Abstract
Background
To understand the clinical and economic outcomes of treatments for managing complications of ischemic central retinal vein occlusion (iCRVO).
Methods
We conducted a systematic literature review by searching multiple databases and ophthalmology conferences from 2004 to 2015. Studies published in English language and populations of age ≥45 years were included. For clinical endpoints, we defined eligibility criteria as randomized controlled trials, prospective before-and-after study designs, and non-randomized studies reporting on treatments in patients with iCRVO. For economic endpoints, all types of study design except cost-of-illness studies were included. We evaluated the definitions of ischemia, clinical and economic endpoints, and rate of development of complications. Risk of bias was assessed for clinical studies using the Cochrane risk-of-bias tool.
Results
A total of 20 studies (1338 patients) were included. Treatments included anti-vascular endothelial growth factors (anti-VEGFs), steroids, and procedures primarily targeting macular edema and neovascularization. Ischemia was not defined consistently in the included studies. The level of evidence was mostly low. Most treatments did not improve visual acuity significantly. Development of treatment complications ranged from 11 to 57 %. Incremental cost-effectiveness ratios reported for anti-VEGFs and steroids were below the accepted threshold of GB£30,000, but considering such treatments only ameliorate disease symptoms they seem relatively expensive.
Conclusions
There is a lack of evidence for any intervention being effective in iCRVO, especially in the prevention of neovascularisation. iCRVO poses a significant clinical and economic burden. There is a need to standardize the definition of ischemia, and for innovative treatments which can significantly improve visual outcomes and prevent neovascular complications.
Similar content being viewed by others
Background
Central retinal vein occlusion (CRVO) is a vascular disorder of the eye and a known cause of significant visual morbidity, including sudden blindness [1]. The global burden of CRVO in adults is estimated to be 2.5 million [2]. The incidence of CRVO increases with age by greater than 10-fold from 40 years of age to 65 years and older [3, 4]. The estimated annual direct cost for managing CRVO in the Medicare population was approximately $1.3 billion in 2006 [5]. In addition, the economic burden of CRVO is significantly higher than for glaucoma. The 1- and 3-year per-patient direct medical costs associated with CRVO are 24 and 15 % higher, respectively, than costs associated with glaucoma [6], despite the prevalence of glaucoma being 24-fold greater than CRVO [7].
The available treatments for iCRVO are used off-label and are directed towards minimizing or delaying the onset of complications associated with CRVO, such as macular edema (ME) and neovascularization (NV) [3]. Complications of NV include neovascular glaucoma (NVG) and vitreous hemorrhage (VH), which can lead to severe visual morbidity and blindness [8].
CRVO has two forms: ischemic and non-ischemic. Non-ischemic CRVO is the milder form of the disease that may resolve on its own or may progress to the ischemic form. Ischemic CRVO (iCRVO) is more severe, resulting in NVG and/or VH. Diagnosis and characterization of the severity of CRVO can be achieved through funduscopy, fluorescein angiography, and optical coherence tomography [9, 10]. Ischemia in CRVO is identified by using various criteria based on findings from these examinations or tests [10]. The exact epidemiology of iCRVO remains unknown; however, one study suggests that iCRVO constitutes about one-fifth of all CRVO cases [11]. Another study estimates 15 % of patients with non-ischemic CRVO progress to iCRVO within 4 months and that 34 % progress within 3 years [8].
More than 90 % of patients suffer from partial or complete vision loss if complications of NVG or iris NV are left untreated [12]. Current management of complications of CRVO include intravitreal anti-vascular endothelial growth factors (anti-VEGFs), intravitreal steroid depots, laser treatments, and a range of surgical interventions [3]. The exact rates of complications in patients with iCRVO receiving these off-label treatments remain unknown and have not been systematically evaluated. There is also a need for a comprehensive systematic review documenting evidence on the full range of treatments for iCRVO, their respective complication rates, and the costs associated with these treatments. The objective of this systematic literature review is to document the clinical outcomes, rates of post-treatment complications associated with interventions, and economic outcomes of treatments used to manage complications of iCRVO.
