Abstract
Purpose
To evaluate the long-term efficacy and safety of GP and TPF sequential chemotherapy regimens in patients with locoregionally advanced nasopharyngeal carcinoma (LA-NPC).
Methods
From 2005 to 2016, a total of 408 LA-NPC patients treated with GP or TPF sequential chemoradiotherapy were retrospectively included. Propensity Score Matching (PSM) was employed to balance the baseline variables. Survival outcomes and acute toxicities were compared between both groups.
Results
A total of 230 patients were selected by 1:1 PSM. At a median follow-up of 91 months, no significant differences were observed between the matched GP and TPF groups regarding 5-year overall survival, progression-free survival, distant metastasis-free survival, and locoregionally relapse-free survival (83.4% vs. 83.4%, P = 0.796; 75.6% vs. 68.6%, P = 0.301; 86.7% vs. 81.1%, P = 0.096; and 87.4% vs. 87.2%, P = 0.721). Notable disparities in adverse effects were identified, with higher incidences of grade 3/4 thrombocytopenia in the GP group while grade 3/4 leukopenia and neutropenia in the TPF group. Though not recorded in our cohort, combined with the FAERS database, thrombotic adverse reactions are a concern for the GP regimen, while the TPF regimen requires vigilance for life-threatening adverse reactions such as septic shock, acute respiratory distress syndrome, and laryngeal edema.
Conclusion
No significant difference in long-term outcomes was observed between the GP and TPF sequential chemotherapy regimens for LA-NPC. Differences in adverse effects should be noted when choosing the regimen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nasopharyngeal carcinoma (NPC) exhibits endemic in Southern China, Southeast Asia, and North Africa [1,2,3,4]. Despite the long-term survival for early-stage NPC patients, nearly 80% patients are diagnosed at late stages due to the insidious symptoms [5, 6]. With the widespread application of Intensity-Modulated Radiation Therapy (IMRT), local control has been greatly improved in patients with locoregionally advanced NPC (LA-NPC) [7]. However, distant metastasis remains the primary cause of treatment failure [8]. Therefore, current research on treating LA-NPC primarily focuses on systemic chemotherapy.
Systemic chemotherapy is essential for improving overall survival and reducing the risk of distant metastasis in LA-NPC [9,10,11]. Consequently, the integration of systemic chemotherapy with radiotherapy has emerged as the standard treatment approach for LA-NPC. Presently, the commonly prescribed systemic chemotherapy regimens include GP (gemcitabine plus cisplatin) and TPF (docetaxel, cisplatin, and fluorouracil), each having shown effectiveness in clinical trials [10, 12]. Thus, the best choice of systemic chemotherapy regimen remains debate.
This study performs a retrospective analysis on the long-term prognosis and safety profile of LA-NPC patients treated with combined IMRT and either GP or TPF sequential chemotherapy. Additionally, this research screens adverse reactions associated with both GP and TPF regimens reported in the FAERS database. This study aims to provide improved guidance for future clinical decision-making.
Methods
Patients
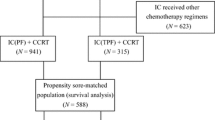
We included patients diagnosed with LA-NPC at Fudan University Shanghai Cancer Center between September 2005 and December 2016. Staging was performed according to the AJCC 8th edition. Initial evaluations included physical exams, laboratory tests, nasopharyngoscopy, enhanced magnetic resonance imaging (MRI) of the nasopharynx and enhanced MRI/computed tomography (CT) of cervical regions. Positron emission and computer tomography (PET/CT) or a combination of chest CT scans, abdominal ultrasound/CT scans, and ECT bone scans were used to exclude metastasis. Oral examinations were conducted prior to radiotherapy to address any dental issues, with extractions performed when necessary. Inclusion criteria were: (1) pathologically confirmed NPC; (2) stage III-IVA; (3) received GP or TPF sequential chemotherapy; (4) received IMRT. Exclusion criteria included: (1) distant metastasis; (2) prior history of cancer. This study was approved by the hospital’s ethics committee, and informed consent was obtained from all patients before treatment.
Treatment
All patients in this study received sequential chemotherapy combined with IMRT. The chemotherapy regimens included the GP regimen (gemcitabine 1,000 mg/m² on days 1 and 8, cisplatin 25 mg/m² on days 1–3, q3w) and the TPF regimen (docetaxel 60 mg/m² on day 1, cisplatin 25 mg/m² on days 1–3, fluorouracil 500 mg/m² per day, continuously infused over 120 h, q3w).
