Abstract
Background
Prospective, population-based study of an 8-year follow up.
To determine the direct cost of diabetic retinopathy [DR], evaluating our screening programme and the cost of treating DR, focusing on diabetic macular oedema [DMO] after anti-vascular endothelial growth factor [anti-VEGF] treatment.
Methods
A total of 15,396 diabetes mellitus [DM] patients were studied. We determined the cost-effectiveness of our screening programme against an annual programme by applying the Markov simulation model. We also compared the cost-effectiveness of anti-VEGF treatment to laser treatment for screened patients with DMO.
Results
The cost of our 2.5-year screening programme was as follows: per patient with any-DR, €482.85 ± 35.14; per sight-threatening diabetic retinopathy [STDR] patient, €1528.26 ± 114.94; and €1826.98 ± 108.26 per DMO patient. Comparatively, an annual screening programme would result in increases as follows: 0.77 in QALY per patient with any-DR and 0.6 and 0.44 per patient with STDR or DMO, respectively, with an incremental cost-effective ratio [ICER] of €1096.88 for any-DR, €4571.2 for STDR and €7443.28 per DMO patient. Regarding diagnosis and treatment, the mean annual total cost per patient with DMO was €777.09 ± 49.45 for the laser treated group and €7153.62 ± 212.15 for the anti-VEGF group, with a QALY gain of 0.21, the yearly mean cost was €7153.62 ± 212.15 per patient, and the ICER was €30,361.
Conclusions
Screening for diabetic retinopathy every 2.5 years is cost-effective, but should be adjusted to a patient's personal risk factors. Treatment with anti-VEGF for DMO has increased costs, but the cost-utility increases to 0.21 QALY per patient.
Similar content being viewed by others
Background
Diabetes mellitus [DM] is defined as a group of metabolic diseases whose common feature is an elevated blood glucose level [hyperglycaemia]. DM is a major health problem worldwide; by 2010 more than 200 million people had been diagnosed with diabetes and is predicted to increase by 62 % over the period 1995 to 2025 [1]. Diabetic retinopathy [DR] is DM microangiopathy in the retina, which is the most common cause of blindness in Europe [2]. To diagnose DR, the use non-mydriatic fundus retinography and telemedicine [3]. A screening programme obviously brings with it a cost to the public health system [4] that depends on the number of patients diagnosed with DR and the frequency of screening. The expansion of screening programmes that can diagnose patients with further ocular complications, such as diabetic macular oedema [DMO] has brought with it an increase in the overall cost of treatment. In the present study, we determine the direct cost of DR to our Health Care Area [HCA] between 2007 and 2014, including screening and the cost of diagnosis and treatment of those patients who had DR and DMO, focusing on the impact of that cost and the cost of the more recent anti-VEGF treatment.
Methods
A population of 15,396, Caucasian, DM patients, which is 86.53 % [15,396/17,792] of all DM patients in our HCAs, were evaluated over an 8-year follow-up period. Our population is homologous with other areas in Spain.
Study design
A prospective, population-based study, conducted from 1st January 2007 to 31st December 2014. All diabetes patients screened annually within this period were included in the study.
The inclusion criteria were: patients with diabetes mellitus type 1 or 2 referred to our HCAs, the screening programme include the detection of DM patients by Family Physicians, who send the patients to non-mydriatic fundus camera unit, patients with HbA1c >7 %, insulin treatment or DM duration >10 years, are focused as high risk patients.
The exclusion criteria were: patients with other specific types of diabetes, those with gestational DM and patients with DR who live outside our HCA.
Methods
We evaluated the cost of visits, examinations and interventions carried out for each patient during the 8-year follow-up. The classification used in the present study, taking into account an eye with a high level of DR, is: [i] no diabetic retinopathy [No-DR], [ii] any diabetic retinopathy [level 20 to 35 of ETDRS] [any-DR], [iii] sight-threatening diabetic retinopathy, defined as level 43 retinopathy or worse by the ETDRS [STDR], and/or [iv] sight-threatening maculopathy [STM]. DMO was classified as 'extrafoveal' or 'clinically significant [CSMO]' according to the ETDRS classification.
Statistical methods
For the cost analysis, we used the Markov model with TreeAge Pro 6.0 statistical software. The sensitivity of our study was 90.2 % for DR, and the specificity was 98.6 %. Costs were standardized using data published by the Health Department of Catalonia [CatSalut] and the costs of pharmaceuticals, surgery material [5] and health staff spending of Hospital Universitari Sant Joan. In the present study we determined only direct costs. An annual 3 % discount rate was applied for future costs and utilities, consistent with the standard UK approach. The analysis was calculated in three steps: [i] a comparison of the cost of a 2.5-year screening programme with the cost of an annual programme, [ii] an evaluation of the cost of diagnosis and treatment of DR at the hospital, [iii] an analysis of the DMO treatment cost-utility, focusing on the inclusion of anti-VEGF drugs.
Our DR screening programme was evaluated by a cost-effectiveness study, comparing the current 2.5-year programme to a theoretical annual programme. Quality-adjusted life-years [QALYs] were used as the primary model outcome measure, using visual acuity as related time-trade off data for evaluating utility, and using life expectancy statistics for Spain in DM patients [78 years]. We also determined the incremental cost-effective ratio [ICER] in the analysis of annual costs against the cost of our current 2.5-year programme [6]. The rates of progression of DR is according to the meta-analysis of Wong et al., [7].
The cost of DMO [only CSMO type] treatment with anti-VEGF intravitreal drugs was evaluated by a cost-utility study based on the QALYs gained [8], considering the utility values most commonly accepted in the ophthalmology literature [8, 9]. We compare DMO patients treated with laser [2007 to 2010] and patients treated only by anti VEGF [2011 to 2014]. The comparison with patients treated with laser takes into account only the first eye treated. A descriptive statistical analysis of quantitative data was made. We used the analysis of frequency and percentage in each category. Differences between those included in the analyses were examined using the two-sample tailed t-test to compare two variables or a one-way ANOVA as if we were comparing more than two variables. Data evaluation and analysis was carried out using SPSS 22.0 statistical software package and p < 0.05 was considered statistically significant.
Results
All 15,396 patients were screened, with a mean follow up of 3.18 ± 1.11 times for each patient over the 8 years. Screening took place every 2.5 years. The mean of the number of patients screened annual was 5507 ± 491.9 [5089, 6337], a mean percentage of 35.09 %. The whole sample included more males [m = 8168, f = 7227], which does in fact reflect the prevalence of diabetes in the population as a whole. The mean age of patients without DR was 65.66 ± 12.23 years, patients with any DR was 63.91 ± 11.85 years, patients with sight threatening diabetic retinopathy [STDR] was 61.48 ± 10.91 years and patients with DMO was 60.67 ± 10.37 years.
Table 1 shows the number of patients screened annually, and the incidence of DR at its different levels. The 8-year incidence of any-DR was 24.12 %, with an annual mean incidence value of 8.37 ± 2.19 % [8.09–8.99 %]. For STDR, the 8-year incidence was 7.59 % with an annual mean incidence value of 2.64 ± 0.15 % [2.48–2.88 %], and for DMO it was 6.36 % with an annual mean incidence value of 2.19 ± 0.18 % [2–2.49 %].
Analysis of cost of DR screening
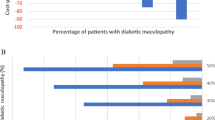
The cost of screening remained stable from 2007 to 2014 [Table 2]. The mean cost was € 223,568 ± 20,956 [€201,840 to € 258,480]. Statistical analysis using the two-sample tailed t-test showed the differences in the total cost of screening were not significant [p = 0298; 95 % CI 201,802–237,717]. The annual mean screening cost per patient was € 40.53 ± 1.21. For a patient with any-DR the mean annual cost was € 482.85 ± 35.14; the annual mean cost of screening for a patient with STDR was € 1528.26 ± 114.94; and the mean annual cost for a patient with only DMO was € 1826.98 ± 108.26.
Table 3 shows the cost of screening annually compared with the current programme. For a patient with any DR, the cost would be €482.32 with a QALY of 11.28. Screening every year would result in an increase of 0.77 in the annual QALY but at a cost of €1347.89. For a patient with STDR or DMO, the annual QALY would increase to values of 0.6 and 0.44 respectively. The ICER would increase the cost to €1096.88 for any-DR, € 4571.2 for STDR and € 7443.28 for DMO patients.
Cost of DR diagnosis and treatment
The mean annual cost of diagnosis and treatment of patients with any-DR was €94,902 ± 19,576 [€77,995 to €128,359], with a mean cost per patient of €285.18 ± 21.61. Table 4 shows the cost of diagnosis and treatment of patients with DR by years, and Table 5 shows the analysis of the cost of diagnosis and treatment of patients with DR.
Analysis of cost-utility of diagnosis and treatment of DMO patients
Table 6 shows the total cost of patients with DMO over the 8 years. The group of patients treated by anti-VEGF drugs [2011 to 2014] was given an average of 5.7 injections the first year, 4.7 injections the second year and 2.9 the third year.
There were two groups of patients treated for CSMO:
-
Group 1 treated by grid or focal laser [2007 to 2010] show a mean annual cost of €67,024 ± 8102€ [€56,880 to €75,317] or €777.09 ± 49.45 per patient.
-
Group 2 treated by anti-VEGF intravitreal injections [2011 to 2014] show a mean annual cost of €545,464 ± 69,128 [€459,384 to € €626,264] or €7153.62 ± 212.15 per patient per year.
For group 1, the mean VA gain after laser treatment was 0.68 ± 1.78 [−11 to 6] letters, and the mean gain in VA for group 2 increased to 6.84 ± 3.22 [0 to 10] letters.
The mean cost per patient of total DMO treatment during a 3 year follow up, was €17791.99, which is from data only available for patients recruited between 2007 to 2013.
For the cost-utility analysis in DMO patients, we used the utility value according Brown et al. [8] for anti-VEGF drug differences with laser, and only the first year of treatment was taken into account. By mean age in DMO in the population [60.67 ± 10.37 years], we considered a further 17.33 years life expectancy in a Spanish population with DM [78 year], and the QALY gain value was 0.21. The yearly mean cost per patient was €7153.62 ± 212.15 per QALY, and the ICER was €30,361 per QALY.
Discussion
In the present study, there are two key points to the cost of DR, the cost of screening and the cost of the DMO treatment, which has increased hugely since the introduction of anti-VEGF drugs. When screening for DR, retinography is the method recommended by scientific societies because it has proven to be cost effective [10, 11]. However, the present study shows that an 8.37 ± 2.19 % detection rate of DR per year [463 ± 63.61 patients] has a total cost of €223,568 ± 20,956. Furthermore, the annual percentage of patients with DR whose condition can lead to poor vision or blindness [STDR], is 2.64 ± 0.15 % [an average of 146.1 ± 19.45 patients] and DMO is detected in 2.19 ± 0.18 % of patients [122.4 ± 20.65 patients]. If these patients go on to develop poor vision or blindness it will represent a significant cost to society, so early detection is of great cost benefit [12].
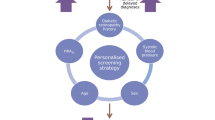
Telemedicine screening for DR has been evaluated in different studies. Rein et al. in 2011 [13] demonstrated that a biannual eye examination by an ophthalmologist is more cost-effective than annual telemedicine. Examinations every 2 years might be cost-effective after one or more normal results and in a population with well-controlled type 2 diabetes [14]. There was essentially no risk of developing significant retinopathy within 3 years of a normal examination result [15]. The American Diabetes Association [2016] position statement concluded that if there is no evidence of DR in one or more eye examinations, then controls every 2 years can be considered adequate. If DR is present, subsequent examinations for patients with type 1 and type 2 DM should be repeated annually by an ophthalmologist or optometrist [16]. The present study demonstrates that for our population, a screening interval of 2.5 years is cost-effective, and annual screening would not improve the management of diabetes patients. For patients with STDR or DMO the results are not so conclusive, but these patients have poor vision, and therefore come for screening earlier than other patients with mild-DR. Furthermore, these patients have worse metabolic status, which requires more attention from their family doctor. It would seem sensible, therefore, to focus screening on patients whose characteristics can be defined as high-risk for developing DR, and we need to identify the patients who need more attention.
The present study also focused on the diagnosis and treatment of patients with diabetic retinopathy. Results show that treatment costs increased from €199.66 ± 19.92 in 2007 to €232.95 ± 21.48 in 2014. This increase can be explained by an increase in more severe forms of DR in recent years, related to poor metabolic control, as we described previously [17].
Finally, the study of DMO treatment costs was focused on the new intravitreal drugs. The introduction of anti-VEGF drugs for treatment of DMO [18] has been replacing laser treatment as the gold standard for DMO since 2011 according to ETDRS protocol [19] and for non-responsive DMO, we have recently been using corticoids, [20, 21]. In the present study, we only included anti-VEGF drugs because non-responsive cases appeared later than our study period. Table 6 shows the cost impact of DMO treatment by these drugs. It shows an exponential increase in the mean cost before 2011 of €67,024 ± 8102 to €545,464 ± 69,128 after 2011. Statistical analysis shows that anti-VEGF drugs are more effective than laser treatment for DMO patients, with an increase in visual acuity of 6.84 ± 3.22 letters in anti-VEGF treated patients against 0.86 ± 0.78 letters in the group treated with laser. This is an increase of 1.23 lines in ETDRS letters similar to the increase in VA observed in the READ 2 study, [22]. The QALY gain value was 0.21, a value similar to the 0.17 QALY gain in the Mitchell et al. study [23]. That study applied its model at 15 years from a baseline of 63 years whereas our model is based on 17 years with a mean patient age of 60 years. Applying the 15-year model to our results predicts a 0.18 QALY gain value, and for a younger population the anti-VEGF cost-effectiveness might be higher because of their longer rest-of-life expectancy.
The ICER of €30,361 per QALY gained relative to laser therapy is lower than the willingness-to-pay [WTP] that is required to be considered cost-effective by the National Institute for Health and Care Excellence [€38,460 or £30,000].
We can conclude, then, that treatment with anti-VEGF drug is cost-useful. Despite the present study being limited to the first year treatment follow up, the increase in VA remains stable, as found in other studies [24]. A difference worth noting is that in the first year the treatment, a mean of 5.7 injections of anti-VEGF per patient is fewer than in other studies such as RESTORE [23], with 10 injections the first year, or the RISE and RIDE clinical trials with 12 injections [25].
A potential weakness of our study is the use of medical record diagnoses and treatment from an integrated health care delivery system. The results of the present study do not represent all of the ophthalmology service costs of diabetes patients, because only patients referred from the screening programme have been evaluated rather than other patients from other hospitals. Weaknesses, also include the use of a screening programme can reduce the number of patients diagnosed with severe forms of DR, also the severity scale could vary if we use wide field image techniques, and the number of patients with STDR can increase. Finally, the number of patients who developed proliferative DR is small and can bias the statistical analysis.
The DMO treatment only takes into account the first treated eye, that which has a more advanced degree of oedema. In addition, the limitation of a 2-year follow up of each patient can reduce the total cost effectiveness for DMO. One strength is that it is a prospective, population-based study, representative of the Spanish population and representative of the standard management of patients with diabetic retinopathy, as opposed to studies that use the Markov or Monte Carlo models that simulate hypothetical cohorts [14, 26].
Conclusion
A screening programme of 2.5 years by telemedicine is cost effective. The cost of screening can be reduced by better selection of time lapse individually tailored to each patient based on their personal risk factors. The cost of DMO increased after anti-VEGF drugs were introduced, but is cost-useful.
References
International Diabetes federation. IDF DIABETES ATLAS, 6TH Edition. Brussels. Belgium. 2013 http://www.idf.org/diabetesatlas
Bourne RR, Jonas JB, Flaxman SR, et al. Vision Loss Expert Group of the Global Burden of Disease Study. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98(5):629–38.
Bragge P, Gruen RL, Chau M, et al. Screening for presence or absence of diabetic retinopathy: a meta-analysis. Arch Ophthalmol. 2011;129:435–44.
Edwards JS. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med. 2010;27(3):249–56.
Departament de salut ordre SLT/42/2012, de 24 de febrer, per la qual es regulen els supòsits i conceptes facturables i s’aproven els preus públics corresponents als serveis que presta l’Institut Català de la Salut. Diari Oficial de la Generalitat de Catalunya. 2012, Núm. 6079-2.3: 10706–856. http://www.gencat.cat/dogc.
Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8.
Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care. 2009;32(12):2307–13.
Brown MM, Brown GC, Sharma S, et al. Utility values and diabetic retinopathy. Am J Ophthalmol. 1999;128:324–30.
Sharma S, Bakal J, Sharma SM, et al. Drug pricing for a novel treatment for wet macular degeneration: using incremental cost-effectiveness ratios to ensure societal value. Can J Ophthalmol. 2005;40:369–77.
Ahmed J, Ward TP, Bursell S-E, Aiello LM, Cavallerano JD, Vigersky RA. The sensitivity and care.diabetesjournals.org Position Statement S65 specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205–9.
Royal College of Ophthalmologist. Diabetic retinopathy guidelines. Section 11. 2012; 103–11. http://www.rcophth.ac.uk.
Yau JW, Rogers SL, Kawasaki R, et al. Meta-Analysis for Eye Disease [META-EYE] Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Rein DB, Wittenborn JS, Zhang X, et al. Vision Cost-Effectiveness Study Group. The cost-effectiveness of three screening alternatives for people with diabetes with no or early diabetic retinopathy. Health Serv Res. 2011;46:1534–6.
Hooper P, Boucher MC, Cruess A, et al. Canadian Ophthalmological Society evidence based clinical practice guidelines for the management of diabetic retinopathy. Can J Ophthalmol. 2012;47 Suppl 2:S12S30.
Agardh E, Tababat-Khani P. Adopting 3-year screening intervals for sight-threatening retinal vascular lesions in type 2 diabetic subjects without retinopathy. Diabetes Care. 2011;34:1318–9.
ADA statement. 9. Microvascular complications and foot care. Diabetes care. 2016;39:S74–6.
Romero-Aroca P, de la Riva-Fernandez S, Valls-Mateu A, et al. Changes observed in diabetic retinopathy. Eight-year follow-up of a Spanish population. Br J Ophthalmol BJO Online First, published on January 14, 2016 as 10.1136/bjophthalmol-2015-307689
Nguyen QD, Brown DM, Marcus DM, et al. RISE and RIDE Research Group. Ranibizumab for diabetic macular oedema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular oedema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806.
Pearson PA, Comstock TL, Ip M, et al. Fluocinolone acetonide intravitreal implant for diabetic macular oedema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–7.
The Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello Ll P, Beck RW, et al. Randomized Trial Evaluating Ranibizumab Plus Prompt or Deferred Laser or Triamcinolone Plus Prompt Laser for Diabetic Macular oedema. Ophthalmology. 2010;117:1064–107.
