Abstract
Background
To compare the visual and refractive outcomes after myopic LASEK using three different excimer lasers and standardized surgical and mitomycin C (MMC) application protocols.
Methods
In this retrospective, observational cohort study, we examined 122 eyes treated with Allegretto, 135 eyes treated with Esiris and 137 eyes treated with Technolas excimer lasers. All eyes were treated under the same surgical protocol, and a standardized MMC dosage was used. The three groups were refraction-matched, and both visual and refractive outcomes were evaluated at 1 and 7 days and 1 and 3 months after surgery.
Results
At 3 months postsurgery, Allegretto provided significantly better outcomes than Esiris and Technolas in terms of postoperative uncorrected distance visual acuity (UDVA) (1.11 ± 0.2 vs 1.01 ± 0.2 vs 0.98 ± 0.2) (P = 0.0001), corrected distance visual acuity (CDVA) (1.13 ± 0.2 vs 1.10 ± 0.1 vs 1.04 ± 0.2) (P = 0.0001), residual sphere (− 0.01 ± 0.2 vs + 0.29 ± 0.7 vs + 0.27 ± 0.6) (P = 0.0001), and efficacy index (0.99 ± 0.2 vs 0.90 ± 0.2 vs 0.91 ± 0.2) (P = 0.0004).
Conclusions
We found slightly better visual and refractive outcomes in the Allegretto group at 3 months post-op after LASEK with MMC to correct myopia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Both laser in situ keratomileusis (LASIK) and surface ablation (SA) have been proven to be safe, effective and predictable procedures, becoming the gold standard for myopia correction [1,2,3]. LASIK flap creation has been suggested to induce higher corneal biomechanical changes [4, 5] and higher order aberrations (HOAs) than SA [6, 7]. In addition, the use of corneal wound healing modulators and the technological development of last excimer laser platforms have peaked interest in SA techniques in recent years.
The introduction of mitomycin C (MMC) for corneal wound healing modulation after SA procedures has led to an increase in the treatment range, thus being comparable to LASIK indications. The use of MMC in SA results in a lower incidence of haze [8, 9], higher predictability [10, 11] for low and moderate myopia and an improvement of long-term refractive stability [12].
The development of new excimer laser devices with advanced ablation profiles, faster ablation rates, more accurate eye trackers, and lower stromal ablations for equal refractions in older excimer lasers has led to better refractive outcomes [13], providing similar results for myopia correction in SA and LASIK [1, 2]. Recently, significant interest has been shown in the outcome variability among different laser platforms. Refractive outcomes and/or biological corneal responses might be different between excimer laser platforms due to differences in ablation profiles, energy levels, laser energy stability, etc. The available excimer laser devices include the WaveLight Allegretto® (WaveLight Laser Technologie AG, Erlangen, Germany), the Esiris® (Schwind eye-tech-solutions Gmbh & Co, Kleinostheim, Germany) and the Technolas® (Bausch & Lomb Surgical, Claremont, CA) excimer lasers. Several comparative studies have been published describing excellent results when myopic LASIK is performed with different excimer laser platforms [14,15,16,17,18], including these three devices [19,20,21,22,23].
Although the Allegretto®, Esiris® and Technolas® platforms have been widely studied individually in several publications [24,25,26,27,28,29,30], few comparative studies among them have been published [31,32,33,34,35]. Furthermore, only four of the previous studies were designed to compare visual and refractive results [31,32,33,34]. In those comparisons, the heterogeneous age ranges [31], the different ablation profiles for each laser studied [32,33,34], the lack of MMC protocol in some cases [35] or the restriction of MMC protocols for the same degree of depth ablation [34] should be considered as certain biases that could have influenced the final results.
Thus, we compared the visual and refractive outcomes obtained with three different excimer laser platforms used for myopic laser-assisted subepithelial keratectomy (LASEK) correction in a young adult population (≤ 40 years old), performed by the same surgeon, using conventional ablation profiles in all devices and following the same surgical protocol in all treated eyes.
Material and methods
This study is a retrospective cohort study of 394 eyes from consecutive patients younger than 40 years who underwent LASEK for the correction of myopia with or without astigmatism between 2005 and 2014.