Patients received two cycles of induction chemotherapy (IC), then followed by IMRT and two cycles of adjuvant chemotherapy (AC) using the same regimen, unless the patient refused or showed intolerance or progressive disease. If acute myelosuppression or organ dysfunction occurred, chemotherapy was delayed. Up to a 2-week delay of chemotherapy was allowed, or the chemotherapy will be terminated. A 20% reduction in the dose for the next cycle was applied in the event of grade 4 hematological or ≥ Grade 3 non-hematological toxicities. Modifications up to 2 times are allowed, or chemotherapy will be terminated. IMRT should begin within 21–28 days from the first day of the last induction chemotherapy cycle [13], the target volume delineation and dose prescription were as with previously published studies [14]. AC was administrated 4 weeks after completing radiotherapy, up to a 2-week delay of AC was allowed, or the AC will be terminated.
Following up
After completing all treatments, patients were followed up every three months during the first two years, every six months from the third to the fifth year, and annually thereafter. Follow-up assessments included physical examinations, nasopharyngoscopy, hematologic tests, and imaging evaluations. The primary endpoints of this study were overall survival (OS) and progression-free survival (PFS). OS was defined as the time from treatment initiation to the patient’s death or last follow-up, while PFS was defined as the time from treatment initiation to the occurrence of recurrence/distant metastasis/death or last follow-up. Secondary endpoints included distant metastasis-free survival (DMFS) and locoregional relapse-free survival (LRFS). DMFS was defined as the time from treatment start to the detection of distant metastasis, and LRFS was defined as the time from treatment start to the detection of locoregional relapse. Weight loss was defined as the percentage difference between post-treatment and pre-treatment weight relative to pre-treatment weight.
FAERS database
The FAERS database, developed by the Food and Drug Administration (FDA), is an open-access database for spontaneously reporting adverse drug reactions. Data can be obtained from the official website: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html. The Medx_UIMA_1.3.8 software is used for standardizing drug names, and adverse reactions are categorized using MedDRA 26.1.
Statistical analysis
In the baseline data section, chi-square tests or Fisher’s exact tests were employed. Patient matching between the GP and TPF groups was conducted using Propensity Score Matching (PSM) at a 1:1 ratio. Survival rates were calculated using the Kaplan-Meier (KM) method, and differences between groups were assessed using the log-rank test. Multivariate analysis was performed using Cox regression. Statistical analyses were carried out in SPSS, while graphs were generated using Prism 8. A two-tailed P-value of less than 0.05 was considered statistically significant. Data from the FAERS database were processed using R software, which included removing duplicate reports based on case ID and report date, extracting major adverse drug reactions related to medications, and applying signal detection methods such as ROR, PRR, and BCPNN.
Results
Patients
The baseline characteristics of 408 patients with LA-NPC were shown in Table 1. Statistical differences were noted between both groups in terms of gender and the number of chemotherapy cycles. We employed PSM based on patient age, gender, KPS score, pathological type, T stage, N stage, clinical stage, and the number of chemotherapy cycles to match the two groups. After matching, as shown in Table 1, there were no statistical differences in baseline data between both groups.
Survivals
For the entire cohort, the median follow-up duration was 91 months (ranging from 38 to 177 months). The patterns of treatment failure for both groups are displayed in Table 2. No differences were observed in nasopharyngeal recurrence, retropharyngeal recurrence, cervical recurrence, or distant metastasis between both groups either in the original cohort or in the matched cohort.
By the last follow-up, 103 patients had died across the entire cohort (44 in the GP group and 59 in the TPF group), while in the matched cohort, 70 patients had died (40 in the GP group and 30 in the TPF group). As illustrated in Fig. 1, the five-year OS, PFS, DMFS, and LRFS were 84.4%, 75.1%, 85.3%, and 88.6% respectively for the entire cohort, and 83.4%, 72.1%, 83.9%, and 87.3% for the matched cohort. After matching, there were no significant statistical differences in the five-year OS (83.4% vs. 83.4%, P = 0.796), PFS (75.6% vs. 68.6%, P = 0.301), DMFS (86.7% vs. 81.1%, P = 0.096), or LRFS (87.4% vs. 87.2%, P = 0.721) between the GP and TPF groups.
Recognizing the potential impact of chemotherapy cycles on treatment efficacy, we used PSM to align the GP and TPF groups based on patient demographics and clinical characteristics such as age, gender, KPS score, pathological type, T stage, N stage, and clinical stage. After matching, as shown in Table S3, there were no statistical differences in baseline data across both groups, except in the number of chemotherapy cycles. Furthermore, Fig. S1 illustrated that there were no significant differences in the five-year OS (81.9% vs. 82.0%, P = 0.972), PFS (74.6% vs. 68.9%, P = 0.185), DMFS (85.8% vs. 81.5%, P = 0.073), and LRFS (87.3% vs. 87.0%, P = 0.724) between the GP and TPF groups in the matched cohort.
Furthermore, we categorized the TPF group into two subgroups based on the number of completed chemotherapy cycles: the TPF-C0 group, which did not complete 4 cycles, and the TPF-C1 group, which did complete 4 cycles. As illustrated in Fig. S2, there were no significant statistical differences in the five-year OS (82.5% vs. 88.3%, P = 0.091), PFS (73.4% vs. 76.7%, P = 0.410), DMFS (82.7% vs. 86.9%, P = 0.140), LRFS (88.3% vs. 89.8%, P = 0.627) between both groups.
As shown in Table 3, multivariate Cox regression analysis was conducted to calculate potential prognostic confounders, incorporating multiple factors including gender (male vs. female), age (< 48 years vs. ≥ 48 years), KPS (< 90 vs. ≥ 90) T stage (T1-2 vs. T3-4), N stage (N0-1 vs. N2-3), Clinical stage (III vs. IVa), chemotherapy regimen (GP vs. TPF), chemotherapy cycle (1–3 vs. 4), and weight loss (≤ 10% vs. > 10%). The analysis indicated that the GP and TPF chemotherapy regimens were not associated with patient prognosis. However, we found that age was predictive of OS (HR 0.594; 95% CI 0.354–0.996; P = 0.048), clinical stage was strongly predictive of OS (HR 0.462; 95% CI 0.278–0.769; P = 0.003) and PFS (HR 0.468; 95% CI 0.297–0.735; P < 0.001), weight loss (HR 0.408; 95% CI 0.222–0.748; P = 0.004) and N stage (HR 0.361; 95% CI 0.141–0.927; P = 0.034) were predictive of DMFS.
Treatment compliance
Acute toxicities during chemoradiotherapy in the original and matched cohorts for patients are depicted in Table 4. Significant differences were observed in the incidence of Grade 3/4 leukopenia, neutropenia, and thrombocytopenia between the GP and TPF groups, with P values less than 0.001 in both cohorts. Although the TPF group experienced more Grade 3/4 mucositis compared to the GP group, this difference was not statistically significant (P = 0.052 in original cohort and P = 0.059 in PSM cohort).
These acute toxicities may prevent patients from completing all four cycles of chemotherapy. As detailed in Table 1 and Table S4, approximately half of the patients could not complete all four cycles of TPF. Specifically, 64 (22.4%) patients were unable to complete the regimen due to myelosuppression. An additional 2 (0.7%) patients discontinued treatment due to disease progression, while 29 (10.1%) patients chose to stop treatment. The remaining 35 (12.2%) patients could not finish due to various reasons, including herpes zoster, renal dysfunction, liver dysfunction, diarrhea, facial neuritis, periodontitis, and common colds.
Furthermore, several factors related to chemotherapy side effects may influence efficacy. As shown in Table S5, there were no significant statistical differences in time to radiotherapy (TTR) (P = 0.397) or radiotherapy interruption (RTI) (P = 0.350) between GP and TPF groups [13, 15]. Notably, there was a statistical difference (P = 0.048) in the radiotherapy completion rates between both groups. However, given the extremely small proportion of patients who did not complete radiotherapy, and the fact that it was caused by sudden diseases such as cerebral hemorrhage or economic constraints, this finding require validation with a larger sample size.
In the FAERS database, we screened for adverse events (AEs) related to GP and TPF treatments, excluding irrelevant System Organ Classes (SOCs). The top 20 AEs for each chemotherapy regimen are displayed in Table S1. Anemia and thrombocytopenia are the most common AEs associated with the GP regimen, while neutropenia and anemia are the most prevalent AEs for the TPF regimen, which was consistent with the data of our cohort.
Although severe AEs like neutropenic sepsis were rare in our cohort, occurring in only three cases, they require careful consideration and assessment. Therefore, we identified 310 strong signal AEs with an IC025 value of ≥ 1.0 associated with the GP regimen and 195 with the TPF regimen according to the FAERS database to highlight some of the AEs of interest (Table S2). Both treatments showed strong infection signals within the ‘Infections and infestations’ category, with septic shock and sepsis occurring more prevalent with TPF. Similarly, peripheral arterial thrombosis rates were higher in the GP regimen, which also uniquely reported arterial thrombosis, ischemic necrosis, extremity necrosis, peripheral ischemia, capillary leak syndrome, and embolism. In the gastrointestinal system, the TPF regimen exhibited stronger signals for neutropenic colitis and enterocolitis, whereas esophageal perforation and gastrointestinal necrosis were specific in the TPF regimen. Both regimens caused cardiac disorders such as arteriospasm coronary, but acute coronary syndrome and acute myocardial infarction were unique in the GP regimen, and cardiotoxicity and ventricular fibrillation were specific in the TPF regimen. In the ‘Blood and lymphatic system disorders’ category, the GP regimen showed higher rates of hematotoxicity, leukopenia, thrombocytopenia, and anemia, while neutropenia was more frequent with TPF. In ‘Hepatobiliary disorders’, cholangitis was more common in the GP regimen. In ‘Respiratory, thoracic and mediastinal disorders’, TPF had a higher incidence of acute respiratory distress syndrome, while pulmonary embolism and laryngeal edema were unique to GP and TPF, respectively. In ‘General disorders and administration site conditions’, mucosal inflammation was unique to TPF. Neurotoxicity was more frequent in GP in the ‘Nervous system disorders’ category, and in ‘Renal and urinary disorders,’ toxic nephropathy was higher in GP.
Discussion
Under the combined use of IMRT and concurrent chemotherapy, patients with LA-NPC achieve good local control [10]. However, distant metastasis remains a significant challenge [16]. Current research primarily focuses on systemic chemotherapy, with the addition of adjuvant or induction chemotherapy potentially helping to manage distant metastasis [12]. Clinical studies indicate that concurrent chemoradiotherapy combined with AC can help control distant metastasis, though it may have poor tolerability [11]. Other studies suggest that IC combined with concurrent chemoradiotherapy can not only improve quality of life but also enhance patient tolerability [17]. However, the adverse reactions during concurrent chemoradiotherapy remain a concern [18]. In this study, we employed sequential chemoradiotherapy (IC + IMRT + AC), and over 80% of patients treated with the GP regimen completed the full course of chemotherapy. This suggests that in addition to induction/adjuvant chemotherapy combined with concurrent chemoradiotherapy, sequential chemotherapy alongside radiotherapy is also a viable option worth considering [18,19,20].
The commonly used systemic chemotherapy regimens include PF, TPF, GP, and TP. Previous research has shown TPF to be superior to PF and TP, although it leads to a higher incidence of grade 3/4 adverse reactions, particularly leukopenia and neutropenia [21,22,23,24,25,26]. Therefore, it is essential to explore alternative options. Previous multicenter randomized study indicated that GP is more effective than PF in treating recurrent and metastatic nasopharyngeal carcinoma [27]. Additionally, other studies have demonstrated the effectiveness of the GP regimen in locally advanced stages of the disease [28, 29]. Research also suggests that the GP regimen outperforms PF and TP in locally advanced nasopharyngeal carcinoma, with comparable adverse reactions [14, 30, 31]. However, the optimal choice of systemic chemotherapy for patients with locally advanced nasopharyngeal carcinoma remains to be determined in clinical practice. In this study, the efficacy of the GP and TPF regimens was found to be comparable, which aligns with previous reports [32,33,34,35].