Nguyen QD, Shah SM, Khwaja AA, et al. READ-2 Study Group. Two-year outcomes of the ranibizumab for oedema of the macula in diabetes [READ-2] study. Ophthalmology. 2010;117(11):2146–51.
Mitchell P, Annemans L, Gallagher M, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema [DME] causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96:688–93.
Pershing S, Enns EA, Matesic B, et al. Cost-effectiveness of treatment of diabetic macular oedema. Ann Intern Med. 2014;160(1):18–29.
Brown GC, Brown MM, Turpcu A, et al. The Cost-Effectiveness of Ranibizumab for the Treatment of Diabetic Macular oedema. Ophthalmology. 2015;122:1416–2.
Kawasaki R, Akune Y, Hiratsuka Y, et al. Cost-utility analysis of screening for diabetic retinopathy in Japan: a probabilistic Markov modeling study. Ophthalmic Epidemiol. 2015;22:4–12.
Acknowledgements
To Arnau BESORA [AB] who contributed to statistical analysis, and Phil Hoddy [PH] who edited the English text.
Funding
This study was funded by the research projects FI12/01535 June 2013, and FI15/01150 Jul 2015 [Instituto de Investigaciones Carlos III [IISCIII] of Spain] and and “Fondo Europeo de Desarrollo Regional” [FEDER].
Availability of data and materials
All the data supporting our findings is contained within the manuscript, and described in the tables. The database will not be published to preserve the patients privacy but can be available from authors upon request.
Authors’ contribution
[PRA] Pedro ROMERO-AROCA contributed to study conception and design, collected research data, carried out the statistical analysis, wrote the Discussion, wrote and edited the manuscript and contributed to the final approval of the version for publication. [SRF] Sofia DE LA RIVA-FERNANDEZ contributed to study conception and design, to the ophthalmology data collection, diagnosed diabetic macular oedema, carried out mathematical studies, wrote the Discussion, and made a critical review, contributing to the final approval of the version for publication. [AVM] Aida VALLS-MATEU contributed to study design, and to the mathematical data study, interpreted the research data, made a critical review and reviewed the translation, contributed to the final approval of the version for publication. [RSA] Ramon SAGARRA-ALAMO contributed to study conception and design, contributed to diabetes mellitus data collection, carried out the retinographies interpreted the research data, and helped to write the manuscript, contributed to final approval of the version for publication. [AMR] Antonio MORENO-RIBAS contributed to study design and the statistical analysis, research data interpretation and contributed to the interpretation of the study findings, contributed to final approval of the version for publication. [NS] Nuria SOLER contributed to ophthalmology data collection, carried out ophthalmic retinographies and OCT procedures and interpreted the research data, contributed to final approval of the version for publication. [DP] Domenec PUIG contributed to image analysis and statistical revision, and final approval for publication.
Competing interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Consent for publication
The present study not contains details, images, or videos relating to individual participants, no consent to publish for participants in the study was required.
Ethics approval and consent to participate
The study was carried out according to local ethics committee protocols, CEIC Hospital Universitario Sant Joan, approval no. 14-12-18/12obs3, and to the revised guidelines of the Declaration of Helsinki. All patients signed an informed consent to participate in the study and for the use of images and its metabolic data. The study was approved and supported by Instituto Carlos III [IISCIII], Spain, nos. FI12/01535 June 2013, and FI15/01150 July 15, and “Fondo Europeo de Desarrollo Regional” [FEDER].
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by: This study was funded by the research projects FI12/01535 June 2013, and FI15/01150 Jul 2015 [Instituto de Investigaciones Carlos III [IISCIII] of Spain] and “Fondo Europeo de Desarrollo Regional” [FEDER].
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Romero-Aroca, P., de la Riva-Fernandez, S., Valls-Mateu, A. et al. Cost of diabetic retinopathy and macular oedema in a population, an eight year follow up. BMC Ophthalmol 16, 136 (2016). https://doi.org/10.1186/s12886-016-0318-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-016-0318-x