Patients with unstable refraction, keratoconus suspects (defined as any even mild localized steepening observed with Placido corneal topography or slight bowing of the posterior corneal surface detected by corneal tomography), prior ocular surgeries, or systemic diseases that could alter refractive or visual outcomes were excluded.
The preoperative examination, which included corrected distance visual acuity (CDVA) (Nidek autochart projector CP 670, Nidek, Gamagori, Japan), manifest and cycloplegic refraction, ultrasound corneal pachimetry (DGH 5100 contact pachymeter, DHG Technology Inc., Exton, PA; OcuScan RXP, Alcon Laboratories, Inc., Fort Worth, TX), topography/tomography and keratometry (Dicon CT200, Vismed Inc., San Diego, CA; CSO Construzione Strumenti Oftalmici, Italy), mesopic infrared pupillometry (Colvard Pupillometer, Oasis 78 Medical Inc., Glendora, CA), slit-lamp biomicroscopy, Goldmann tonometry and funduscopy, was performed by a masked observer.
All patients provided written informed consent, and institutional review board approval was obtained (regional committee of clinical research of the Community of Madrid. REF 216/3). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Surgical technique
An experienced surgeon (M.A. T) performed all procedures in private practice ophthalmic clinics.
A povidone-iodine solution 5% was applied on the eyelid and conjunctiva before the sterile surgical drape and an eyelid rigid speculum was positioned. All surgeries were performed under topical anaesthesia (lidocaine 2%). A 20% ethanol solution diluted in balanced salt solution (BSS) was instilled for 40 s inside an 8.5-mm corneal trephine (ASICO, Westmont, IL) centred on the pupil. The ethanol solution was eliminated with a cellulose sponge (Merocel®, Medtronic Ophthalmics) and gently rinsed with a cannula connected to a BSS syringe. Once the edges of the epithelial flap were dried with a cellulose sponge, the flap was peeled back with a crescent blade (Alcon Surgical, Orlando, FL), leaving a superior hinge (12-o’clock position). The stromal ablations were performed with the following excimer lasers depending on the date each device remained at the facilities: Wavelight Allegretto 400 Hz®, WaveLight Laser Technologies AG, hereafter “A device”, Esiris®, Schwind Eye Tech Solutions, hereafter “E device” or Technolas 517c®, Bausch & Lomb Surgical, excimer lasers, hereafter “T device”. The A device used 0.95 mm spot separation; fluence: 200 mJ/cm2; and repetition rate: 400 Hz. The E device used 0.95 mm spot separation; fluence: 650 mJ/cm2; and repetition rate: 200 Hz. The T device used 0.95 mm spot separation; fluence: 600 mJ/cm2; and repetition rate: 50 Hz. A 6–7.5 mm optical zone (larger than or equal to the mesopic pupillary size) was ablated using a conventional ablation algorithm (non-customized) in all treated eyes, according the manufacturer’s recommendations.
After stromal ablation, a sponge soaked in MMC 0.02% was applied over the stromal bed for 15 s for every 50-μm ablation depth. For ≤50-μm ablation depths, 15 s of MMC were applied, avoiding leakage to the epithelial flap and the limbus. The programmed spherical ablation was 10% less than the intended correction to avoid overcorrection caused by MMC. The residual stromal bed was gently rinsed with balanced salt solution, and the epithelial flap was repositioned over the stromal bed. A therapeutic soft contact lens (AcuVue; Johnson & Johnson Vision Care, Inc., Jacksonville, FL) was carefully placed on the eye, and antibiotic drops (ciprofloxacin 3 mg/mL, Oftacilox®, Alcon Cusí, Barcelona, Spain) and nonsteroidal anti-inflammatory drops (ketorolac trometamol 5 mg/mL, Acular®, Allergan, Madrid, Spain) were instilled before the speculum was removed.
Postoperative follow-up
Ciprofloxacin 3 mg/mL and steroid drops (dexamethasone alcohol 1 mg/mL, Maxidex®, Alcon Cusí) were applied four times daily during the first postoperative week. The therapeutic contact lens was removed at 1 week after surgery. The steroid drop dosage was tapered over the next 2 months as follows: three times daily for the first month, twice daily for the following 15 days and once daily for the last 15 days. Preservative-free artificial tears were applied as needed.
