Abstract
Background
F-627 (efbemalenograstim alfa) is a novel long acting granulocyte colony-stimulating factor (G-CSF) that contains two human G-CSF fused to a human immunoglobulin G2 (hIgG2) -Fc fragment with a peptide linker. This studyevaluated the efficacy and safety of F-627, also known as efbemalenograstim alfa (Ryzneuta®) in reducing neutropenia compared with filgrastim (GRAN®).
Methods
This was a multicenter, randomized, open-label, active-controlled non-inferiority study. Two hundred thirty nine (239) patients were enrolled in thirteen centers and received the chemotherapy with epirubicin (100 mg/m2) and cyclophosphamide (600 mg/m2) on day 1 of each cycle for a maximum of four cycles. Patients were randomized to receive either a single 20 mg subcutaneous (s.c.) injection of F-627 on day 3 of each cycle or daily s.c. injection of filgrastim 5 µg/kg/d starting from day 3 of each cycle. The primary endpoint was the duration of grade 3 or 4 neutropenia in cycle 1. The safety profile was also evaluated.
Results
The mean (SD) duration of grade 3 or 4 neutropenia in cycle 1 was 0.68 (1.10) and 0.71 (0.95) days for the F-627 and the filgrastim groups, respectively. The Hodges-Lehmann estimate of the between-group median difference (F-627 vs filgrastim) in the duration of grade 3 or 4 neutropenia in cycle 1 was 0 day and the upper limit of the one-sided 97.5% CI was 0 day, which was within the prespecified non-inferiority margin of 1-day. Results for all efficacy endpoints in cycles 2 − 4 were consistent with the results in cycle 1, however a trend towards a lower incidence and a shorter duration of grade 3 or 4 neutropenia and grade 4 neutropenia was observed in the F-627 group compared with the filgrastim group. The ANC nadir in the F-627 group was significantly higher than that in the filgrastim group in each cycle. A single fixed dose of F-627 was well tolerated and as safe as standard daily filgrastim.
Conclusions
A single fixed dose of 20 mg of F-627 in each cycle was as safe and effective as a daily dose of filgrastim 5 µg/kg/d in reducing neutropenia and its complications in patients who received four cycles of EC.
Trial registration
ClinicalTrials.gov: NCT04174599, on 22/11/2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Although there are great advancements in targeted therapy and immune therapy, chemotherapy still plays a critical role in cancer treatment strategies. Neutropenia is one of the most common toxicities in cancer patients receiving myelosuppressive chemotherapy regimens [1,2,3]. Patients who develop neutropenia are at increased risk for infection with fever and febrile neutropenia (FN) [4]. FN often requires hospitalization and treatment with intravenous antibiotics and may necessitate chemotherapy dose reductions and/or delays. Moreover, FN can be life-threatening and is associated with a high risk of mortality [5].
Granulocyte-colony stimulating factor (G-CSF) is often used to manage chemotherapy-associated FN and allow anticancer drugs to be administered more effectively by stimulating neutrophil survival, proliferation, differentiation, and activation [6]. The use of recombinant human G-CSF (rhG-CSF) based on the risk of FN had been long established as the standard of care in treatment guidelines of most major cancer-focused medical associations [7, 8]. According to these guidelines, prophylactic G-CSF use is recommended for patients with a clinically significant risk of FN, based on the chemotherapy regimen and patient-specific risk factors.
Several rhG-CSFs therapies, such as filgrastim and pegfilgrastim, have been developed and approved to treat neutropenia, particularly in the management of chemotherapy-induced neutropenia. Filgrastim is a non-glycosylated form of rhG-CSF that has a short elimination half-life [2] and typically requires repeated daily s.c. injection during chemotherapy. Pegfilgrastim (Neulasta®), a pegylated form of filgrastim, is long-acting and requires less frequent administration in comparison to filgrastim, typically once per cycle of chemotherapy [9]. Although the benefits of pegfilgrastim have been well established by randomized and placebo-controlled trials and real-world experience [10,11,12], US- and EU-approved pegfilgrastim (Neulasta®) have not yet been approved in China, and thus filgrastim remains the primary treatment option for oncologists and patients in China.
As an alternative method to pegylation for extending elimination half-life, a novel Fc-infusion technology was used to develop F-627, a 413 amino acid recombinant fusion protein that contains two human G-CSF and a human IgG2-Fc fragment, with a 16 amino acid peptide linker. Consistent with its molecular design, F-627 has the same mechanism of action as other rhG-CSFs; it binds to the G-CSF receptor (G-CSFR) and activates signal transducer and activator of transcription 3 (STAT3), resulting in the proliferation, differentiation, and activation of neutrophils. As such, F-627 is intended to be used to decrease the duration of neutropenia and the incidence of infection, as manifested by FN, in patients with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of FN. Similar to pegfilgrastim and unlike daily filgrastim, F-627 has demonstrated a prolonged in vivo half-life [13] and is intended to be administered once per cycle of chemotherapy. F-627, therefore, has the potential to be a non-pegylated, long-lasting (administered once per cycle) substitute for pegfilgrastim.