Methods
Search methods for identifying studies
We conducted a systematic review using search strategies with Medical Subject Heading (MeSH) terms for iCRVO and clinical outcomes to identify relevant studies. A similar search was performed for economic outcomes; however, the search strategy was not restricted by ischemia-related terms. We searched PubMed, EMBASE, PsycINFO, Education Resources Information Center (ERIC), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE), Cochrane Central Register of Controlled Trials (CENTRAL), Health Technology Assessment (HTA) database, and the Campbell Collaboration Library of Systematic Reviews. In addition to the above literature sources the UK National Health Service (NHS) Economic Evaluation Database (NHS EED) was searched for economic studies. The searches were limited to the period January 2004–March 2015, as guidelines on management of CRVO by the UK Royal College of Ophthalmologists were first published in 2004, and to the English language and human studies. Conference proceedings from EURetina, Royal College of Ophthalmologists (available for 2013 and 2014), American Academy of Ophthalmology (AAO), and International Society of Pharmacoeconomics and Outcomes Research (ISPOR) were also searched for 2004–2015. The search strategy for the PubMed database is shown in Table 1.
Eligibility criteria
We included studies assessing individuals 45 years or older with complications of iCRVO. Study populations were considered ischemic if at least one of the following was present: a) the study mentioned the population had ischemia or non-perfusion and b) the inclusion criteria of the study included at least one of the Hayreh [9] or Central Retinal Vein Occlusion Study (CVOS) [10] criteria. Hayreh’s criteria [9] include: a) presence of multiple dark deep intraretinal hemorrhages, b) presence of multiple cotton wool spots, c) degree of retinal vein dilatation and tortuosity, d) relative afferent pupillary defect, and e) electroretinographic tests showing reduced b-wave amplitude, reduced b:a ratio, and prolonged b-wave implicit time. The CVOS criteria [10] include: a) poor visual acuity of <6/60 (equivalent decimal scale = 0.10 and logarithm of the minimum angle of resolution (LogMAR) = 1.00) and b) fluorescein angiography showing greater than 10 disc areas of retinal capillary non-perfusion. Clinical studies were excluded if the results were not reported separately by ischemic status. If a study reported results by ischemic status then the results for the ischemic subpopulation were included in this review. However, if ischemia was not explicitly mentioned in the economic studies, ischemia was determined manually by authors during the study selection phase. Economic studies with CRVO population having complications such as persistent ME and NV were considered as ischemic, and hence were included.
We included studies of interventions used in clinical practice to manage iCRVO or its complications against any comparator (sham, placebo, other active treatment/intervention). Studies without comparator but reporting before-and-after outcomes were also included. We focused on studies reporting clinical outcomes such as visual acuity and retinal thickness, and/or rates of complication development, prognosis of complications, relationship between complications and economic outcomes such as cost of treatment, cost per quality-adjusted life year (QALY), and incremental cost-effectiveness ratio (ICER).
Randomized controlled trials (RCTs), non-randomized trials, and prospective uncontrolled (before-and-after) study designs were included to assess clinical outcomes. All economic studies except cost-of-illness studies were included. Retrospective studies, case studies, commentaries, and case series were excluded. Systematic reviews and meta-analysis were used to cross-reference bibliographies to ensure relevant studies were not inadvertently excluded.
Study selection
Abstracts identified by the search were screened independently by two reviewers and any differences were resolved by consulting a third arbitrator.
Data collection and risk-of-bias assessment
Data from eligible studies were extracted and information was collected for country of investigation, sample size, inclusion and exclusion criteria, patient characteristics at baseline, efficacy outcomes, rate of complication development, relationship between complications, type of economic analysis, perspective of the analysis, cost year, quality of life, and economic outcomes. All best corrected visual acuity (BCVA) values were converted to LogMAR units [13]. All costs were converted to 2015 GBP using the Organisation for Economic Co-operation and Development gross domestic product purchasing power parity conversion rates [14]. Data were extracted by one reviewer and 100 % verified by a second reviewer. Risk of bias for each clinical study was assessed using the Cochrane risk-of-bias assessment tool [15].
Results
Study selection
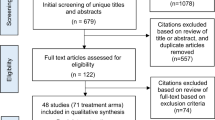
A total of 1891 de-duplicated study abstracts including 130 conference abstracts were screened, of which 20 studies (13 reporting clinical outcomes and seven reporting economic outcomes) were included in the final assessment. A flow diagram summarizing the study attrition is shown in Fig. 1.
Study characteristics
In the included 13 clinical studies shown in Table 2, six studies reported ME complications, four studies reported NV complications, and three studies did not mention complications. Seven studies [16–22] were RCTs and two [23, 24] were before-and-after prospective uncontrolled studies. Other prospective study designs included non-RCTs [25, 26], a randomized trial assessing two doses of the same drug [27], and a cohort study [28]. The included studies were conducted in various countries across the world, including Iran [21, 23, 25] (n = 3), USA [27, 28] (n = 2), Germany [24, 26] (n = 2), Sweden [16] (n = 1), Italy/USA [22] (n = 1), and Japan [20] (n = 1). Three were multinational [17–19]. Anti-VEGF treatments included aflibercept, bevacizumab, and ranibizumab; steroid treatments included triamcinolone; procedural treatments included pars plana vitrectomy with radial optic neurotomy, panretinal photocoagulation (PRP), selective PRP, photodynamic therapy with verteporfin, retinal endovascular lysis, and surgical induction of chorioretinal venous anastomosis; aspirin was used as an anticoagulant.