Recent studies indicate that IC combined with concurrent chemoradiotherapy leads to weight loss in patients with LA-NPC [36,37,38]. Pre-treatment obesity is a protective prognostic factor for this cancer, and weight loss during treatment is associated with poorer outcomes [39,40,41]. Multivariate analysis of this study also proved that significant weight loss is an independent prognostic factor for distant metastasis (HR 0.408; 95% CI 0.222–0.748; P = 0.004). Therefore, it is crucial to monitor patients’ weight during treatment and provide timely nutritional support. Considering the high frequency incidence of mucositis in TPF regimen, which was usually thought to influence the food intake, the GP regimen may be more suitable for patients with nutritional risks.
With the advancement of IMRT and systemic chemotherapy, the prognosis for patients with LA-NPC has significantly improved. Consequently, there is increasing focus on the toxic side effects caused by treatment. Our study indicates that the GP regimen results in a lower incidence of grade 3 and 4 acute toxic reactions compared to the TPF regimen, aligning with previous findings [32,33,34,35]. From the FAERS database, we observed differences in reported adverse reactions between the GP and TPF regimens. The GP regimen is strongly associated with thrombosis, suggesting the need for monitoring coagulation parameters and performing pulmonary artery CTA and lower limb Doppler ultrasound during treatment. On the other hand, the TPF regimen may cause life-threatening conditions such as septic shock, ARDS, and laryngeal edema. While these adverse reactions are rare and not occurred in our cohort, they are critical and require vigilance during use. Overall, the adverse reactions caused by the GP regimen are generally manageable.
This study has several limitations. First, it is retrospective, inherently subject to selection bias. Second, despite subsequent matching efforts, initial disparities in baseline data existed between patients receiving different chemotherapy regimens. Third, plasma EBV-DNA load is associated with prognosis, but it had not yet been established as standard at our institution in the period of this study (2005–2016). In future follow-ups, we plan to incorporate this data.
Conclusion
In summary, there is no significant difference in long-term prognosis for LA-NPC between the groups receiving GP and TPF sequential chemotherapy regimens. However, there are notable differences in the incidence of adverse reactions between the GP and TPF regimens which should be concerned when choosing the regimens. Further phase III clinical trials are needed for comparison.
Data availability
No datasets were generated or analysed during the current study.
References
Chang ET, Ye W, Zeng YX, et al. The evolving epidemiology of nasopharyngeal Carcinoma[J]. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1035–47.
Bai R, Sun J, Xu Y, et al. Incidence and mortality trends of nasopharynx cancer from 1990 to 2019 in China: an age-period-cohort analysis[J]. BMC Public Health. 2022;22(1):1351.
Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma[J]. Lancet. 2019;394(10192):64–80.
Zhang Y, Rumgay H, Li M, et al. Nasopharyngeal Cancer incidence and mortality in 185 countries in 2020 and the projected Burden in 2040: Population-based global epidemiological Profiling[J]. JMIR Public Health Surveill. 2023;9:e49968.
Ji MF, Sheng W, Cheng WM, et al. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China[J]. Ann Oncol. 2019;30(10):1630–7.
He YQ, Wang TM, Ji M et al. A polygenic risk score for nasopharyngeal carcinoma shows potential for risk stratification and personalized screening[J]. Nat Commun. 2022; 13(1): 1966.
Xu T, Zhou X, Shen C, et al. Suggestions for surveillance and radiation strategy in nasopharyngeal carcinoma treated with IMRT: based on hazard-rate and patterns of recurrence[J]. Oral Oncol. 2018;76:61–7.
Sheng J, Lam S, Zhang J, et al. Multi-omics fusion with soft labeling for enhanced prediction of distant metastasis in nasopharyngeal carcinoma patients after radiotherapy[J]. Comput Biol Med. 2024;168:107684.
Petit C, Lee A, Ma J, et al. Role of chemotherapy in patients with nasopharynx carcinoma treated with radiotherapy (MAC-NPC): an updated individual patient data network meta-analysis[J]. Lancet Oncol. 2023;24(6):611–23.
Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in Combination with Radiotherapy for definitive-intent treatment of stage II-IVA nasopharyngeal carcinoma: CSCO and ASCO Guideline[J]. J Clin Oncol. 2021;39(7):840–59.
Lee NY, Sherman EJ. Nasopharynx cancer: induction or adjuvant? That is the question[J]. Cancer. 2020;126(16):3620–3.
Ng WT, Choi CW, But B, et al. Exploratory study of NPC-0501 Trial: optimal cisplatin dose of concurrent and Induction/Adjuvant chemotherapy for Locoregionally Advanced Nasopharyngeal Carcinoma[J]. Clin Cancer Res. 2022;28(12):2679–89.