Current study was designed to evaluate the efficacy and safety of a single subcutaneous injection of F-627 20 mg versus daily subcutaneous injection of filgrastim (GRAN®) 5 µg/kg/day in prophylactic treatment of chemotherapy-induced neutropenia in Chinese patients with breast cancer receiving up to four cycles of chemotherapy with epirubicin and cyclophosphamide.
Methods
The study was conducted in accordance with Declaration of Helsinki. The institutional review boards and ethics committees at each participating center reviewed and approved the study protocol. Informed consent was obtained from all patients before any study-related procedure was performed. The study was registered on www.chinadrugtrials.org.cn (CTR20170705, 02/01/2018) and clinicaltrials.gov (NCT04174599, 22/11/2019).
Patients who met the following inclusion criteria were eligible for study entry: female patients aged 18–75 years old who required adjuvant chemotherapy after radical mastectomy for breast cancer and were scheduled to receive 4 cycles of EC chemotherapy (epirubicin [Pharmorubicin] 100 mg/m2 + cyclophosphamide 600 mg/m2); Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; absolute neutrophil count (ANC) ≥ 2.0 × 109/L; hemoglobin (Hb) ≥ 11.0 g/dl, and platelet (PLT) ≥ 100 × 109/L; total bilirubin ≤ 1.5 × upper limit of normal (ULN); alanine transaminase (ALT) and aspartate transaminase (AST) ≤ 2.5 × ULN, serum creatinine ≤ 1.5 × ULN; and left ventricular ejection fraction greater than 50%.
Patients were excluded if they had undergone radiation therapy within 4 weeks prior to enrollment; had received neoadjuvant chemotherapy before surgery; had prior bone marrow or stem cell transplant; comorbid with malignancies other than breast cancer; had received a treatment with rhG-CSF within 6 weeks prior to randomization; intolerant to hormone pretreatment; diagnosed with acute cardiac failure congestive, cardiomyopathy, or myocardial infarction; with any disease that may cause splenomegaly; with acute infection; with chronic active Hepatitis B within 1 year (unless patients tested negative for HBsAg prior to enrollment), or Hepatitis C; were pregnant or breastfeeding; were known to be human immunodeficiency virus (HIV) positive or had been diagnosed with AIDS; with active tuberculosis (TB); with history of TB exposure, unless negative for tuberculin test; with sickle-cell anemia; with known allergy to G-CSF or excipients; had received any other study drug within 1 month or 5 half-lives of the study drugs prior to enrollment (whichever was longer).
Study design
This was a multicenter, randomized, open-label, active-controlled non-inferiority phase III study to evaluate the efficacy and safety of a single injection of F-627 compared with daily injection of filgrastim per chemotherapy cycle in Chinese patients with breast cancer. Patients were randomized via central randomization method and were assigned in a 1:1 ratio to F-627 20 mg or filgrastim 5 µg/kg/day treatment groups.
Study drug
F-627 comprises of a dimeric recombinant human G-CSF and a human Immunoglobulin G (IgG) 2-Fc fragment, with a 16 amino acid peptide linker, which is produced by recombinant DNA technology and expressed in Chinese Hamster Ovary (CHO) cells.
Patients randomized to the F-627 group received a single subcutaneous injection of F-627 20 mg per chemotherapy cycle on day 3 of each cycle, approximately 48 ± 4 h after receiving one standard dose of chemotherapy. Selection of the 20 mg fixed dose used in this Phase III study was based on the comprehensive clinical efficacy/safety data and PK/PD evaluations from the Phase I and II studies.
Patients randomized to the filgrastim group received daily subcutaneous injection of filgrastim 5 µg/kg/day, based on actual body weight, beginning on day 3 of each cycle, approximately 48 ± 4 h after starting chemotherapy, and continuing until ANC ≥ 5 × 109/L after the expected nadir or for up to 14 days, whichever occurred first.
Chemotherapy treatment
On day 1 of each chemotherapy cycle, patients received an intravenous bolus infusion of epirubicin (100 mg/m2) and cyclophosphamide (600 mg/m2). Chemotherapy was repeated every 21 days for up to four cycles. To begin chemotherapy on day 1 of the next cycle (day 22 of the previous cycle), patients should meet the following criteria: ANC ≥ 2.0 × 109/L, PLT ≥ 80 × 109/L, total bilirubin ≤ 1.5 × upper limit of normal (ULN), ALT and AST ≤ 2.5 × ULN, serum creatinine ≤ 1.5 × ULN. A 14-day recovery period was permitted if patients did not meet these criteria, however, if the criteria described above were still not met after 14 days, the subject was eliminated from the study.
If a non-hematologic toxicity in previous cycle of EC therapy occurred in a patient, and as such, dose reduction was deemed necessary by the investigator, the dosage of each chemotherapy drug was reduced in the next cycle, i.e., the actual dosage was determined by the investigator based on a patient’s condition.
RhG-CSF (including recombinant human granulocyte–macrophage colony-stimulating factor, rhGM-CSF), traditional Chinese medicines known to promote neutrophil production, lithium, and prophylactic antibiotics were prohibited from use in patients enrolled in the study.
Efficacy measurements
The primary efficacy end point was the duration of grade 3 or grade 4 neutropenia (defined as ANC < 1.0 × 109/L) in cycle 1. Other efficacy endpoints included the duration of grade 3 or grade 4 neutropenia in cycles 2–4, the incidence of grade 3 or grade 4 neutropenia in cycle 1 to 4, the duration and incidence of grade 4 neutropenia (defined as ANC < 0.5 × 109/L) in cycle 1 to 4, the duration and incidence of grade 2 or greater neutropenia (defined as ANC < 1.5 × 109/L) in cycle 1 to 4, the depth of ANC nadir between day 3 and 13 in each of the cycles (1–4), incidence of FN, and the time of ANC recovery to 2.0 × 109/L post the nadir in cycle 1 to cycle 4. Blood samples were collected to determine ANC on days 1 (before the chemo), 3, 5, 7, 8, 9, 10, 11, 13, 15, and 21 in cycle 1, as well as on days 3, 5, 7, 9, 11, 13, 15, and 21 in cycles 2–4. To avoid measurement bias, the ANC in cycle 1 were determined by central laboratory, the ANC in cycles 2–4 can be determined by local site laboratory.
Safety measurements
The safety end points of this study were incidence of adverse events, changes in clinical laboratory values, vital signs, physical examination, 12-lead ECG, abdominal ultrasound and the presence of anti-F627 antibodies. Serum was only collected for detection of antibodies in the F-627 group before the start of the study drug injection and at various time points during the study.
Statistical methods
The sample size was calculated using a non-inferiority design. The non-inferiority margin was set to be 1 day, the standard deviation (SD) of the duration of grade 3 or 4 neutropenia in each treatment group was assumed to be 1.75 days based on a previous phase II trial, and a 20% dropout rate was assumed. Using these estimates, a planned sample size of 120 subjects for each treatment group was chosen to provide 80% power to establish non-inferiority of F-627 compared with filgrastim.
Treatment differences in the duration of grade 3 or 4 neutropenia were assessed by confidence intervals (CIs) estimated via the Hodges–Lehmann method. An upper 97.5% one-sided CI (95% two-sided) was evaluated with respect to a non-inferiority margin of 1 day. Treatment differences in other efficacy endpoints between the F-627 and filgrastim groups were assessed with a 95% CI. No adjustments for multiplicity were made.
Efficacy analyses were performed in both PPS (Per-Protocol set, including PPS A and B. PPS A: subjects in FAS (Full analysis set, defined as all randomized subjects who received the study drug and undergone at least one postbaseline efficacy evaluation) and without major protocol deviations, serious medication noncompliance, loss to follow-up, or withdrawal during cycle 1. PPS B: subjects in FAS and without major protocol deviations, serious medication noncompliance, loss to follow-up, or withdrawal in any cycle) and FAS. The primary efficacy analyses were performed on FAS following the ICH-E9 guideline, the missing ANC values in cycle 1 were imputed using covariate adjustments, and the PPS and the FAS without imputation were also used for a sensitivity analysis. In addition, a sensitivity analysis of the treatment differences in duration of grade 3 or 4 neutropenia in cycle 1 was performed based on both PPS and FAS by bootstrap resampling methods. Because the results from these analyses led to the same conclusions, for simplicity, only the results from the FAS with imputation were reported in this paper.
Adverse events were tabulated by organ class system, severity, relationship to study drug, and treatment group. Changes in laboratory variables were depicted by use of shift tables and through the tabulation of summary statistics for each variable.
All analyses were performed using SAS software (version 9.4).
Results
Patients disposition
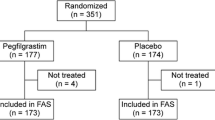
The study was conducted in 13 centers in China between April 2018 and June 2019. A total of 242 Chinese women with breast cancer were enrolled and randomized: 122 patients to F-627 and 120 patients to filgrastim. Finally, a total of 239 randomized patients were included in the FAS.
A total of 25 patients withdrew from the study after receiving the study drug, including 7 patients in the F-627 group and 18 patients in the filgrastim group. Overall, 214 (88.4%) patients completed the study (Fig. 1).
There were 229 patients in PPS A after excluding 3 patients with major deviations and 7 patients without valid ANC in cycle 1 due to early withdrawal from FAS. There were 209 patients in PPS B after excluding the patients with major protocol deviations or lacking valid ANC assessments due to withdrawal early in cycles 2–4 (Fig. 1). Because the efficacy analysis from FAS and PPS led to the same conclusions, only the results from the FAS with imputations are reported in this paper.
Patient baseline characteristics in the FAS population are summarized in Table 1. Patient characteristics were similar between the F-627 and filgrastim groups. All patients were Chinese females with breast cancer diagnosed with standard histopathological methods. No patient received chemotherapy or radiotherapy within 1 year before screening.
Study drug administration
The mean (SD) number of injections administered to patients treated with filgrastim was 7.3 (1.25) in cycle 1, 7.6 (1.03) in cycle 2, 7.8 (0.86) in cycle 3 and 7.8 (0.82) in cycle 4. Patients treated with F-627 received a single 20 mg injection of study drug per chemotherapy cycle.
Chemotherapy administration
Overall, the exposures of chemotherapeutic drugs in both groups were comparable in each cycle. A median of four cycles of EC chemotherapy was administered in both groups. In cycle 1, except for one patient in the filgrastim group who received a chemotherapeutic drug with a relative dose intensity of 85.3%, patients in both groups received all EC doses as planned. In cycles 2–4, the dosage of each anticancer drug was reduced for some patients due to toxicity. However, the relative dose intensities of each chemotherapeutic drug were all > 80% in both groups (except for one patient in filgrastim with a relative dose intensity of 67.5% which was recorded as a major protocol deviation).
Efficacy
The duration and incidence of grade 3 or 4 neutropenia, grade 4 neutropenia in cycle 1
The mean (SD) duration of grade 3 or 4 neutropenia in cycle 1, the primary endpoint, is listed in Table 2. The Hodges-Lehmann estimate of the between group median difference (F-627 − Filgrastim) in the duration of grade 3 or 4 neutropenia in cycle 1 was 0 day, and the upper limit of the one-sided 97.5% CI was 0 day, which was within the prespecified non-inferiority margin of 1 day, thereby establishing non-inferiority of F-627 to filgrastim. The sensitivity analysis results from both the PP population and the FAS population without ANC imputation were consistent with the primary analysis results. No significant differences between F-627 and filgrastim groups were observed in the duration of grade 4 neutropenia, the incidence of grade 3 or 4 neutropenia and the incidence of grade 4 neutropenia in cycle 1 (Table 2).
The duration and incidence of grade 3 or 4 neutropenia, grade 4 neutropenia in cycles 2–4
The durations and incidences of grade 3 or 4 neutropenia and grade 4 neutropenia in cycles 2–4 were summarized in Table 2. No differences between the two groups were observed in the durations of grade 3 or 4 neutropenia and grade 4 neutropenia in cycles 2–4. As expected, the incidences of grade 3 or 4 neutropenia and grade 4 neutropenia were lower during cycles 2–4 compared with cycle 1. The incidences tended to be lower in the F-627 group than in the filgrastim group. The incidences of grade 3 or 4 neutropenia and grade 4 neutropenia in the F-627 and filgrastim groups were similar across cycles except cycle 3.
Depth of ANC nadir, cycles 1–4
The ANC values reached a nadir at day 9 of each cycle in both groups and the ANC nadir in cycle 1 was lower than that in subsequent cycles. In cycle 1, the mean (SD) of ANC nadir was 2.059 × 109/L (1.502) in the F-627 group and 1.603 × 109/L (1.206) in the filgrastim group. The nadir in the F-627 group was higher than that in filgrastim group and the Hodges-Lehmann estimate of the between group median difference (F-627 − Filgrastim) in the ANC nadir in cycle 1 was 0.3 × 109/L (95% CI: 0.04 × 109/L, 0.65 \(\times\) 109/L). In cycles 2–4, the nadir in F-627 group was also higher than that in the filgrastim group. Table 2 summarizes the depth of ANC nadir for all cycles.
The incidence of FN
One (0.8%) patient in the F-627 group and 2 (1.7%) patients in the filgrastim group experienced FN in cycle 1. The incidence of FN was low in both group and no significant difference was observed between the two groups. During cycles 2–4, no patients developed FN in both groups.
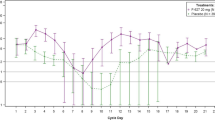
The ANC-time profile
Figure 2 displays the ANC profile of the median ANCs of each treatment group in cycle 1. The F-627 and filgrastim treatment groups displayed similar ANC values through day 11. ANC values of both groups reached the peak on day 5 and the nadir on day 9. After day 11, the ANC of the F-627 group continued to increase until day13, while the ANC of filgrastim group gradually decreased.
Safety
All adverse events with an incidence rate of ