Abstract
Background
The use of Spectral domain optical coherence tomography (SD-OCT) to evaluate the predictors of visual acuity-recovery in patients treated with conbercept for macular edema (ME) secondary to central retinal vein occlusion (CRVO) has rarely been seen. We collected 26 CRVO-ME patients with different OCT measures at 6 months follow-up to identify the factors that are most strongly correlated with the best-corrected visual acuity (BCVA) post-treatment in CRVO-ME patients treated with conbercept.
Purpose
To evaluate the effectiveness of intravitreal conbercept injections for the treatment of CRVO-ME and to determine the major predictors of best-corrected visual acuity (BCVA) post-treatment.
Methods
A retrospective study methodology was used. Twenty-six eyes from 26 patients with CRVO-ME were enrolled in the study. After an initial intravitreal injection of conbercept (0.5 mg/0.05 ml), monthly injections for up to 6 months were given following a 1 + PRN (pro re nata) regimen. Data collected at monthly intervals included measurements of the logMAR BCVA, central subfield thickness (CST), macular volume (MV), photoreceptor layer thickness (PLT), outer nuclear layer thickness (ONLT), and the disrupted ellipsoid zone (DEZ). The correlation between BCVA, before and after injections, and each of CST, MV, PLT, ONLT, DEZ was analyzed.
Results
The logMAR BCVA in months 3 and 6 post-injection was significantly improved relative to the baseline. In this same period the CST, MV, PLT, ONLT and DEZ were also significantly improved relative to the baseline. There was a negative correlation between PLT and logMAR BCVA at months 3 and 6 after treatment (r = − 0.549, P < 0.001; r = − 0.087, P < 0.001).
Conclusion
Intravitreal injection of conbercept is an effective treatment for CRVO-ME. With 6 months of follow-up, logMAR BCVA and CST, MV, PLT, ONLT, DEZ improved. PLT was negatively correlated with the visual function in CRVO-ME patients after conbercept treatment, which may be a predictor of vision recovery in patients with CRVO-ME.
Similar content being viewed by others
Background
Central retinal vein occlusion (CRVO) is one of the leading causes of vision loss among people aged 50 years or older. The incidence rate of CRVO in this group is approximately 1.36% [1]. Macular edema (ME) is the most common complication and a major cause of visual impairment in people with CRVO [2].
During CRVO formation, vascular endothelial growth factor (VEGF) levels increase resulting in an increase in the permeability of the retinal vessel walls. This causes the breakdown of the blood retinal barrier and ultimately leads to ME [3]. Many studies have shown that intravitreal anti-VEGF therapy is considered safe and effective for the treatment of CRVO-ME [4, 5]. Conbercept, an anti-VEGF medication, is a humanized, soluble, VEGF receptor (VEGFR) protein comprising extracellular domain-2 of VEGFR1, and extracellular domains-3 and-4 of VEGFR-2. Intravitreal injection of conbercept has been proven safe and effective for the treatment of CRVO-ME [6, 7]. However, clinically, repeated administration and follow-up visits are required, and some patients do not respond to treatment or have a poor visual outcome after repeated administration [8, 9]. Thus, it is very important to explore the predictive factors of a successful clinical outcome from conbercept therapy.
SD-OCT has been demonstrated to be an effective technique for analyzing macular edema through the evaluation of retinal structures. For example, using this technique, Xu et al. discovered that the ganglion cell layer and the inner plexiform layer are associated with visual gains in patients with diabetic macular edema [10]. Liu et al. discovered that external limiting membrane integrity is a prediction factor of visual function in CRVO-ME patients that have received ranibizumab injections [11]. However, SD-OCT assessment is still rarely to predict the visual acuity in CRVO-ME patients after conbercept therapy.
In this study, we investigated the retinal microstructure in patients with CRVO-ME after treatment with intravitreal conbercept during six-months follow-up period. We aim to identify the factors that are most strongly correlated with the best-corrected visual acuity (BCVA) post-treatment.