All patients were examined at 1-day, 1-week, and 1- and 3-month postoperative visits by two experienced optometrists, who recorded, under standardized registration, the uncorrected distance visual acuity (UDVA) and corrected visual acuity (CDVA) in the same room under the same illumination adjusted to mesopic conditions. At the three-month visit, a complete ocular examination was performed, including manifest residual refraction, CDVA and topography.
Statistical analysis
Statistical analysis was performed with the “Statview SE + Graphics”™ (Abacus Concepts Inc., Berkeley, CA) program and a Macintosh PowerBook 1400cs/117 personal computer (Apple Computer Inc., Cupertino, CA, USA). A decimal scale was used for visual acuity measurements and converted to LogMAR quotation using a conversion chart for statistical analysis. The data included the mean, standard deviation, standard error and range. The Kolmogorov-Smirnov test was used to check normality of the distribution, and a factorial ANOVA test was used for analysis. In addition, an intra-group linear regression analysis was performed. A 95% confidence interval was set up, and a P value < 0.05 was considered statistically significant.
Results
This study included 394 consecutive myopic eyes that were divided into three refraction-matched groups: 122 eyes treated with device A, 135 eyes treated with device E, and 137 eyes treated with device T. The preoperative sphere and cylinder were matched in ±0.50 D between groups.
Preoperative sphere in all groups was ≤ − 11.00 D and cylinder was ≤ − 4.50 D. Some statistically significant differences were found in terms of keratometry, pachymetry and age due to the large sample size of this study. Nevertheless, these differences were small and not clinically relevant. Preoperative data are shown in Table 1.
At 1 day, 1 week and 1 month postoperatively, UDVA showed no statistically significant differences among groups, in both decimal and LogMAR notations. However, at the three-month visit, UDVA showed statistically significant better results in the A device group compared to both the E and T device groups (P = 0.0001), in both decimal and LogMAR notations (Table 2). Cumulative UDVA is shown in Fig. 1.
Cumulative histogram of three-month uncorrected distance visual acuity after myopic LASEK with MMC of 394 eyes treated with the Allegretto, Esiris and Technolas excimer lasers. LASEK = Laser-assisted subepithelial keratomileusis; MMC = Mitomycin C; VA = Visual acuity. a- Allegretto, b- Esiris, c-Technolas
Three months postoperatively, statistically significant better results in CDVA were obtained, in decimal notation, with the A device and E device compared to the T device (P = 0.0001), whereas in LogMAR notation, these differences were not detected among the groups, although a slight tendency to significance was noted between the E device and the T device (P = 0.05) (Table 3).
At the last follow-up visit, residual sphere and cylinder showed statistically significant differences among groups. Thus, the A device provided a lower and statistically significant residual sphere (P = 0.0001) and a lower and statistically significant residual cylinder (P = 0.0001) than those of the E and T device groups (Table 3).
The efficacy index (defined as the postoperative UDVA/preoperative CDVA) showed better outcomes in the A device group than in the E and T device groups. These differences were statistically significant (P = 0.0004) (Table 3). The percentage of cumulative UDVA at the three-month visit is shown in Fig. 1. The safety index (defined postoperative CDVA/preoperative CDVA) showed statistically better results between the A device or E device group and the T device group (P = 0.03), whereas significant differences between the A device and E device groups were not detected (Table 3). Changes in lines of CDVA are shown in Fig. 2. The mean changes in lines of preoperative and postoperative CDVA are shown in Table 3.
Changes in lines of corrected distance visual acuity at 3 months after myopic LASEK with MMC of 394 eyes treated with Allegretto, Esiris and Technolas excimer lasers. LASEK = Laser-assisted subepithelial keratomileusis; MMC = Mitomycin C; CDVA = Corrected distance visual acuity. a- Allegretto, b- Esiris, c-Technolas
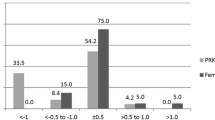
Predictability for residual spherical equivalent (SE) ± 0.50 D showed statistically significant differences among groups (p = 0.0001) (Fig. 3). Predictability for residual SE ± 1.00 D is shown in Fig. 3. No differences were detected among groups (p = 0.08). Linear regression analysis showed a high, positive and statistically significant correlation between preoperative SE and the effectively corrected refraction in all evaluated groups (Fig. 4).