Cost and economic outcome data were available from seven studies, of which four [29–32] used cost-utility analysis and three [33–35] used cost-effectiveness analysis. Of the seven studies, six [29–32, 34, 35] were obtained from relevant conference proceedings and only one [33] was a full-text article, leading to lack of comprehensiveness in reported data. All studies were conducted in CRVO patients, without the ischemic status provided explicitly. However, NV complications and/or persistent ME are typically associated with ischemia; hence we considered these economic studies relevant to the iCRVO population. Table 3 reports the key characteristics of the studies included.
Definitions of ischemia
Korobelnik et al. [17], Brown et al. [19], Parodi et al. [22], and Feltgen et al. [24] classified eyes with greater than 10 disc areas of non-perfusion as ischemic; Hayreh et al. [28] used Hayreh’s classification to identify ischemia. Boyer et al. [18] defined ischemia as eyes with a BCVA of 20/40 (+0.3) to 20/320 (+1.2) and greater than 10 disc areas of non-perfusion. Campochiaro et al. [27] considered eyes with a BCVA of 20/30 (+0.2) to 20/400 (+1.3) as ischemic. Asano et al. [20] classified ischemia as eyes having large non-perfusion areas, severe hemorrhages, and severe dye leakage, whereas Ramezani et al. [21] classified ischemia as eyes with capillary non-perfusion, presence of relative afferent pupillary defect (RAPD), poor BCVA, and severe intraretinal hemorrhages. Mirshahi et al. [25] considered a BCVA of less than 20/200 (+1.0), presence of RAPD, extensive hemorrhages, and more than 10 disc areas of non-perfusion as indicating ischemic eyes. Wittstrom et al. [16], Jonas et al. [26], and Tabatabaii et al. [23] did not report their method of classifying ischemia. Definitions of ischemia were not available in the economic studies as none of the studies evaluated costs in ischemic population.
Clinical outcomes
The commonly reported efficacy and effectiveness endpoints used in studies were BCVA and central retinal thickness (CRT; also referred to as central macular thickness). All studies reported changes in BCVA whereas only six studies [17–21, 27] reported changes in CRT. Among the included studies, BCVA was calculated in different units, such as Snellen visual acuity, LogMAR, and the decimal system.
Studies reported improvement in BCVA and/or reduction in CRT in the treatment group compared to the comparator group; however, most studies did not provide the level of significance of the improvement, and overall the quality of evidence was low mostly owing to the risk of bias and small population sizes (Table 4). BCVA data as reported in studies are shown in Table 5. Data on rate of complication development during or post-treatment were provided by five studies (Table 4) [17, 22–25]. Development of treatment complications ranged from 11 to 57 % [17, 22–25], with NV as the most commonly reported complication during or after treatment. No studies were found that demonstrated a relationship between the different complications of iCRVO.
Economic outcomes
Key economic data reported across all studies were cost of treatment, administration costs, cost per QALY, and ICER, and key therapies studied were ranibizumab, dexamethasone intravitreal implants, and aflibercept. For two studies conducted in the UK, the analysis was carried out from a UK NHS perspective; [33, 34] in contrast, studies from the USA (n = 2) [31, 35], Sweden (n = 1) [29], and Canada (n = 2) [30, 32] used a payer/healthcare perspective, and another study from Canada used a societal perspective. All included studies calculated the costs of ME secondary to iCRVO but lacked economic data for NV complications. One study expressed costs in 2011 GBP [33], another in 2012 Canadian dollars [30], and another in 2011 USD [31]. For the rest of the studies, which did not report the currency-year, the year of publication was assumed to be the currency-year [29, 32, 34]. Sensitivity analysis was reported in all [29–31, 33–35] but one study [32]. All costs are reported in 2015 values.
In the UK, the ICER of ranibizumab versus observation was £18,381, which included cost of treatment, adverse events, and cost of blindness [33]. In Sweden, aflibercept was dominant, being both less costly (incremental cost of − £2654) and more effective (incremental QALY of 0.061) than ranibizumab [29]. In the USA, the ICER for ranibizumab was £24,882 versus dexamethasone intravitreal implant from a payer perspective [31]. For a patient cohort aged 66–68 years, Haig et al. [32] found that the ICERs for ranibizumab were £16,243 and £1218 (2015 values) if conducted through a Canadian payer perspective and a societal perspective, respectively.
The incremental cost-utility ratios for dexamethasone intravitreal implant versus observation were £12,492 and £8168 as conducted through a Canadian payer perspective and a societal perspective, respectively [30]. This analysis included cost of treatment, cost of adverse events, and cost of blindness [30]. In the USA, the ICER for dexamethasone intravitreal implant was £13,913 versus observation from a payer perspective [31]. The ICER of dexamethasone intravitreal implant versus observation was £17,757, which included only the cost of treatment [34]. In another cost-effectiveness analysis conducted in the USA, the ICER of dexamethasone intravitreal implant compared to observation was reported to be £14,983, which was sensitive to the percentage of patients incurring CRVO in the best-seeing eye, the risk of fellow eye occurrence, and cost of vision loss [35]. Additional details about the included studies are shown in Table 6.
Risk of bias
Using the Cochrane risk-of-bias assessment, the types of bias evaluated for clinical studies were: selection bias: patients not assigned to an intervention or control group using random sequence generation (eight studies [20, 21, 23–28]), or the allocation of participants not concealed (seven studies [20, 21, 23–26, 28]); performance bias: lack of blinding of participants and personnel (seven studies [20, 21, 23–26, 28]); detection bias: blinding of investigators was not done as blinding reduces confounding related to the knowledge of intervention assignment (seven studies [20, 21, 23–26, 28]); attrition bias: incomplete outcomes data due to omission of some participants from the reports of analyses (seven studies [17–21, 26, 27],); reporting bias: selective reporting of study measures (six studies [17–19, 21, 26, 27]); and other biases inherent in various study designs (no studies) [15]. A summary of the risk of bias among the included studies are shown in Table 7. Overall, the risk of bias was high.
Discussion
Our systematic review found studies reporting treatments for iCRVO that included anti-VEGFs, steroids, anticoagulants, and procedural treatments. Treatments commonly targeted the complications of ME and NV. Although complications secondary to iCRVO were successfully treated, BCVA failed to improve and patients continued to have severe vision loss or near-blindness. The rate of development of complications during treatment or follow-up was only reported for procedural treatments. There were no data in the studies on the relationship between the various complications of iCRVO. Additionally, there was a lack of economic evidence for iCRVO population. A number of definitions were used for iCVRO, but they mainly used a combination of criteria within the Hayreh and CVOS classifications.
Treatments for ischemic central retinal vein occlusion
Treatments for iCRVO complication of ME with anti-VEGFs included aflibercept and ranibizumab. Aflibercept treatment improved BCVA in iCRVO patients but population size of iCRVO in the trials was small, and trials with larger sample sizes may be needed for more conclusive results [17–19]. Ranibizumab showed an encouraging improvement in BCVA and also decreased excess foveal thickness in iCRVO patients [27]. However, the numbers of patients were smaller, follow-up was short, and there was a lack of control arm. Thus, these results cannot be considered definitive.
The combination of anti-VEGF bevacizumab injection and PRP resolved anterior-segment NV and prevented an increase in intraocular pressure, but did not lead to an improvement in BCVA in iCRVO patients [16]. Also, bevacizumab caused systemic and ocular adverse events [16]. Aflibercept, ranibizumab, and triamcinolone injections reduced CRT, but the level of significance of this reduction was not reported. Anti-VEGFs reduced ME in iCRVO patients effectively; however, their effect on neovascular complications was not clear. The authors of the rubeosis anti-VEGF (RAVE) trial concluded that anti-VEGFs only delay the neovascular complications in iCRVO and do not treat the underlying blockage of the blood flow in the central retinal vein [36]. Overall, it appears that anti-VEGF treatments provide a short-term impact.
Among various steroids which are available for treating ME [37] (such as triamcinolone acetonide, dexamethasone, and fluocinolone), clinical efficacy of triamcinolone was studied in iCRVO and economic evidence was available for dexamethasone [30, 31, 34, 35] but its clinical efficacy has not been studied recently. Similar to anti-VEGFs, the effects of triamcinolone acetonide on BCVA were sustained only for the short term (less than 6 months) [20, 21, 26].
Our review found that procedural treatments are not successful in improving the vision or even preventing further vision loss in iCRVO. Retinal endovascular lysis, PRP, selective PRP, and photodynamic therapy with verteporfin did not improve BCVA [22, 24]. Moreover, the majority of procedural treatments caused vision to deteriorate. The surgical induction of chorioretinal venous anastomosis may improve BCVA and prevent NV in iCRVO [25], but randomized studies with larger sample sizes are needed. These findings are similar to another review that evaluated the effectiveness of surgical treatments in CRVO patients [38]. In that review, while laser and other surgical interventions were still important treatment modalities, they were mostly reserved for severe cases of ischemia. Hence, lack of visual improvement may have been due to the overall poor prognosis of ischemic eyes requiring surgery. However, the number of post-operative complications were high.
One study used aspirin for its anticoagulant properties [28], but this also did not improve vision. In fact, patients in the aspirin study showed worse vision, more retinal hemorrhages, and more visual-field loss than non-ischemic patients [28]. Aspirin was not recommended in ischemic patients.
At present, therapies used for the acute treatment of CRVO may include medical therapy with anticoagulants, fibrinolytics, corticosteroids, acetazolamide, and isovolemic hemodilution [3], all of which aim to improve venous blood flow in the acute setting. However, such early treatments are generally controversial and off-license, and few patients get detected that early. Even with the use of current therapies, some eyes with iCRVO end up blind and painful and, ultimately, enucleation (removal of the eyeball) may be necessary to provide comfort to patients [39]. Thus, there is a need for curative treatments and better preventative treatments in iCRVO.
Definitions of ischemia
It is possible that differences in the results of BCVA could arise from the lack of a standardized definition of ischemia. Hayreh et al. differentiated ischemic eyes based on the propensity for neovascular complications using functional tests such as visual acuity, visual fields, RAPD, electroretinography, and two morphologic tests (slit-lamp ophthalmoscopy and fluorescein fundus angiography); [9] whereas CVOS defined iCRVO when there is fluorescein angiographic evidence of more than 10 optic disc areas of capillary non-perfusion [10]. As observed in the literature, few studies used only one of these criteria; indeed, most studies used a mix of these criteria to define ischemia. Also, as pointed out in the interim guidelines published by the Royal College of Ophthalmologists, no evidence of the correct combination of these two leading definitions exists that can best define iCRVO [40]. Since this systematic review was completed, the results of the CRYSTAL study have been published, which looked at the effectiveness of ranibizumab in CRVO [41]. In this study by Larsen et al. a new definition of ischaemia was proposed based on fluorescein angiography macular subfield analysis [41]. This new definition does not conform to the definition used by Hayreh et al. [9], but it is valuable contribution to the field. More work is needed in this area as a need exists to standardize the definition of ischemia that can help disease prognosis and treatment decisions.
Complications in ischemic central retinal vein occlusion
Ischemia in CRVO leads to complications such as ME, NV, or VH. The relationship between these complications is often under-examined. A retrospective study conducted by Chen et al. found that the incidence of developing NVG in pre-existing glaucoma eyes was significantly higher in groups with ischemia and an intraocular pressure greater than 20 mmHg [42]. It is important to detect such relationships between the prominent complications of iCRVO as this can help change the treatment paradigms and reduce the clinical burden of the disease.
In order to treat the complications of iCRVO, various treatments are employed but these can often lead to their own complications or adverse events. Serious ocular adverse events are observed in anti-VEGF treatments; [18, 19, 27] however, they are not reported separately by ischemic status of the patient. Complications often develop following the procedural treatments, hence surgical options should be selected with caution. In order to treat the complications caused by the treatments, additional therapies or procedures are required [24], which further increases the disease burden.
Economic outcomes
We did not find any study reporting data on the cost of therapies to prevent or treat complications in the population defined as ischemic, suggesting a major gap in the literature for this population. In lieu of a defined ischemic population, we assumed the presence of NV complications and persistent ME to be an indicator of ischemia in the CRVO population. All economic studies reported cost outcomes in the CRVO population with persistent ME, and no data were found for other complications such as NV or NVG. We also did not identify any publications assessing economic outcomes for bevacizumab, triamcinolone, and procedural treatments, which are often used in iCRVO.
Cost components included across all analyses also varied to some extent. All economic studies considered only direct costs or components of direct costs. For example, two studies included cost of treatment and its administration in their analysis [29, 31], whereas four included costs associated with adverse events in their analysis [30, 31, 33, 35]. Since most therapies are associated with complications, cost models cannot be considered robust with consideration of cost of these complications and adverse events. Moreover, none of the studies evaluated indirect costs of complications of iCRVO. Since iCRVO can lead to severe vision loss, it can be assumed that the indirect cost burden will be high. Commonly, observation or no treatment was considered as the comparator. We found only two analyses making direct comparison between active treatments. These were for aflibercept versus ranibizumab and for ranibizumab versus dexamethasone intravitreal implants [29, 31]. Figure 2 shows the ICER values reported across studies with monetary findings converted to 2015 GBP values and grouped by cost components considered in the analysis. All but one of the ICER values are below the accepted £30,000/QALY threshold [43]. Although these therapies stay under the ICER threshold, it is important to note that they are not curative treatments and they only ameliorate the symptoms of the disease. However, the low ICER values are a reflection of significant impacts on quality of life and/or QALYs. Thus, further research is needed in this population to further understand both the clinical effects and the quality-of-life aspects.