Materials and methods
Patients and inclusion criteria
This was a retrospective study. The patient group in this study consisted of 26 individuals diagnosed with CRVO-ME (26 eyes). All patients were examined and treated in the Department of Ophthalmology, the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), China, from February 2016 and December 2019. Inclusion criteria were: (1) fundus fluorescein angiography diagnosis of CRVO; (2) OCT showed macular edema with macular thickness > 250 μm; (3) no prior treatment for CRVO-ME; (4) intraocular pressure within the normal range; (5) no other eye diseases present, including severe cataract, glaucoma, uveitis, fundus diseases, myopia more than 3.00 diopters (D); (6) no history of eye surgery; and (7) no other serious medical diseases. All patients had a detailed understanding of the treatment benefits and risks, and of the study’s medical ethics requirements. The study conformed to the tenets of the Declaration of Helsinki and was approved by the Ethical Review Committee of the First Affiliated Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), China (LLSC-2021-095). Written informed consent was obtained from each patient prior to participation in the study.
Treatments and ophthalmic examination
All patients initially received one intravitreal injection of 0.5 mg/0.05 mL of conbercept (Chengdu Kang Hong Biotech Co, Ltd., Sichuan, China) and PRN (pro re nata) thereafter. All patients were evaluated monthly, including fluorescein angiography (FA) and optical coherent tomography (OCT) (Heidelberg Engineering, Heidelberg, Germany). The diagnostic criteria are according to previous studies [12]. The occlusion of the vein trunk and avascular zones in the retina are according to the performance of FA. If persistent or recurrent edema was detected, the 1 + PRN regimen was adopted. A persistent or recurrent edema was defined as a relapse of the cystoid space at the foveal center and an increase of the foveal thickness to 250 μm [13, 14].
For all participants, we used OCT for data collection, including the logMAR BCVA, central subfield thickness (CST), macular volume (MV), photoreceptor layer thickness (PLT), outer nuclear layer thickness (ONLT), and disrupted ellipsoid zone (DEZ) (Fig 1). Before the examination, the patients may be given Compound Topicamide eye drops for convenience of examination. During the examination, the examiner aims the lens at the patient's eye sitting directly in front of OCT, and lets the patient look at a fixed point of view and adjust the fixed point of view until a clear fundus image and OCT scanning line are presented on the fundus imaging display. At this time, relevant data required by OCT can be displayed and collected three times and took the average of them automatically according to the OCT instrument.
Outcome measurements
All patients were followed for 6 months post-treatment. The logMAR BCVA, central subfield thickness (CST), macular volume (MV), photoreceptor layer thickness (PLT), outer nuclear layer thickness (ONLT), and disrupted ellipsoid zone (DEZ) were evaluated at the baseline, week 1, and at months 1, 3, and 6 post-treatment.
Statistical analyses
All analyzes were conducted using SPSSv.18.0 for Windows (SPSS, Chicago, IL). Quantitative data that were normally distributed were analyzed using ANOVA. The correlation assesses between BCVA after medication and the OCT parameters using Pearson’s analysis. All statistical tests were two-sided. A P-value of < 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
Twenty-six eyes of 26 patients (11 females, 15 males) with CRVO-ME met the inclusion criteria between February 2016 and December 2019 (Table 1). The mean age of the participants was 57.70 ± 12.91 years. The mean intraocular pressure (IOP) of patients was 12.64 ± 2.13 mmHg. All patients received the 1 + PRN regimen. The total number of injections was 53, while the average number of injections per patient was 3.08 ± 0.21 (minimum = 1; maximum = 5). Three cases of subconjunctival hemorrhage occurred post-injection, with each undergoing self-absorption after 1 week of observation. There were no special complications in the remaining cases.
Baseline and follow-up characteristics
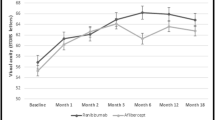
The mean logMAR BCVA was 1.00 ± 0.73 at baseline, and the respective values for the mean logMAR BCVA in week 1, and months 1, 3, and 6 were 0.53 ± 0.33, 0.44 ± 0.29, 0.40 ± 0.25, and 0.36 ± 0.08. Comparison of the logMAR BCVA pre-injection and post-injection showed a statistically significant difference in months 3 (P = 0.007) and 6 (P = 0.003) post-injection (Fig. 2A).