Attempted versus achieved spherical equivalent refraction scatterplots at 3 months after LASEK with MMC for myopia correction in the Allegretto, Esiris and Technolas groups. The linear regression equation and coefficient of determination (r2) are displayed. LASEK = Laser-assisted subepithelial keratomileusis; MMC = Mitomycin C. a- Allegretto, b- Esiris, c-Technolas
No intraoperative or postoperative complications were found in any group.
Discussion
Marginally better predictability and efficacy results were noted with device A at the three-month follow-up visit. However, we found that LASEK surgery is safe, predictable and effective using any of the three excimer laser platforms studied. Although significant differences were detected among the three devices, it should be noted that this difference is so small that it has no clinical impact.
All groups showed initial slow UDVA recovery, as previously described [36]. No differences were found until the first month follow-up visit, but at the 3 month follow-up visit, the A device patients showed significantly better UDVA and efficacy index results. Although variations in the energy level of the laser beam, which are common when using the first excimer devices during the treatments, could affect the predictability, these deficiencies have been improved considerably by manufacturers in the modern laser platforms, achieving optimal energy stability and very precise nomograms. In addition, a higher time of exposure of the stroma alters the corneal hydration, and thus, a greater number of treatment interruptions because of eye movements and primitive eye-tracking systems could also affect the predictability of the ablation. These improvements, together with the greater speed of the laser, could also justify the differences we observed in our study.
Published series for SA using the A device showed comparable results to ours, even with older excimer laser versions (A device 200 Hz) [24, 29, 30]. However, slightly worse results in UDVA were found using the A device 400 Hz in other published series [26]. Isolated E device series showed results comparable to ours, with subtle differences among groups [25, 27]. Prior isolated T device series also obtained similar results [28]. The slight differences observed might be related to higher age range recruitment requirements or the different refraction ranges studied, in addition to the lack of certain data (i.e., excimer laser version or age range), which may hinder the analysis.
Five studies were found that compared SA refractive results with different excimer lasers [31,32,33,34,35], but none of these studies included the three excimer laser platforms examined in our study. Nassiri et al. published two different PRK series [32, 33] in which slightly better UDVA was obtained with the A device compared to the T device, as found in the present study. Compared with VISX Star S4 [34, 35] the A device obtained better UDVA. In these studies, the authors described MMC protocols, refraction ranges and ablation profiles that were different from ours and among these studies, which is problematic as these difference interfere with the proper comparison of these studies. As previously described, using the same surgical protocol, standard ablation procedures for each platform and the same MMC protocol, those biases were diminished.
No statistically significant differences were found among groups in preoperative corrected distance visual acuity (CDVA) in decimal and LogMAR charts. The three-month postoperative CDVA was statistically better for the A device and E device groups than for the T device group, whereas in LogMAR notation, these differences were not detected among the groups. Similar results were found in the safety index analysis, and the number of eyes with vision lines lost was worse with T device data than with data from the other devices. The A device and E device results were similar to previous publications [25,26,27], but our T device results were worse than other results [32, 28]. It Some degree of epithelial alteration, subclinical haze, etc., improves the visual results of surface ablation, even at 3 months after surgery. Nevertheless, since the purpose of the current paper was not to establish the efficacy and safety of surface ablation but to compare the results obtained with different laser platforms, evaluating all results at the same point in the postoperative examination is mandatory, even if the visual results are still not definitive.
In the last follow-up visit of our study, the spherical and cylindrical residual refraction with the A device was different than those with the T and E devices, in both paired comparison and regression analyses. Regarding spherical residual refraction, the A device obtained near to plano refraction with myopic tendency, whereas the E and T devices overcorrected. With cylindrical residual refraction, the A device was also near the plano, with E and T devices undercorrecting. Regression analysis showed low residual refraction dispersion, despite planned treated refraction in eyes treated with the A device, whereas higher dispersion was found both in the E and T devices.
Predictability (SE ±0.50 D) was also better for the A device than for the other two devices. Knowing the optimal predictability results of every excimer laser platform available and its improvement compared to initial models [37, 38], we suggest that the predictability analysis might circumscribe to the ±0.50 D analysis, thus overcoming the prior SE measurement of ±1.00 D.