Zhou X, Xu T, Yang Y, et al. Survival impact of increasing time to IMRT initiation following induction chemotherapy in nasopharyngeal carcinoma: a propensity score-matched analysis[J]. Oral Oncol. 2021;122:105506.
Wu M, Ou D, Hu C, et al. Comparing long-term survival and late toxicities of different sequential chemotherapy regimens with intensity-modulated Radiotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma[J]. Transl Oncol. 2020;13(7):100765.
Yao JJ, Zhang F, Gao TS, et al. Survival impact of radiotherapy interruption in nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a big-data intelligence platform-based analysis[J]. Radiother Oncol. 2019;132:178–87.
Liang Y, Li J, Li Q, et al. Plasma protein-based signature predicts distant metastasis and induction chemotherapy benefit in nasopharyngeal Carcinoma[J]. Theranostics. 2020;10(21):9767–78.
Lee AWM, Ngan RKC, Ng WT, et al. NPC-0501 trial on the value of changing chemoradiotherapy sequence, replacing 5-fluorouracil with capecitabine, and altering fractionation for patients with advanced nasopharyngeal carcinoma[J]. Cancer. 2020;126(16):3674–88.
Tang LL, Guo R, Zhang N, et al. Effect of Radiotherapy alone vs Radiotherapy with Concurrent Chemoradiotherapy on Survival without Disease Relapse in patients with low-risk nasopharyngeal carcinoma: a randomized clinical Trial[J]. JAMA. 2022;328(8):728–36.
Zheng Y, Xue F, Ou D, et al. Deletion of concurrent chemotherapy on the basis of sequential chemoradiotherapy for non-metastatic stage T4 nasopharyngeal carcinoma in IMRT era[J]. Cancer Med. 2024;13(4):e6578.
Liao S, Diao Y, Ling Q, et al. The effects of different induction chemotherapy cycles and adjuvant chemotherapy on the survival outcomes of patients with locally advanced nasopharyngeal Carcinoma[J]. Front Oncol. 2022;12:845704.
Liu GY, Lv X, Wu YS, et al. Effect of induction chemotherapy with cisplatin, fluorouracil, with or without taxane on locoregionally advanced nasopharyngeal carcinoma: a retrospective, propensity score-matched analysis[J]. Cancer Commun (Lond). 2018;38(1):21.
Jin T, Qin WF, Jiang F, et al. Cisplatin and fluorouracil induction chemotherapy with or without Docetaxel in Locoregionally Advanced Nasopharyngeal Carcinoma[J]. Transl Oncol. 2019;12(4):633–9.
Peng H, Chen L, Mao YP, et al. Nomogram-aided individual induction chemotherapy regimen selection in advanced nasopharyngeal carcinoma[J]. Oral Oncol. 2021;122:105555.
Peng H, Tang LL, Chen BB, et al. Optimizing the induction chemotherapy regimen for patients with locoregionally advanced nasopharyngeal carcinoma: a big-data intelligence platform-based analysis[J]. Oral Oncol. 2018;79:40–6.
Peng H, Chen B, He S, et al. Efficacy and toxicity of three induction chemotherapy regimens in Locoregionally Advanced Nasopharyngeal Carcinoma: outcomes of 10-Year Follow-Up[J]. Front Oncol. 2021;11:765378.
Liu SL, Sun XS, Xie HJ, et al. Comparing three induction chemotherapy regimens for patients with locoregionally advanced nasopharyngeal carcinoma based on TNM stage and plasma Epstein-Barr virus DNA level[J]. BMC Cancer. 2020;20(1):89.
Hong S, Zhang Y, Yu G, et al. Gemcitabine Plus Cisplatin Versus Fluorouracil Plus Cisplatin as First-Line therapy for recurrent or metastatic nasopharyngeal carcinoma: final overall survival analysis of GEM20110714 phase III Study[J]. J Clin Oncol. 2021;39(29):3273–82.
Kong XY, Lu JX, Yu XW, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as a first-line concurrent chemotherapy regimen in nasopharyngeal carcinoma: a prospective, multi-institution, randomized controlled phase II study[J]. Cancer Chemother Pharmacol. 2019;84(1):155–61.