The mean CST was 747.58 ± 149.38 μm at baseline, and the respective values for the mean CST in week 1, and in months 1, 3, and 6 were 609.92 ± 187.73 μm, 404.50 ± 129.49 μm, 299.50 ± 74.24 μm, and 274.08 ± 29.30 μm. Mean CST decreased between the baseline period and week 1 (P = 0.003), month 1 (p ≤ 0.001), month 3 (p ≤ 0.001), and month 6 (p ≤ 0.001) post-injection. Mean CST decreased between week 1 post-injection and month 1 (p ≤ 0.001), month 3 (p ≤ 0.001), and month 6 (p ≤ 0.001) post-injection. Mean CST also decreased between month 1 post-injection and both month 3 (P = 0.018) and month 6 (P = 0.004) post-injection (Fig. 2B).
The mean MV was 30.87 ± 2.63 μm3 at baseline, and the respective values for the mean MV in week 1, and in months 1, 3, and 6 were 28.62 ± 1.41 μm3, 12.70 ± 2.10 μm3, 11.57 ± 1.35 μm3, and 10.93 ± 0.52 μm3. Mean MV in decreased between the baseline and week 1 (P = 0.005), month 1 (p ≤ 0.001), 3 (p ≤ 0.001), and 6 (p ≤ 0.001) post-injection. Mean MV decreased between week 1 post-injection and month 1 (p ≤ 0.001), month 3 (p ≤ 0.001), and month 6 (p ≤ 0.001) post-injection. Mean MV also decreased between month 1 post-injection and month 6 post-injection (P = 0.023, Fig. 2C).
The mean PLT was 58.96 ± 8.56 μm at baseline, and the respective values for the mean MV in week 1, and in months 1, 3, and 6 were 51.85 ± 8.22 μm, 39.04 ± 9.03 μm, 20.92 ± 5.36 μm, and 19.15 ± 1.68 μm. PLT decreased between the baseline period and week 1 (P = 0.006), month 1 (p ≤ 0.001), month 3 (P = 0.006), and month 6 (P = 0.006) post-injection. PLT decreased between week 1 post-injection and month 1 (p ≤ 0.001), month 3 (p ≤ 0.001), and month 6 (p ≤ 0.001) post-injection. PLT also decreased between month 1 post-injection and both month 3 (p ≤ 0.001) and month 6 (p ≤ 0.001) post-injection (Fig. 2D).
The mean ONLT was 432.58 ± 45.24 μm at baseline, and the respective values for the mean MV in week 1, and in months 1, 3, and 6 were 389.31 ± 42.45 μm, 317.96 ± 41.57 μm, 217.04 ± 48.86 μm, and 157.92 ± 8.87 μm. The ONLT decreased between the baseline period and week 1 (P = 0.002), month 1 (P ≤ 0.001), month 3 (P ≤ 0.001), and month 6 (p ≤ 0.001) post-injection. The ONLT decreased between week 1 post-injection and month 1 (P ≤ 0.001), month 3 (P ≤ 0.001), and month 6 (P ≤ 0.001) post-injection. The ONL also decreased between month 1 post-injection and both month 3 (P ≤ 0.001) and month 6 (P ≤ 0.001) post-injection. Furthermore, the ONLT decreased between month 3 and month 6 post-injection (P = 0.001, Fig. 2E).
The mean DEZ is 2921.04 ± 536.37 μm at baseline, and the respective values for the mean MV in week 1, and in months 1, 3, and 6 were 2401.23 ± 543.33 μm, 1944.73 ± 522.30 μm, 1440.88 ± 542.88 μm, and 1245.42 ± 105.19 μm. The DEZ decreased between the baseline and week 1 (P = 0.002), month 1 (P ≤ 0.001), month 3 (P ≤ 0.001), and month 6 (P ≤ 0.001) post-injection. The DEZ decreased between week 1 post-injection and month 1 (P = 0.005), month 3 (P ≤ 0.001), and month 6 (P ≤ 0.001) post-injection. The DEZ also decreased between month 1 post-injection and both month 3 (P = 0.002) and month 6 (P ≤ 0.001) post-injection (Fig. 2F).
Correlation analysis of BCVA after injection
The results of the Pearson’s correlation analysis showed that there was a statistically significant correlation between the baseline of PLT and the post-injection BCVA correlated at month 3 (r = − 0.549, P < 0.001) and month 6 (r = − 0.087, P < 0.001, Fig. 3).
Discussion
Macular edema is the major complication secondary to central retinal vein occlusion that can lead to severe impairment of central vision [15]. Previous studies have shown that CRVO-ME can destroy the normal structure of macular causing photoreceptor dysfunction [16, 17]. Therefore, it is necessary to assess the changes in macular structure and visual function associated with gains in visual acuity after an intravitreal conbercept injection. In this study, we found that after conbercept injection, CST, MV, PLT, ONL, and DEZ all improved with observation time relative to baseline measurements. The mean logMAR BCVA improved significantly in the 3 months after treatment and stabilized by 6 months. Furthermore, we showed that the baseline of PLT and BCVA at months 3 and 6 after treatment have a negative correlation, which indicated that PLT may be a predictor for vision recovery of CRVO-ME.
Although treatment of macular edema typically follows a 3 + PRN regimen due to regional economic influences [18], our treatment regimen was monthly intravitreal conbercept injections for 1 month followed by 1 + PRN regimen. It was found that the structure and function of the macular area were improved relative to the baseline and the visual acuity improved over the duration of the study. This suggests that the treatment program is effective for treating CRVO-ME. The results reported here are consistent with previous studies [19, 20]. However, the macular edema has not yet disappeared significantly in many cases 1 week after the first injection in this study. The mean CST of greater than 400 μm 1 month after the first injection, less than 400 μm after 3 months after treatment. The results suggests that macular edema may not soon disappeared after treatment initiation in many cases. Previous studies discovered that CST does not soon decreased in RVO patients with the longer course, with the phenomenon of antagonism of VEGF or recurrent edema occurs [21]. Thus, it may be due to the longer course of RVO-ME, as well as ischemic cases in some patients, the macular edema has not yet disappeared in some cases after treatment.
Previous studies have noted that vision recovery was limited even though retinal thickness was reduced to normal levels after treatment [22, 23]. The reason for this is not clear. It is known that macular edema was noted mostly in the outer nuclear layer, causing photoreceptor cell loss and fovea dysfunction [23]. Akagi-Kurashige et al. found that macular photoreceptor abnormalities of RVO patients could cause a decrease in parafoveal cone density and disrupt the cone mosaic spatial arrangement [24]. Therefore, the assessment of the retinal layers, especially those consisting of photoreceptor layers, is useful for evaluating visual prognosis.
In our study, we found a negative correlation between BCVA and PLT. The photoreceptor layer is the area where photoreceptor cells are distributed on the OCT. Therefore, the variation of photoreceptor thickness reflects the loss of photoreceptors. Histologic studies have showed that macular edema secondary to RVO is mostly detected in the outer nuclear layer, along with cystoid spaces and causes liquefaction necrosis, which results in loss of photoreceptor cells and photoreceptor dysfunction in the fovea [25]. To recover visual acuity that was adversely affected by macular edema, a reduction in retinal thickness may be necessary. However, the loss of photoreceptor cells may lead to limited visual recovery. Therefore, preservation of the photoreceptor cells would thus be necessary [26].
In this study, we measured the thickness of the photoreceptor layer, and found there was a negative correlation between photoreceptor layer thickness and logMAR BCVA at months 3 and 6 after treatment. We hypothesized that macular edema secondary to CRVO may result in structural damages, disorganization, and loss of photoreceptor cells. Especially when macular edema exceeds its elastic limit of outer nuclear layer, the damage of photoreceptor cells is irreversible. Following macular edema subsiding, the thinning of the PLT may reflect the loss of photoreceptor cells, which was responsible for the limited improvement in vision. Of course, the sample size of this study is small and the observation time is short, which may lead to the deviation of the results. In the future, large samples, multi-center, long-term observations are needed to be to be carried out.
There are some methodological limitations that should be considered when evaluating the results of this study. First, the study was performed at a single center and the sample size was relatively small. A larger sample size and longer follow-up period will be needed to validate our results. Second, CRVO is a chronic disease, while the observation time in this study is short. It is therefore difficult to fully assess the long-term prognosis with conbercept treatment. Third, we manually measured the various retinal layer thicknesses, although automated software would have allowed a more objective evaluation and erased any potential bias.
In sum, the study demonstrated the efficacy of conbercept treatment for CRVO-ME using a 1 + PRN regimen. Improvements with observation time over the 6 months follow-up period were found in the central subfield thickness, macular volume, photoreceptor layer thickness, outer nuclear layer and disrupted ellipsoid zone. The mean logMAR BCVA are improved significantly in the 3 months after treatment and stabilized by 6 months post-treatment. PLT is associated with the visual function in CRVO-ME patients after conbercept treatment and may be a predictor for vision recovery.
Availability of data and materials
The data are available from the corresponding author upon reasonable request.
Abbreviations
- SD-OCT:
-
Spectral domain optical coherence tomography
- BCVA:
-
Best-corrected visual acuity
- CRVO-ME:
-
Macular edema secondary to central retinal vein occlusion
- CST:
-
Central subfield thickness
- MV:
-
Macular volume
- PLT:
-
Photoreceptor layer thickness
- ONLT:
-
Outer nuclear layer thickness
- DEZ:
-
The disrupted ellipsoid zone
References
Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011;56(4):281–99.
Rhoades W, Dickson D, Nguyen QD, Do DV. Management of macular edema due to central retinal vein occlusion - The role of aflibercept. Taiwan J Ophthalmol. 2017;7(2):70–6.
McIntosh RL, Rogers SL, Lim L, Cheung N, Wang JJ, Mitchell P, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1113–23 e1115.
Daien V, Eldem BM, Talks JS, Korobelnik JF, Mitchell P, Finger RP, et al. Real-world data in retinal diseases treated with anti-vascular endothelial growth factor (anti-VEGF) therapy - a systematic approach to identify and characterize data sources. BMC Ophthalmol. 2019;19(1):206.
Callizo J, Ziemssen F, Bertelmann T, Feltgen N, Vogeler J, Koch M, et al. Real-world data: Ranibizumab treatment for retinal vein occlusion in the OCEAN study. Clin Ophthalmol. 2019;13:2167–79.
Zhang J, Liang Y, Xie J, Li D, Hu Q, Li X, et al. Conbercept for patients with age-related macular degeneration: a systematic review. BMC Ophthalmol. 2018;18(1):142.
Deng Y, Zhong QW, Zhang AQ, Cai XJ, Lu MZ, Zhang SC, et al. Microvascular changes after conbercept therapy in central retinal vein occlusion analyzed by optical coherence tomography angiography. International journal of ophthalmology. 2019;12(5):802–8.
Ip MS, Oden NL, Scott IU, VanVeldhuisen PC, Blodi BA, Ghuman T, et al. Month 12 outcomes after treatment change at month 6 among poor responders to Aflibercept or bevacizumab in eyes with macular edema secondary to central or Hemiretinal vein occlusion: a secondary analysis of the SCORE2 study. JAMA Ophthalmol. 2019;137(3):281–7.
Eldeeb M, Chan EW, Dedhia CJ, Mansour A, Chhablani J. One-year outcomes of ziv-aflibercept for macular edema in central retinal vein occlusion. Am J Ophthalmol Case Rep. 2017;8:58–61.
Xu Y, Qu Y, Suo Y, Gao J, Chen X. Correlation of retinal layer changes with vision gain in diabetic macular edema during conbercept treatment. BMC Ophthalmol. 2019;19(1):123.
Liu H, Li S, Zhang Z, Shen J. Predicting the visual acuity for retinal vein occlusion after ranibizumab therapy with an original ranking for macular microstructure. Exp Ther Med. 2018;15(1):890–6.
Turczynska MJ, Krajewski P, Brydak-Godowska JE. Wide-field fluorescein angiography in the diagnosis and Management of Retinal Vein Occlusion: a retrospective single-center study. Med Sci Monit. 2021;27:e927782.
Jung SH, Kim KA, Sohn SW, Yang SJ. Association of aqueous humor cytokines with the development of retinal ischemia and recurrent macular edema in retinal vein occlusion. Invest Ophthalmol Vis Sci. 2014;55(4):2290–6.