When we designed this study, our goal was to unmask the subtle differences between excimer laser platforms by creating an ideal surgical workflow where the possible biases were diminished as much as possible, although some of these platforms might not be commercially available at the present time. We used a standardized surgical procedure by a single surgeon to avoid surgical biases. Refraction-matched patients were recruited to avoid postoperative refractive results biases among groups. Finally, a standard MMC protocol [3, 8, 11, 39, 40] was used for biological response homogenization.
Different biological responses that exceed the MMC effect might be expected for each excimer laser studied due to their technical differences. While we did not find haze in any studied group, we propose that the biological homogenization induced by MMC affects all groups in the same way. For these reasons, we can hypothesize that the differences found are real and related to the specific technical features of each excimer laser platform studied.
The studied excimer laser platforms have also been studied in LASIK surgeries [19,20,21,22,23]. Given the known differences between LASIK and SA, the direct comparison of our results with previous publications exceeds the aim of our work. Table 4 shows the results of previous studies on similar laser platforms for SA and LASIK procedures.
Certain possible biases in our results analysis are avoided by delimiting the recruitment maximum age (under 40) and by matching the spherical and cylindrical refraction; the preoperative significant differences obtained in the keratometry and pachymetry values were small and not clinically relevant.
Conclusions
In conclusion, we found subtle postoperative differences favouring the Allegretto excimer laser after myopic LASEK surgery with a standardized surgical and MMC protocol when studying matched populations. However, it should be noted that these differences are so small that they have no clinical impact.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- A:
-
Allegretto excimer laser
- BSS:
-
Balanced salt solution
- CDVA:
-
Corrected distance visual acuity
- D:
-
Dioptre
- E:
-
Esiris excimer laser
- LASEK:
-
Myopic laser-assisted subepithelial keratectomy
- LASIK:
-
Laser in situ keratomileusis
- MMC:
-
Mitomycin C
- SA:
-
Surface ablation
- SE:
-
Spherical equivalent
- T:
-
Technolas excimer laser
- UDVA:
-
U
References
de Benito-Llopis L, Teus MA, Sánchez-Pina JM, Hernández-Verdejo JL. Comparison between LASEK and LASIK for the correction of low myopia. J Refract Surg. 2007;23(2):139–45.
Teus MA, de Benito-Llopis L, Sanchez-Pina JM. LASEK versus LASIK for the correction of moderate myopia. Optom Vis Sci. 2007;84(7):605–10.
de Benito-Llopis L, Teus MA, Sánchez-Pina JM. Comparison between LASEK with mitomycin C and LASIK for the correction of myopia of −7.00 to −13.75 D. J Refract Surg. 2008;24(5):516–23.
Ambrosio R Jr, Wilson S. LASIK vs LASEK vs PRK: advantages and indications. Semin Ophthalmol. 2003;18(1):2–10.
Dawson DG, Randleman JB, Grossniklaus HE, O’Brien TP, Dubovy SR, Schmack I, Stulting RD, Edelhauser HF. Corneal ectasia after excimer laser keratorefractive surgery: histopathology, ultrastructure, and pathophysiology. Ophthalmology. 2008;115(12):2181–91.
Chung SH, Lee IS, Lee YG, Lee HK, Kim EK, Yoon G, Seo KY. Comparison of higher-order aberrations after wavefrontguided laser in situ keratomileusis and laser-assisted subepithelial keratectomy. J Cataract Refract Surg. 2006;32(5):779–84.
Buzzonetti L, Iarossi G, Valente P, Volpi M, Petrocelli G, Scullica L. Comparison of wavefront aberration changes in the anterior corneal surface after laser-assisted subepithelial keratectomy and laser in situ keratomileusis: preliminary study. J Cataract Refract Surg. 2004;30(9):1929–33.
Teus MA, de Benito-Llopis L, Alió JL. Mitomycin C in refractive refractive surgery. Surv Ophthalmol. 2009;54(4):487–502.
Chen SH, Feng YF, Stojanovic A, Wang QM. Meta-analysis of clinical outcomes comparing surface ablation for correction of myopia with and without 0.02% mitomycin C. J Refract Surg. 2011;27(7):530–41.
