophic areas. (Fig. 3 a to h) Analysis of OCT revealed a significant thinning of the retinal nerve fiber layer in the macula of the OD and detachment of the neuroepithelial layer in the macula. The outer retina in the atrophic area had thinned. There was no RPE in the area with severe atrophy and thinning of the choroid was confirmed. (Fig. 4 a to d) Results of the ERG showed a drop in photopic and scotopic amplitudes of a-and b-wave, more significantly in the OS. EOG revealed a bimanual Arden ratio of 1.2, which was below the standard value. (Fig. 5) The axial length of the OD was 21.09 mm and the OS was 20.15 mm. These findings lead to the diagnosis of PPRCA, AACG, and PSC in both eyes.
a, b Wide angle under-eye photography of the OD and OS showing retinal choroidal atrophy distributed along the large retinal veins, with large choroidal vessels visible around the optic disc. c, d Fundus autofluorescence of the OD and OS indicating significant low fluorescence surrounding the high fluorescence band corresponding to the atrophy zone. e, f Early fluorescein fundus angiography of the OD and OS showing transmitted fluorescence at the edges of atrophy, and fluorescence masking the pigmented areas. g, h Late FFA of the OD and OS showing weak fluorescence in the atrophic areas of both eyes, with some areas of fluorescence staining. Cataract occlusion in the macular region of the OS
This patient underwent trabeculectomy combined with cataract phacoemulsification aspiration followed by IOL implantation in the OD and Nd-YAG laser iridotomy in the OS. Intraoperative dilatation of the pupil in the OD revealed that the patient’s lens suspensory ligament was relaxed. Three months after surgery, the patient’s BCVA was 0.6 in the OD and 0.2 in the OS, IOP was 17 mmHg in the OD and 13 mmHg in the OS. A further OCT showed the area of intraretinal capsule-like hyporeflective signal in the macula had disappeared, but the other fundus symptoms did not improve significantly. (Fig. 4 a to d) The patient was examined for the entire exome gene, and no ocularly significant mutation loci were identified.
Discussion
PPRCA is a rare degenerative disease of the fundus of the eye with unknown etiology. It was originally discovered by Brown et al. during fundus examination of a male patient with mottled baldness [3]. Typically, PPRCA is characterized by RPE and choroidal atrophy along the large retinal veins with osteoblast-like pigmentation. The disorder is prevalent in men, and is always symmetrical in both eyes, develops slowly, rarely affects the macula, and it does not affect central vision. As a consequence, it is difficult to identify this condition in patients. There have been previous reports of PPRCA with strabismus [4], amblyopia [5], nystagmus [5], macular cystoid edema [6], macular hole [7], etc., but its relationship with concomitant diseases has not been reported. Huang et al. suggested that PPRCA is an additional, incomplete, self-limiting form of retinitis pigmentosa (RP) [1]. Mckay et al. identified heterozygosity for a val162-to-met (V162M; 604,210.0010) mutation within the fourth EGF-like domain of the CRB1 gene in PPRCA patients that was very close to the mutated CRB1 gene locus in RP patients [2]. We have found no ocularly significant mutation loci. The genetic test we made could only detect known mutated loci, and there might be some unknown mutant loci that have not been identified in RP and PPRCA. Therefore, even if the test was negative, it might not prove that PPRCA is not associated with RP. The 55-year-old woman presented with severe PSC in bilateral eyes, posterior subcapsular cataract more in left eye than in the right eye, most probably due to a more serious PPRCA in the OS. During surgery, we discovered laxity of the lens zonules and suspensory ligaments, which seemed like RP complicated by cataract. We hypothesized that the PPRCA was linked to RP, and that the mechanisms of co-occurrence of AACG and PSC are similar to that of RP complicated by the latter two diseases.
This patient showed severe PSC in bilateral eyes and laxity of the lens zonules and suspensory ligaments, which is similar to the symptoms of RP with PSC. We speculate that PPRCA triggered RPE and choroidal atrophy, and the degenerated retinal tissue produced cytokines which broke through the damaged blood-retinal barrier and to injure the lens. The ensued long-term inflammatory state triggered laxity of the suspensory ligament [8,9,10]. This patient had a relaxed lens suspensory ligament, short axial length, and anterior segment crowding which predisposed her to AACG. The patient had a plasma exudate in the macular region of the OD, which was absorbed after IOP stabilization. PPRCA triggered RPE and choroidal atrophy, making the patient less tolerant to high IOP than an average patient with AACG. The fluid exuded from the choroidal vessels broke the blood-retinal intraocular barrier to accumulate in the neuroepithelium, which was gradually absorbed after IOP stabilization.
Conclusions
In conclusion, we reported a rare case of PPRCA accompanied with AACG and PSC. We speculated that PPRCA might be an incomplete manifestation of RP. Given the poor understanding of PPRCA etiology, further genetic testing and more case studies are needed to investigate the relationship between PPRCA and RP.
Availability of data and materials
All date generated and analyzed during this study are included in this article.
Abbreviations
- PPRCA:
-
Pigmented paravenous retinochoroidal atrophy
- AACG:
-
Acute angle-closure glaucoma
- PSC:
-
Posterior subcapsular cataract
- OD:
-
Oculus dexter
- OS:
-
Oculus sinister
- RPE:
-
Retinal pigment epithelium.
- RP:
-
Retinitis pigmentosa
- BCVA:
-
Best corrected visual acuity
- UBM:
-
Ultrasound biomicroscopy
- HFA:
-
Humphrey field analyser
- OCT:
-
Optical coherence tomography
- FAF:
-
Fundus autofluorescence
- FFA:
-
Fluorescein fundus angiography
- ERG:
-
Electroretinogram
- EOG:
-
Electrooculography
- IOP:
-
Intraocular pressure
References
Huang HB, Zhang YX. Pigmented paravenous retinochoroidal atrophy (Review). Exp Ther Med. 2014;7(6):1439–45.
McKay GJ, Clarke S, Davis JA, Simpson DAC, Silvestri G. Pigmented Paravenous Chorioretinal Atrophy Is Associated with a Mutation within the Crumbs Homolog 1 (CRB1) Gene. Investigative Opthalmology & Visual. Science. 2005;46(1):322–8.
Brown TH. Retino-Choroiditis Radiata. Br J Ophthalmol. 1937;21(12):645–8.
Choi WJ, Joo K, Park KH. Pigmented Paravenous Retinochoroidal Atrophy. Korean J Ophthalmol. 2020;34(1):90–1.
Traboulsi EI, Maumenee IH. Hereditary pigmented paravenous chorioretinal atrophy. Arch Ophthalmol. 1986;104(11):1636–40.
Figueiredo R, Morais Sarmento T, Garrido J, Ramalho A. Pigmented paravenous retinochoroidal atrophy associated with unilateral cystoid macular oedema. BMJ Case Reports. 2019;12(8):e230633.
Lessel MR, Thaler A, Heilig P. ERG and EOG in progressive paravenous retinochoroidal atrophy. Doc Ophthalmol. 1986;62(1):25–9.
Hong Y, Li H, Sun Y, Ji Y. A Review of Complicated Cataract in Retinitis Pigmentosa: Pathogenesis and Cataract Surgery. J Ophthalmol. 2020;2020:6699103.
ten Berge JC, Fazil Z, Born LI, Wolfs RCW, Schreurs MWJ, Dik WA, et al. Intraocular cytokine profile and autoimmune reactions in retinitis pigmentosa, age-related macular degeneration, glaucoma and cataract. Acta Ophthalmol. 2018;97(2):185–92.
Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011;22(2):124–32.
Acknowledgements
Thank you for the help of Home for Researchers editorial team (www.home-for-researchers.com).
Funding
This work was supported by the Jilin Scientific and Technological Development Program (20190303186SF). The role of this funding was in the collection and analysis of genetic data.
Author information
Authors and Affiliations
Contributions
YS acquired data and drafted the article. JL and LY performed acquisition and analysis of the data. YZ performed the surgery, critically revised the manuscript, and substantively revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethical approval required.
Consent for publication
The patient gave written permission for clinical details and images in this study. This report does not contain any personal information that could lead to the identification of the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Patient perspective.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, Y., Li, J., Yu, L. et al. Pigmented paravenous retinochoroidal atrophy with acute angle-closure glaucoma and posterior subcapsular cataract: a case report. BMC Ophthalmol 22, 184 (2022). https://doi.org/10.1186/s12886-022-02355-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-022-02355-5