Study strengths and limitations
To the best of our knowledge this is the first systematic review to assess clinical outcomes and economic outcomes in iCRVO. Also, it is the only report that presents the various definitions of ischemia and rates of complication development from published studies. A major strength of this research is the comprehensive, structured, and systematic approach undertaken to search the literature and conference proceedings to identify all studies that report clinical and economic outcomes in the iCRVO segment. Moreover, BCVA, which is reported in the literature with various units, was converted to a single unit of LogMAR. This homogenizes the results for easier understanding. It should be noted that there are considerable methodological limitations in the included studies. While evaluating clinical outcomes, except for two studies [16, 22], all treatments are compared to sham injection, no treatment, pre-treatment, or the same treatment but with a different dose. Thus, there is a lack of head-to-head trials demonstrating the relative efficacy of treatments. Although meta-analyses exist for CRVO [44] there are no meta-analyses comparing various treatments for different complications in the iCRVO population. Among the included economic studies, two studies made direct comparisons between active treatments, but various other treatments often used to iCRVO complications were not studied.
Additionally, there was a lack of RCTs with long follow-up durations and the ischemic population was poorly represented in bigger trials. Only a few trials included in this review had a follow-up of more than 12 months [17, 19, 25, 28]. A trial conducted in iCRVO patients found that the complication of edema reoccurred after the discontinuation of ranibizumab. When ranibizumab injections were withheld for 3 months, about half of patients had recurrent edema along with the loss of visual acuity gains through the treatment [36]. Trials with longer follow-up can provide long-term patient outcomes which may be helpful in understanding the treatment. Furthermore, the proportion of patients with ischemia is dramatically smaller than that of non-ischemic patients in trials concerning the CRVO population. Even when a trial recruits only iCRVO patients, the sample size is very small. Thus, there are no trials with large numbers of ischemic patients, leading to uncertainties in the robustness of the evidence for this group of patients. The majority of cost evidence was obtained from conference proceedings, which leads to limited understanding of the economic aspect of iCRVO. It was difficult to compare studies on key cost drivers in order to understand the differences because of the lack of detail being reported.
A few limitations should be considered when interpreting these findings. BCVA and CRT were presented at various time points, and this varied between studies, it was difficult to make a direct comparison. It was not possible to convert the change in BCVA into LogMAR units for two studies [17, 18] as the baseline BCVA data were not available. Any indirect comparisons must be made with extreme caution as the patient population, complications secondary to CRVO, follow-up period, treatments, economic analysis perspective, and countries differ from study to study.
Researchers can expand the review findings by adding the results from retrospective case series and individual case studies, which comprises the majority of literature on iCRVO. Combination therapies can be explored in iCRVO, which may have the potential to improve vision and reduce complications. A trial in our review shows the benefits of bevacizumab injection in combination with PRP [16], while another trial highlights the avoidance of PRP in all iCRVO patients by choosing selective PRP [22]. A combination of selective PRP and bevacizumab injections may be an effective strategy in iCRVO patients suffering from anterior-segment NV. Extensive research is still needed on the role of anti-VEGFs in treating the complications of iCRVO. Researchers can add to economic evidence of iCRVO by conducting cost analyses specific to iCRVO patient population. The therapeutic care of iCRVO awaits an innovative therapy that can improve the blood flow to the center of the retina.
Conclusions
In conclusion, there is no high-level evidence for any current intervention being effective in a population of exclusively iCRVO cases. Furthermore, there is no solid evidence that anti-VEGF treatment, which is highly effective in CRVO without ischemia, does anything to prevent neovascularization in iCRVO. According to published studies, existing treatments reduce only the complications of iCRVO and do not significantly improve vision impairment, or do so only temporarily. Notwithstanding the scarcity of studies, there is a pressing need for innovative curative and preventive treatments in iCRVO as none of the current treatments solve the significant clinical and economic burden of this blinding condition.
Abbreviations
AAO, American Academy of Ophthalmology; BCVA, best-corrected visual acuity; CDSR, Cochrane Database of Systematic Reviews; CENTRAL, Cochrane Central Register of Controlled Trials; CRT, Central Retinal Thickness/Macular Thickness; CRVO, central retinal vein occlusion; DARE, Database of Abstracts of Reviews of Effects; ERIC Education Resources Information Center; HTA, Health Technology Assessment; ICER, incremental cost-effectiveness ratio; iCRVO, ischemic central retinal vein occlusion; ISPOR, International Society of Pharmacoeconomics and Outcomes Research; LogMAR, logarithm of the minimum angle of resolution; ME, macular edema; MeSH, Medical Subject Headings; NHS EED UK, National Health Service Economic Evaluation Database; NV, neovascularisation; NVG, neovascular glaucoma; PRP, pan-retinal photocoagulation; QALY, quality-adjusted life year; RAPD, relative afferent pupillary defect; RAVE, rubeosis anti-vegf trial; RCT, randomised-controlled trial; VH, vitreous hemorrhage
References
Yau J, Lee P, Wong T, et al. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38:904–10.
Rogers S, McIntosh RL, Cheung N, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117:313–9.
Mohamed Q, McIntosh RL, Saw SM, Wong TY. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507–19.
Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8.
Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye. 2011;25:981–8.
Fekrat S, Shea AM, Hammill BG, et al. Resource use and costs of branch and central retinal vein occlusion in the elderly. Curr Med Res Opin. 2009;26:223–30.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117(6):1113–23.
Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201–17.
Group CVOS. A randomised clinical trial of early panretinal photocoagulation for ischemic central vein occlusion: the Central Vein Occlusion Study Group N Report. Ophthalmology. 1995;102(10):1434–44.
Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41.
Williamson TH. Central retinal vein occlusion: what’s the story? Br J Ophthalmol. 1997;81:698–704.
Colenbrander A. Consilium Ophthalmologicum Universale Visual Functions Committee. Visual acuity measurement standard. Ital J Ophthalmol. 1984;2:5–19.
The Campbell and Cochrane Economics Methods Group (CCEMG), Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre). CCEMG-EPPI-Centre Cost Converter (version 1.4). http://eppi.ioe.ac.uk/costconversion/default.aspx. Accessed 10 July 2015.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928.
Wittstrom E, Holmberg H, Hvarfner C, Andreasson S. Clinical and electrophysiologic outcome in patients with neovascular glaucoma treated with and without bevacizumab. Eur J Ophthalmol. 2012;22:563–74.
Korobelnik JF, Holz FG, Roider J, et al. Intravitreal Aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO Study. Ophthalmology. 2014;121:202–8.
Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119:1024–32.
Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429–37.
Asano S, Miyake K, Miyake S, Ota I. Relationship between blood-aqueous barrier disruption and ischemic macular edema in patients with branch or central retinal vein occlusion: effects of sub-tenon triamcinolone acetonide injection. J Ocul Pharmacol Ther. 2007;23:577–84.
Ramezani A, Entezari M, Moradian S, et al. Intravitreal triamcinolone for acute central retinal vein occlusion; a randomised clinical trial. Graefes Arch Clin Exp Ophthalmol. 2006;244:1601–6.
Parodi MB, Friberg TR, Pedio M, et al. Panretinal photocoagulation and photodynamic therapy for anterior segment neovascularization secondary to ischemic central retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 2007;38:94–9.
Tabatabaii SA, Rasoolnejad SA, Moghimi S, et al. The results of radial optic neurotomy for treatment of central retinal vein occlusion. Acta Med Iranica. 2008;46:373–8.
Feltgen N, Junker B, Agostini H, Hansen LL. Retinal endovascular lysis in ischemic central retinal vein occlusion: one-year results of a pilot study. Ophthalmology. 2007;114:716–23.
Mirshahi A, Roohipoor R, Lashay A, et al. Surgical induction of chorioretinal venous anastomosis in ischaemic central retinal vein occlusion: a non-randomised controlled clinical trial. Br J Ophthalmol. 2005;89:64–9.
Jonas JB, Akkoyun I, Kamppeter B, et al. Intravitreal triamcinolone acetonide for treatment of central retinal vein occlusion. Eur J Ophthalmol. 2005;15:751–8.
Campochiaro PA, Hafiz G, Shah SM, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Mol Ther. 2008;16:791–9.
Hayreh SS, Podhajsky PA, Zimmerman MB. Central and hemicentral retinal vein occlusion: role of anti-platelet aggregation agents and anticoagulants. Ophthalmology. 2011;118:1603–11.
Eriksson M, Castelo-Branco A, Nilsson J. Cost-effectiveness of aflibercept in the treatment of macular oedema secondary to central retinal vein occlusion in Sweden. Value Health. 2014;17:A608.
Vicente C, Koster B, Zilbershtein R, Piwko C. Cost-effectiveness of dexamethasone intravitreal implant in the treatment of macular edema (ME) following central retinal vein occlusion (CRVO). Value Health. 2013;16:A177–8.
Duff S, Gricar J, Kymes S, et al. PSS20 cost-utility analysis of treatments for macular edema secondary to retinal vein occlusion. Value Health. 2012;15:A571–2.
Haig J, Lawrence D, Barbeau M, et al. PSS19 Economic evaluation of ranibizumab for the treatment of macular edema secondary to branch and central retinal vein occlusion in Canada. Value Health. 2012;15:A571.
Taylor M, Serbetci E, Ferreira A, et al. A United Kingdom-based economic evaluation of ranibizumab for patients with retinal vein occlusion (RVO). J Med Econ. 2014;17:423–34.
Hayward E, Almond C, Trueman D, et al. PSS25 the cost-effectiveness of Ozurdex® (dexamethasone intravitreal implant in applicator) compared with observation for the treatment of macular oedema following central and branch retinal vein occlusion. Value Health. 2011;14:A506.
Kowalski J, Yeh WS, O’Leary B, et al. PSS13 incremental cost-utility analysis of dexamethasone intravitreal implant for the treatment of macular edema following retinal vein occlusion. Value Health. 2011;14:A55.
Brown DM, Wykoff CC, Wong TP, et al. Ranibizumab in preproliferative (ISCHEMIC) central retinal vein occlusion: the rubeosis anti-VEGF (RAVE) trial. Retina. 2014;34:1728–35.
Sarao V, Veritti D, Boscia F, Lanzetta P. Intravitreal steroids for the treatment of retinal diseases. Sci World J. 2014:989501. http://www.hindawi.com/journals/tswj/2014/989501/ Accessed 8 July 2016.
Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245–52.
Ford JA, Clar C, Lois N, et al. Treatments for macular oedema following central retinal vein occlusion: systematic review. BMJ Open. 2014;4:e004120.
Royal College of Ophthalmologists. Interim guidelines for management of retinal vein occlusion. London: Royal College of Ophthalmologists; 2010.
Larsen M, Waldstein SM, Boscia F, et al. Individualized ranibizumab regimen driven by stabilization criteria for central retinal vein occlusion. Ophthalmology 2016. http://dx.doi.org/10.1016/j.ophtha.2016.01.011.
Chen H-F, Chen M-C, Lai C-C, et al. Neovascular glaucoma after central retinal vein occlusion in pre-existing glaucoma. BMC Ophthalmol. 2014;14:119.
McCabe C, Claxton K, Culyer AJ. The NICE cost-effectiveness threshold. Pharmacoeconomics. 2008;26:733–44.
Huang P, Niu W, Ni Z, et al. A meta-analysis of anti-vascular endothelial growth factor remedy for macular edema secondary to central retinal vein occlusion. PloS one. 2013;8(12):e82454.
Dijkers, M. Introducing GRADE: a systematic approach to rating evidence in systematic reviews and to guideline development. 2013; KT Update (1)5. Austin, TX: SEDL, Center on Knowledge Translation for Disability and Rehabilitation Research.
Acknowledgement
Manuscript prepared and copy-edited by Nik Prowse (nikprowse.com); we also acknowledge Katherine Cullen for her input to study design and review of the manuscript.
Funding
The systematic review was funded by Cell Therapy Catapult London. This publication was funded by Valid Insight.
Availability of data and materials
Not applicable.
Authors’ contributions
SB, PK, MN designed the search strategy. SG, AS and MN carried out record searching and screening. MN, AS, SG and SB assessed full records for eligibility. AS, MN and SG were involved in data extraction. SB provided advice on conversion of visual acuity units to logMAR. SB and PK were involved in interpretation of findings. MN, SB and AS were involved in writing the manuscript. SB, MM and PK revised the manuscript critically. All authors read and approved the final manuscript.
Competing interests
The systematic review was funded by Cell Therapy Catapult London. Valid Insight is an expert-led global consultancy with leading expertise in health economics and outcomes research, market access strategies and product lifecycle management solutions (validinsight.com).
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bradshaw, S.E., Gala, S., Nanavaty, M. et al. Systematic literature review of treatments for management of complications of ischemic central retinal vein occlusion. BMC Ophthalmol 16, 104 (2016). https://doi.org/10.1186/s12886-016-0282-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-016-0282-5