Lv J, Wei Y, Yin JH, et al. The tumor immune microenvironment of nasopharyngeal carcinoma after gemcitabine plus cisplatin treatment[J]. Nat Med. 2023;29(6):1424–36.
Chan SK, Chan SY, Tong CC, et al. Comparison of efficacy and safety of three induction chemotherapy regimens with gemcitabine plus cisplatin (GP), cisplatin plus fluorouracil (PF) and cisplatin plus capecitabine (PX) for locoregionally advanced previously untreated nasopharyngeal carcinoma: a pooled analysis of two prospective studies[J]. Oral Oncol. 2021;114:105158.
Zang J, Xu M, LI C, et al. Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China[J]. J Cancer Res Clin Oncol. 2020;146(9):2369–78.
Zhu J, Duan B, Shi H, et al. Comparison of GP and TPF induction chemotherapy for locally advanced nasopharyngeal carcinoma[J]. Oral Oncol. 2019;97:37–43.
Huang YM, Qiao SQ, Lu L, et al. Gemcitabine combined with cisplatin vs. taxane, cisplatin, and fluorouracil in the treatment of locally advanced nasopharyngeal carcinoma: a retrospective case-control study[J]. Eur Rev Med Pharmacol Sci. 2020;24(14):7655–63.
Lian CL, Zhou R, Zhou Y, et al. Assessment of response to different induction chemotherapy regimens in locally advanced nasopharyngeal Carcinoma[J]. Drug Des Devel Ther. 2023;17:551–62.
Wang F, Chuner J, Lei W, et al. Optimal induction chemotherapeutic regimen followed by concurrent chemotherapy plus intensity-modulated radiotherapy as first-line therapy for locoregionally advanced nasopharyngeal carcinoma[J]. Med (Baltim). 2020;99(39):e22283.
Miao J, Wang L, Ong EHW, et al. Effects of induction chemotherapy on nutrition status in locally advanced nasopharyngeal carcinoma: a multicentre prospective study[J]. J Cachexia Sarcopenia Muscle. 2023;14(2):815–25.
Fourati N, Trigui R, Dhouib F, et al. Quality of weight loss during chemoradioherapy in patients with nasopharyngeal cancers[J]. Cancer Radiother. 2023;27(4):281–9.
Leelasawatsuk P, Prapaisit U, Chaiyarukjirakun V, et al. Long-term monitoring and predictive factors of critical weight loss among patients with nasopharyngeal carcinoma in a curative treatment setting[J]. Am J Otolaryngol. 2022;43(3):103407.
Jin YN, Xia TL, Mai DM, et al. The prognostic value of weight loss during radiotherapy among patients with nasopharyngeal carcinoma: a large-scale cohort study[J]. BMC Cancer. 2022;22(1):505.
Du Y, Feng R, Chang ET, et al. Body mass index and body shape before treatment and nasopharyngeal carcinoma prognosis: a population-based patient cohort study in southern China[J]. Int J Cancer. 2023;153(2):290–301.
Duan YY, Deng J, Su DF, et al. Construction of a comprehensive nutritional index and comparison of its prognostic performance with the PNI and NRI for survival in older patients with nasopharyngeal carcinoma: a retrospective study[J]. Support Care Cancer. 2021;29(9):5371–81.
Acknowledgements
We acknowledge the support of the Department of Radiation Oncology, Fudan University Shanghai Cancer Center. And we thanks to Zhang Jing (Second Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his outstanding work on the FAERS database. The views expressed in this publication are those of the authors.
Funding
This work was supported by Scientific and Innovative Action Plan of Shanghai (grant no: 21Y11911900), and Key Clinical Specialty Project of Shanghai. These fundings were research grant for personal researchers from the Shanghai government and had no involvements in this study.
Author information
Authors and Affiliations
Contributions
Ying Zhu: materials and methods, data analysis, manuscript preparation, manuscript editing. Fen Xue: conceptualization, data collection, data analysis, manuscript edits and revision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the hospital’s ethics committee, and informed consent was obtained from all patients before treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhu, Y., Xue, F. Comparing long-term efficacy and safety of GP versus TPF sequential chemoradiotherapy for locoregionally advanced nasopharyngeal carcinoma: a propensity score-matched analysis. BMC Cancer 24, 1145 (2024). https://doi.org/10.1186/s12885-024-12932-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12932-0