Abstract
Background
Usher syndrome (USH) is the most prevalent cause of the human genetic deafness and blindness. USH type II (USH2) is the most common form of USH, and USH2A is the major pathogenic gene for USH2. For expanding the spectrum of USH2A mutations and further revealing the role of USH2A in USH2, we performed the USH2A gene variant screening in Chinese patients with USH2.
Methods
Genomic DNA was extracted from peripheral blood of unrelated Chinese USH2 patients, we designed specific primers for amplifying the coding region (exons 2–72) of the USH2A gene. Sanger sequencing was used to study alleles. Silico prediction tools were used to predict the pathogenicity of the variants identified in these patients.
Results
Five heterozygous pathogenic variants were detected in four patients. Two patients were found to have two-mutations and two patients only have one. Two novel variants c.4217C > A (p.Ser1406X) and c.11780A > G (p.Asp3927Gly)) were predicted deleterious by computer prediction algorithms. In addition, three reported mutations (c.8559-2A > G, c.8232G > C and c.11389 + 3A > T) were also found in this study.
Conclusions
We identified five heterozygous pathogenic variants in the USH2A gene in Chinese patients diagnosed with Usher syndrome type 2, two of which were not reported. It expands the spectrum of USH2A variants in USH.
Similar content being viewed by others
Background
Usher syndrome (USH), an autosomal recessive disorder, is a clinically and genetically heterogeneous disease. USH is characterized by retinitis pigmentosa (RP), bilateral sensorineural hearing impairment and intact vestibular responses [1]. It is the most prevailing cause of the human hereditary deafness and blindness. In worldwide, the general prevalence of USH approximately ranges from 3.3 to 6.4 per 100,000 individuals [2]. Up to now, it is unavailability of a therapy for the USH.
Clinically, according to the severity and progression of vision and hearing loss of patients, USH classified into USH type I (USH1), USH type II (USH2), and USH type III (USH3) [3]. Besides, approximately 20–30% cases are categorized as atypical USH. USH1 is the most serious form in the three types, patients with USH1 have congenital profound hearing loss and begin to lose their vision early in life. Different from the USH1 patients defined as having congenital deafness and blindness within the first decade of life, patients with USH2 exhibit congenital mild-moderate hearing and vision loss in the second decade of life, and generally show normal vestibular function in all their lives. USH2 is the most common form of USH and USH2 patients account for more than 50% of all USH patients [2, 4]. USH3 patients are not born deaf and blind. They usually show a gradual loss of their hearing and vision.
Up to now, 16 genes have been identified that may cause USH (https://sph.uth.edu/retnet/sum-dis.htm), three genes of them (USH2A (usherin) [5], ADGRV1 (Adhesion G Protein-Coupled Receptor V1) [6] and DFNB31 (autosomal recessive deafness 31) [7]) are the USH2 genes. USH2A gene is the major pathogenic gene for USH2 and responsible for more than 74% USH2 cases [8]. USH2A gene is located on chromosome 1q41 and has two alternatively spliced isoforms. The shorter USH2A isoform was first identified in 1998 [5] and the much longer USH2A isoform b was identified by van Wijk et al. in 2004 [9]. To date, all 72 exons of USH2A isoform b have been carried out plenty of mutational analyses and found many pathogenic mutations (including splicing mutations at splice sites) [10, 11]. The protein usherin, encoded by the isoform b of USH2A, is presumed with 5202 amino acids and anchored on the cell membrane [12]. In mammalian photoreceptors, the usherin are expressed specifically in the connecting cilia and involved in the cargo delivery from the inner segment to the outer segment [13]. Previous research has been shown that mutations of USH2A could cause nonsyndromic recessive RP [14, 15]. What is more, USH2A gene also related to tactile sensitivity and acuity [16].
In this study, five deleterious variants and 14 non-pathogenic variants in the USH2A gene were identified in four Chinese USH2 patients by mutation screening. Two of the pathogenic variants we detected were novel.