Ogino K, Tsujikawa A, Murakami T, Muraoka Y, Kurashige Y, Yoshimura N. Grid photocoagulation combined with intravitreal bevacizumab for recurrent macular edema associated with retinal vein occlusion. Clin Ophthalmol. 2011;5:1031–6.
Huang P, Niu W, Ni Z, Wang R, Sun X. A meta-analysis of anti-vascular endothelial growth factor remedy for macular edema secondary to central retinal vein occlusion. PLoS One. 2013;8(12):e82454.
Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011;89(1):e35–40.
Altunel O, Duru N, Goktas A, Ozkose A, Goktas E, Atas M. Evaluation of foveal photoreceptor layer in eyes with macular edema associated with branch retinal vein occlusion after ozurdex treatment. Int Ophthalmol. 2017;37(2):333–9.
Luo W, Jia F, Liu M, Wang Y, Zhang T. The analysis of correlative factors of visual acuity with intravitreal Conbercept injection in macular edema associated with branch retinal vein occlusion. J Ophthalmol. 2018;2018:7348153.
Osaka R, Muraoka Y, Miwa Y, Manabe K, Kobayashi M, Takasago Y, et al. Anti-vascular endothelial growth factor therapy for macular edema following central retinal vein occlusion: 1 initial injection versus 3 monthly injections. Ophthalmologica. 2018;239(1):27–35.
Miwa Y, Muraoka Y, Osaka R, Ooto S, Murakami T, Suzuma K, et al. Ranibizumab for macular edema after branch retinal vein occlusion: One Initial Injection Versus Three Monthly Injections. Retina (Philadelphia, Pa). 2017;37(4):702–9.
Campochiaro PA, Hafiz G, Channa R, Shah SM, Nguyen QD, Ying H, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusions: two-year outcomes. Ophthalmology. 2010;117(12):2387–94 e2381–2385.
Al-Zamil WM, Yassin SA. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313–30.
Yu JJ, Thomas AS, Berry D, Yoon S, Fekrat S, Grewal DS. Association of Retinal Inner Layer Disorganization with Ultra-Widefield Fluorescein Angiographic Features and Visual Acuity in branch retinal vein occlusion. Ophthalmic Surg Lasers Imaging Retina. 2019;50(6):354–64.
Akagi-Kurashige Y, Tsujikawa A, Ooto S, Makiyama Y, Muraoka Y, Kumagai K, et al. Retinal microstructural changes in eyes with resolved branch retinal vein occlusion: an adaptive optics scanning laser ophthalmoscopy study. Am J Ophthalmol. 2014;157(6):1239–49 e1233.
Dysli M, Ruckert R, Munk MR. Differentiation of underlying pathologies of macular edema using spectral domain optical coherence tomography (SD-OCT). Ocul Immunol Inflamm. 2019;27(3):474–83.
Ota M, Tsujikawa A, Murakami T, Yamaike N, Sakamoto A, Kotera Y, et al. Foveal photoreceptor layer in eyes with persistent cystoid macular edema associated with branch retinal vein occlusion. Am J Ophthalmol. 2008;145(2):273–80.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant No. 81700867), the Natural Science Foundation of Anhui Province, China (grant No. 1808085MH253) and the Training Program for Talents from Higher Education of Anhui Province, China (grant No. gxgwfx2019034). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. None of authors have any financial interest in any of the material described herein.
Author information
Authors and Affiliations
Contributions
QZ, PFZ conceived and designed the study. QZ, YFH, XC, RRZ, YPL, CHW, CFW, LXM participated in information gathering and editing, analyzed and interpreted all the data. QZ wrote the first draft of manuscript. Other authors reviewed and edited the manuscript. QZ reviewed and approved the final the version published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Wuhu Yijishan Hospital of Wannan Medical College, China (LLSC-2021-095). Written informed consent was obtained from each patient prior to participation in the study.
Consent for publication
We have obtained explicit written informed consent to publish all data (including individual details, images or videos) related to the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Q., Hou, Y., Cao, X. et al. Predictors of visual recovery in patients with macular edema secondary to central retinal vein occlusion after treatment with Conbercept. BMC Ophthalmol 21, 402 (2021). https://doi.org/10.1186/s12886-021-02174-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-021-02174-0