Sy ME, Zhang L, Yeroushalmi A, Huang D. Hamilton DR effect of mitomycin-C on the variance in refractive outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2014;40(12):1980–4.
Camellin M. Laser epithelial keratomileusis with mitomycin C: indications and limits. J Refract Surg. 2004;20(5):S693–8.
Alio JL, Soria FA, Abbouda A, et al. Fifteen years follow-up of photorefractive keratectomy up to 10D of myopia: outcomes and analysis of the refractive regression. Br J Ophthalmol. 2016;100(5):626–32.
El Bahrawy M, Alió JL. Excimer laser 6(th) generation: state of the art and refractive surgical outcomes. Eye Vis (Lond). 2015;2:6.
Twa MD, Lembach RG, Bullimore MA, Roberts C. A prospective randomized clinical trial of laser in situ keratomileusis with two different lasers. Am J Ophthalmol. 2005;140(2):173–83.
Awwad ST, El-Kateb M, Bowman RW, Cavanagh HD, McCulley JP. Wavefront-guided laser in situ keratomileusis with the Alcon CustomCornea and the VISX CustomVue: three month results. J Refract Surg. 2004;20(5):S606–13.
Fraunfelder FW, Rich LF. Laser in situ keratomileusis using the Nidek EC-5000 or the Alcon LADARVision 4000 systems. J Refract Surg. 2004;20(2):127–31.
Durrie D, Stahl J. Randomized comparison of custom laser in situ keratomileusis with the Alcon CustomCornea and the Bausch & Lomb Zyoptix systems: one-month results. J Refract Surg. 2004;20(5):S614–8.
Seward MS, Oral D, Bowman RW, El-Agha MS, Cavanagh HD, McCulley JP. Comparison of LADARVision and Visx Star S3 laser in situ keratomileusis outcomes in myopia and hyperopia. J Cataract Refract Surg. 2003;29(12):2351–7.
Mearza AA, Muhtaseb M, Aslanides IM. Visual and refractive outcomes of LASIK with the SCHWIND ESIRIS and WaveLight ALLEGRETTO WAVE eye-q excimer lasers: a prospective, contralateral study. J Refract Surg. 2008;24(9):885–90.
Han DC, Chen J, Htoon HM, Tan DT, Mehta JS. Comparison of outcomes of conventional WaveLight(®) Allegretto Wave(®) and Technolas(®) excimer lasers in myopic laser in situ keratomileusis. Clin Ophthalmol. 2012;6:1159–68.
Binder PS, Rosenshein J. Retrospective comparison of 3 laser platforms to correct myopic spheres and spherocylinders using conventional and wavefront-guided treatments. J Cataract Refract Surg. 2007;33(7):1158–76.
Moshirfar M, Betts BS, Churgin DS, Hsu M, Neuffer M, Sikder S, Church D, Mifflin MD. A prospective, randomized, fellow eye comparison of WaveLight® Allegretto Wave ® eye-Q versus VISX CustomVue™ STAR S4 IR™ in laser in situ keratomileusis (LASIK): analysis of visual outcomes and higher order aberrations. Clin Ophthalmol. 2011;5:1339–47.
He L, Liu A, Manche EE. Wavefront-guided versus wavefront-optimized laser in situ keratomileusis for patients with myopia: a prospective randomized contralateral eye study. Am J Ophthalmol. 2014;157(6):1170–8.
Randleman JB, Loft ES, Banning CS, Lynn MJ, Stulting RD. Outcomes of wavefront-optimized surface ablation. Ophthalmology. 2007;114(5):983–8.
O’Brart D, Al-Attar M, Hussein B, Angunawela R, Marshall J. Laser subepithelial keratomileusis for the correction of high myopia with the Schwind ESIRIS scanning spot laser. J Refract Surg. 2006;22(3):253–62.
Gambato C, Catania AG, Vujosevic S, Midena E. Wavefront-optimized surface ablation with the Allegretto Wave eye-Q excimer laser platform: 12-month visual and refractive results. J Refract Surg. 2011 Nov;27(11):792–5.
Arbelaez MC, Vidal C, Arba MS. Comparison of LASEK and LASIK with thin and ultrathin flaps after excimer laser ablation with the SCHWIND aspheric ablation profile. J Refract Surg. 2011;27(1):38–48.
Partal AE, Rojas MC, Manche EE. Analysis of the efficacy, predictability, and safety of LASEK for myopia and myopic astigmatism using the Technolas 217 excimer laser. J Cataract Refract Surg. 2004;30(10):2138–44.
Kymionis GD, Diakonis VF, Kounis G, et al. Effect of excimer laser repetition rate on outcomes after photorefractive keratectomy. J Cataract Refract Surg. 2008;34(6):916–9.
Falavarjani KG, Hashemi M, Modarres M, Sanjari MS, Darvish N, Gordiz A. Topography-guided vs wavefront-optimized surface ablation for myopia using the WaveLight platform: a contralateral eye study. J Refract Surg. 2011;27(1):13–7.
He L, Manche EE. Contralateral eye-to-eye comparison of wavefront-guided and wavefront-optimized photorefractive keratectomy: a randomized clinical trial. JAMA Ophthalmol. 2015;133(1):51–9.
Nassiri N, Safi S, Aghazade Amiri M, Sheibani K, Safi H, Panahi N, Nassiri N. Visual outcome and contrast sensitivity after photorefractive keratectomy in low to moderate myopia: wavefront-optimized versus conventional methods. J Cataract Refract Surg. 2011;37(10):1858–64.
Nassiri N, Sheibani K, Azimi A, Khosravi FM, Heravian J, Yekta A, Moghaddam HO, Nassiri S, Yasseri M, Nassiri N. Refractive outcomes, contrast sensitivity, HOAs, and patient satisfaction in moderate myopia: Wavefront-optimized versus tissue-saving PRK. J Refract Surg. 2015;31(10):683–90.
Moshirfar M, Churgin DS, Betts BS, Hsu M, Sikder S, Neuffer M, Church D, Mifflin MD. Prospective, randomized, fellow eye comparison of WaveLight Allegretto Wave eye-Q versus VISX CustomVueTM STAR S4 IRTM in photorefractive keratectomy: analysis of visual outcomes and higher-order aberrations. Clin Ophthalmol. 2011;5:1185–93.
He L, Manche EE. Prospective randomized contralateral eye evaluation of subjective quality of vision after wavefront-guided or wavefront- optimized photorefractive keratectomy. J Refract Surg. 2014;30(1):6–12.
Taneri S, Weisberg M, Azar DT. Surface ablation techniques. J Cataract Refract Surg. 2011;37(2):392–408.
Trokel SL, Srinivasan R, Braren B. Excimer laser surgery of the cornea. Am J Ophthalmol. 1983;96(6):710–5.
McDonald MB, Kaufman HE, Franzt JM. Excimer laser ablation in a human eye. Arch Ophthalmol. 1989;107(5):641–2.
Lacayo GO 3rd, Majmudar PA. How and when to use mitomycin-C in refractive surgery. Curr Opin Ophthalmol. 2005;16(4):256–9.
LeccisottiA. Mitomycin C in photorefractive keratectomy: effect on epithelialisation and predictability. Cornea. 2008;27(3):288–91.
Acknowledgements
Not applicable.
Funding
The authors declare that they have no sources of funding for the research reported.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception (IRP, JGO, MAT), substantial contribution of the design of the work (IRP, JGO, MAT, RC, MGG), data acquisition and analysis (IRP, JGO, MAT), interpretation of data (IRP, JGO, MAT, RC, MGG), drafted the work (IRP, JGO, RC) and substantively revised the manuscript (MAT, MGG). All the authors (IRP, JGO, MAT, RC, MGG) have approved the submitted version and have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided written informed consent, and institutional review board approval was obtained (regional committee of clinical research of the Community of Madrid. REF 216/3). The study was performed in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rodríguez-Pérez, I., Gros-Otero, J., Teus, M.A. et al. Myopic Laser-Assisted Subepithelial Keratectomy (LASEK) outcomes using three different excimer laser platforms: a retrospective observational study. BMC Ophthalmol 19, 205 (2019). https://doi.org/10.1186/s12886-019-1214-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-019-1214-y