Methods
Sample collection and ethics statement
Unrelated Chinese patients diagnosed with USH2 were included in this study. Two hundred unrelated normal individuals were recruited in this study as healthy controls. All patients underwent careful clinical examinations in Shanghai Tenth People’s Hospital and Clinical diagnosis of Usher syndrome were based on examination of optical coherence tomography (OCT) and electroretinogram (ERG), the typical RP fundus appearance, intact vestibular function, and sensorineural hearing impairment. The reference sequence from NCBI served as controls. This study was granted approval by the Declaration of Helsinki and approved by the institutional review board (IRB) of Tongji Eye Institute of Tongji University School of Medicine (Shanghai, China). Written informed consent was obtained from all participants.
The grading system for severity of hearing impairment and evaluation of vestibular function
The severity of hearing impairment can be judged according to the pure tone hearing threshold: mild hearing loss: 26–40 dB HL, moderate hearing loss: 41–80 dB HL, severe hearing loss: > 80 dB HL. Vestibular function tests include position tests and hot and cold water tests. (1) Position test: Dix-Hallpike technique was used to induce dizziness. Keeping the patient horizontal with his head pressed down by 30°. The head and eyes of the patient first turn to the right and then to the left, and repeated it several times to observe the severity and duration of nystagmus and dizziness. (2) Hot and cold water test: otoscopy should be performed before the test, and it can be performed without tympanic membrane perforation. The patient lies on his back and raises his head 30°to keep the lateral semicircular canal becomes upright. Each external ear canal was filled with cold or warm water for 40 s. Discomfort from warm water is usually lighter than cold water. In normal patients, cold water stimulates the nystagmus of the slow-phase stimulus side and the fast phase deviates from the stimulus side; warm water stimulus has the opposite response; in patients with vestibular cochlear nerve and vestibular nucleus disease, irrigation on the lesion side cannot induce nystagmus or nystagmus appears healthy slightly slower or shorter duration.
Sample preparation and variant screening
Peripheral blood samples from all the participants were collected in EDTA tubes. Standard protocols of RelaxGene Blood DNA System (TianGen, Beijing, China) were used to extract Genomic DNA according to the manufacturer’s instructions. DNA samples were stored at − 80 °C environment before used. Using the Primer3 software (http://primer3.sourceforge.net/) designed specific primers encompassing USH2A exons 2 to 72 (Table S1) (including the intron-exon boundary). The coding region was amplified by polymerase chain reaction (PCR) and using Sanger sequencing which was performed using ABI3730 Automated Sequencer (PE Biosystems, Foster City, CA, USA) study alleles. Nucleotide sequences assayed by Sanger sequencing were compared with the published DNA sequence of the USH2A gene (NCBI Reference Sequence: NM_206933.3(http://genome.ucsc.edu/cgi-bin/hgc?hgsid=785073911_5XSAy4TZHazZdzeKszSK5wYZ4AfE&g=htcCdnaAli&i=NM_206933&c=chr1&l=215796232&r=216596790&o=215796232&aliTable=refSeqAli)). The cDNA numbering + 1 position corresponds to A in the ATG translation initiation codon for USH2A.
Predictions of the pathogenic effect of missense variations and splice-site
We used several different computer algorithms: SIFT and PROVEAN (http://provean.jcvi.org/genome_submit_2.php), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) and MutationTaster (http://www.mutationtaster.org/) to predict the pathogenic effect of missense variants. Human Splicing Finder (HSF) (http://www.umd.be/HSF3/) was used to predict the pathogenicity of Splicing-site. Evolutionary conservation across species was evaluated through the alignment of orthologous USH2A protein sequences (including Mouse, Troglodyte, Bovine, Chicken, Mulatta and Zebrafish) with the human USH2A protein sequence, using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Results
Clinical characteristics of the USH2 patients
According to the data of their families, all the recruited patients followed the pattern of autosomal recessive inheritance. Representative fundus photographs indicated typical RP features (Fig. 1a), and representative OCT imaging suggested significantly diminished retinal thickness in patients (Fig. 1b). Moreover, most patients have moderate-to-severe hearing impairment, and analysis of pure tone audiogram testing demonstrated bilateral decrease of air-conduction and bone-conduction auditory (Fig. 1c). The tympanograms were showed type As which means limited activity of the middle ear transmission system (Fig. 1d). The ERG wave amplitude of patients were undetectable (Fig. 1e). Those features indicate the diagnosis of USH2, and detailed clinical information of the patients is summarized in Table 1.
Representative clinical examination of the USH2 patients. a The appearance of the fundus of patient No.003 shows typical retinal degeneration including irregular pigment clumps in the retina and attenuation of the retinal vessels. b OCT of left eye of patient No.002. c Result of pure tone audiogram testing of patient No.002 indicated bilateral hearing loss, cross or circle labels indicate air-conduction hearing, and right angle labels indicate bone-conduction hearing. d Tympanogram of patient No.003 demonstrated limited sound system activity of the middle ear. e The results of ERG of patient No.003 displayed indistinguishable wave amplitude
Pathogenicity analysis of the USH2A variants
In this study, we found 19 changes among four USH2 patients by exon sequencing of the USH2A gene. According to the result of computer algorithms, five of them were predicted to be pathogenic variants (Table 2). All the other 14 variants predicted non-pathogenic are listed in the Table S2.
These five heterozygous mutations include a nonsense mutation (c.4217C > A (p.Ser1406X)), two splice site mutations (c.8559-2A > G and c.11389 + 3A > T), and two missense mutations (c.8232G > C (p.Trp2744Cys) and c.11780A > G (p.Asp3927Gly)). All of these can be predicted as harmful by the computer prediction tool.
In the five pathogenic variants, two of them (c.4217C > A (p.Ser1406X) and c.11780A > G (p.Asp3927Gly)) were novel (can not be found in the variants in publicly available human genome aggregation data sets) and three (c.8559-2A > G, c.8232G > C (p.Trp2744Cys) and c.11389 + 3A > T) were reported. All the variants predicted to be pathogenic were absent in 200 Chinese unrelated healthy controls.
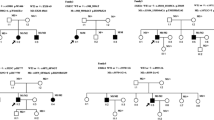
Two novel variants (c.4217C > A (p.Ser1406X) (Fig. 2a) in exon 19 and c.11780A > G (p.Asp3927Gly) (Fig. 2e) in exon 61) were found in patient No.002. In the family of patient No.002, c.4217C > A (p.Ser1406X) was found in his mother (Figure S1 B) and c.11780A > G (p.Asp3927Gly) was found in his father (Figure S1 C). Parents of patient No.002 are normal. Reported intron sequence variant c.8559-2A > G (Fig. 2c) and missense variant c.8232G > C (p.Trp2744Cys) (Fig. 2b) in exon 42 were found in patient No.004. Interestingly, intron sequence variant c.8559-2A > G also found in patient No.001 and his unaffected father (Figure S1 A). Finally, an intron sequence variant c.11389 + 3A > T (rs753886165) (Fig. 2d) was found in patient No.003. However, in patient No.001 and No.003, we do not find the allelic variant in the USH2A gene. The pedigrees of the four patients with variants in USH2A are shown in the Fig. 3.
Direct sequencing analysis of the pathogenic variants in USH2A identified in this study. a Sequence shown the heterozygous nonsense variant c.4217C > A (p.Ser1406X) and the corresponding wild-type sequence. b Sequence shown the heterozygous missense variant c.8232G > C (p.Trp2744Cys) and the corresponding wild-type sequence. c Sequence shown the heterozygous one-base-substitution variant c.8559-2A > G and the corresponding wild-type sequence. d Sequence shown the heterozygous one-base-substitution variant c.11389 + 3A > T and the corresponding wild-type sequence. e Sequence shown the heterozygous missense variant c.11780A > G (p.Asp3927Gly) and the corresponding wild-type sequence. Arrows indicate the position of variants
Pedigree of the Chinese Usher syndrome type II patients’ family. The black filled shapes mean individuals diagnosed with USH2 and the unfilled mean unaffected ones. Males are represented by squares, females circles. Patient number is below the individuals’ symbol. Individuals with available DNA samples were marked with asterisk. Question mark means the unknown allelic variant. M1: c.4217C > A (p.Ser1406X); M2: c.8232G > C (p.Trp2744Cys); M3: c.8559-2A > G; M4: c.11389 + 3A > T; M5: c.11780A > G (p.Asp3927Gly)
For the exon pathogenic variants identified in this study, we examined the location of them along the usherin. Finally, we identified functional domains the exon variants located within (Fig. 4a). Additionally, we aligned USH2A sequences among different species, including Human, Troglodyte, Mulatta, Bovine, Chicken, Mouse and Zebrafish for each of the two novel missense variants by Clustal Omega. The results of the conservative analysis of amino acid sequences were shown in Fig. 4.
a Schematic illustration of the exon pathogenic variants identified in this study along the USH2A isoform b protein domains. SP: signal peptide; Lam GL: Laminin G-like domain; Lam NT: Laminin N-terminal; EGF Lam: EGF-like domain; FN3: fibronectin type-III; LamG: Laminin G domain; TM: transmembrane region; PDB: PDZ-binding domain b Amino acid sequence alignments obtained by Clustal Omega software. Exon missense mutations in this study p.Trp2744Cys (c.8232G > C) and p.Asp3927Gly (c.11780A > G) in Human USH2A gene aligned with other species including Troglodyte, Zebrafish, Chicken, Mulatta, Mouse and Bovine
Discussion
Currently, 16 genes associated with USH have been identified, and three are USH2-causing gene. The USH2A gene causes 30–40% of USH2 cases and 10–15% of recessive RP cases [19]. Usherin is localized to a spatially restricted membrane microdomain in mammalian photoreceptors [13]. Previous researches have shown that congenital usherin protein mutations can induce the connecting cilium disorder and eventually lead to blindness [20].
Up to now, mutation screening in Chinese patients was revealed 25 mutations in previous researches [15, 18, 21,22,23,24]. In Southern population of China, 8.47% of sporadic RP patients are belong to USH [21]. In this study, we identified two novel variants (a missense variant and a nonsense variant) in the USH2A gene of four Chinese patients diagnosed with USH2 and found three reported mutations.
Isoform b of USH2A consists 8 domains, including N-terminal signal peptide (SP), laminin G-like domain (Lam GL), laminin N-terminal (Lam NT), laminin-type EGF-like domain (EGF Lam), fibronectin Type III (FN3), laminin G domain (LamG), transmembrane region (TM), and a PDZ-binding motif (PBM) at its C-terminal end [9]. By the PBM interacted with the PDZ domain of harmonin and whirlin, USH2A integrated into the USH protein network [25].
All of the two novel pathogenic variants are located in the FN3 domain (Fig. 4a). c.4217C > A (p.Ser1406X) is located in the fourth FN3 domain, and c.11780A > G (p.Asp3927Gly) is located in the 24th FN3 domain. In addition, reported mutation c.8232G > C (p.Trp2744Cys) is located in the fourteenth FN3 domain.
Heterozygous nonsense variant c.4217C > A (p.Ser1406X) causing a premature stop codon at 1406 is located on exon 19, and leads to a subsequent loss of 3796 amino acids, which make the protein usherin to lose more than 70% of its amino acids including 30 TM domains, 2 LamG domains, TM domain, and PBM domain. Therefore, heterozygous nonsense variant c.4217C > A (p.Ser1406X) affecting the structure and function of the protein usherin have a great possibility of causing the USH2. Novel missense variant p.Asp3927Gly (c.11780A > G) replaces a polaraspartic acid with a nonpolar hydrophobic glycine at codon 3927. Amino acid substitutions caused by reported missense variant p.Trp2744Cys (c.8232G > C) occur at highly conserved sites among the tested species. Interestingly, sites of novel missense variant p.Asp3927Gly (c.11780A > G) in the Human, Troglodyte, Mulatta, Chicken, Zebrafish and Bovine are conserved while the Mouse not.
For the Family # 2 and Family # 3, the following possibilities could be attributed to the unknown allelic variants: 1. Variants in deep-intronic regions of USH2A were not detected because this part of the genome was not covered in the screening. 2. Variants in regulatory elements except the USH2A gene cannot be excluded. 3. The duplication or deletion of other alleles may not be detected due to the absence of copy number variation analysis.
Because of unknown allelic variants in Family # 2 and Family # 3, we suppose that other pathogenic variants may exist in patients. Data from Family # 2 was supportive for the pathogenicity of the novel nonsense variant c.4217C > A (p.Ser1406X) and novel missense variant c.11780A > G (p.Asp3927Gly). The other three pathogenic variants are known pathogenic mutations that have been reported. However, sufficient biological and clinical evidence was required to reveal the relationship between the identified variants and the USH2. The detailed reasons of these pathogenic mutations leading to visual defects and hearing impairment have not been elucidated, and pending further function and mechanism investigations.
In all the three USH2-causing genes, USH2A gene is the most important causative gene, and the usherin which encoded by USH2A is crucial for the long-term maintenance of mammalian photoreceptors [13]. Accordingly, identification of the mutations in the USH2A gene will not only elucidate the role of USH2A in USH2, but also aid the clinical diagnosis and help to find effective treatments for USH2.
Conclusions
In conclusion, we have described five heterozygous variants may cause USH2 in USH2A in four Chinese patients with USH2, two of which were novel. The specific mechanism for these variants to induce USH2 needs further research to confirm. The findings in this study expand the spectrum of USH2A mutations in USH.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ADGRV1:
-
Adhesion G protein-coupled receptor V1
- DFNB31:
-
Autosomal recessive deafness 31
- EGF Lam:
-
Laminin-type EGF-like domain
- ERG:
-
Electroretinogram
- FN3:
-
Fibronectin Type III
- HSF:
-
Human splicing finder
- IRB:
-
Institutional review board
- Lam GL:
-
Laminin G-like domain
- Lam NT:
-
Laminin N-terminal
- LamG:
-
Laminin G domain
- OCT:
-
Optical coherence tomography
- PBM:
-
PDZ-binding motif
- PCR:
-
Polymerase chain reaction
- RP:
-
Retinitis pigmentosa
- SP:
-
Signal peptide
- TM:
-
Transmembrane region
- USH:
-
Usher syndrome
- USH1:
-
Usher syndrome type I
- USH2:
-
Usher syndrome type II
- USH2A:
-
Usherin
- USH3:
-
Usher syndrome type III
References
Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36(8):595–603.
Keats BJ, Corey DP. The usher syndromes GGS. Am J Med Genet. 1999;89(3):158–66.
Mathur P, Yang J. Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852(3):406–20.
Smith RJ, Berlin CI, Hejtmancik JF, Keats BJ, Kimberling WJ, Lewis RA, et al. Clinical diagnosis of the usher syndromes. Usher syndrome consortium. Am J Med Genet. 1994;50(1):32–8.
Eudy JD, Weston MD, Yao S, Hoover DM, Rehm HL, Ma-Edmonds M, et al. Mutation of a gene encoding a protein with extracellular matrix motifs in usher syndrome type IIa. Science. 1998;280(5370):1753–7.
Weston MD, Luijendijk MW, Humphrey KD, Moller C, Kimberling WJ. Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of usher syndrome type II. Am J Hum Genet. 2004;74(2):357–66.
Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, et al. A novel gene for usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121(2):203–11.
Espinos C, Millan JM, Beneyto M, Najera C. Epidemiology of usher syndrome in Valencia and Spain. Community genetics. 1998;1(4):223–8.
van Wijk E, Pennings RJ, te Brinke H, Claassen A, Yntema HG, Hoefsloot LH, et al. Identification of 51 novel exons of the usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with usher syndrome type II. Am J Hum Genet. 2004;74(4):738–44.
Garcia-Garcia G, Aparisi MJ, Jaijo T, Rodrigo R, Leon AM, Avila-Fernandez A, et al. Mutational screening of the USH2A gene in Spanish USH patients reveals 23 novel pathogenic mutations. Orphanet J Rare Dis. 2011;6:65.
Le Guedard-Mereuze S, Vache C, Baux D, Faugere V, Larrieu L, Abadie C, et al. Ex vivo splicing assays of mutations at noncanonical positions of splice sites in USHER genes. Hum Mutat. 2010;31(3):347–55.
Kremer H, van Wijk E, Marker T, Wolfrum U, Roepman R. Usher syndrome: molecular links of pathogenesis, proteins and pathways. Hum Mol Genet. 2006;15 Spec No 2:R262–70.
Reiners J, Nagel-Wolfrum K, Jurgens K, Marker T, Wolfrum U. Molecular basis of human usher syndrome: deciphering the meshes of the usher protein network provides insights into the pathomechanisms of the usher disease. Exp Eye Res. 2006;83(1):97–119.
Weston MD, Eudy JD, Fujita S, Yao S, Usami S, Cremers C, et al. Genomic structure and identification of novel mutations in usherin, the gene responsible for usher syndrome type IIa. Am J Hum Genet. 2000;66(4):1199–210.
Liu XZ, Hope C, Liang CY, Zou JM, Xu LR, Cole T, et al. A mutation (2314delG) in the usher syndrome type IIA gene: high prevalence and phenotypic variation. Am J Hum Genet. 1999;64(4):1221–5.
Frenzel H, Bohlender J, Pinsker K, Wohlleben B, Tank J, Lechner SG, et al. A genetic basis for mechanosensory traits in humans. PLoS Biol. 2012;10(5):e1001318.
Nakanishi H, Ohtsubo M, Iwasaki S, Hotta Y, Mizuta K, Mineta H, et al. Identification of 11 novel mutations in USH2A among Japanese patients with usher syndrome type 2. Clin Genet. 2009;76(4):383–91.
Xu W, Dai H, Lu T, Zhang X, Dong B, Li Y. Seven novel mutations in the long isoform of the USH2A gene in Chinese families with nonsyndromic retinitis pigmentosa and usher syndrome type II. Mol Vis. 2011;17:1537–52.
Sun LW, Johnson RD, Langlo CS, Cooper RF, Razeen MM, Russillo MC, et al. Assessing photoreceptor structure in retinitis Pigmentosa and usher syndrome. Invest Ophthalmol Vis Sci. 2016;57(6):2428–42.
Liu X, Bulgakov OV, Darrow KN, Pawlyk B, Adamian M, Liberman MC, et al. Usherin is required for maintenance of retinal photoreceptors and normal development of cochlear hair cells. Proc Natl Acad Sci U S A. 2007;104(11):4413–8.
Ng TK, Tang W, Cao Y, Chen S, Zheng Y, Xiao X, et al. Whole exome sequencing identifies novel USH2A mutations and confirms usher syndrome 2 diagnosis in Chinese retinitis pigmentosa patients. Sci Rep. 2019;9(1):5628.
Dai H, Zhang X, Zhao X, Deng T, Dong B, Wang J, Li Y. Identification of five novel mutations in the long isoform of the USH2A gene in Chinese families with usher syndrome type II. Mol Vis. 2008;14:2067–75.
Liu X, Tang Z, Li C, Yang K, Gan G, Zhang Z, Liu J, Jiang F, Wang Q, Liu M. Novel USH2A compound heterozygous mutations cause RP/USH2 in a Chinese family. Mol Vis. 2010;16:454–61.
Shu HR, Bi H, Pan YC, Xu HY, Song JX, Hu J. Targeted exome sequencing reveals novel USH2A mutations in Chinese patients with simplex usher syndrome. BMC Med Genet. 2015;16:83.
Adato A, Lefevre G, Delprat B, Michel V, Michalski N, Chardenoux S, et al. Usherin, the defective protein in usher syndrome type IIA, is likely to be a component of interstereocilia ankle links in the inner ear sensory cells. Hum Mol Genet. 2005;14(24):3921–32.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Key Basic Research Program of China (973 Program 2015CB964601), National Natural Science Foundation of China (81371062 and 81602582), and Thousand Youth Talents Program of China (to J.C.). The funding had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
CJJ and ZZL conceived and designed the experiments, HCH and LXY performed the experiments, CJJ and HCH analyzed the data, CJJ and ZZL contributed reagents/materials/analysis tools. HCH drafted the manuscript. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB) of Tongji Eye Institute of Tongji University School of Medicine in Shanghai (registration number 2013YXY12), China. Written informed consent was voluntarily provided from all participating members.
Consent for publication
All patient’s written consent for their personal or clinical details along with any identifying images to be published in this study was obtained.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Primer information for the USH2A gene exons 2 to 72 sequencing. (XLS 31 kb)
Additional file 2: Table S2.
variants predicted non-pathogenic in this study.
Additional file 3: Figure S1.
Sequencing data of variants c.8559-2A>G, c.4217C>A (p.Ser1406X) and c.11780A>G (p.Asp3927Gly) identified in the father of patient No.001 and the parents of patient No.002.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
He, C., Liu, X., Zhong, Z. et al. Mutation screening of the USH2A gene reveals two novel pathogenic variants in Chinese patients causing simplex usher syndrome 2. BMC Ophthalmol 20, 70 (2020). https://doi.org/10.1186/s12886-020-01342-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-020-01342-y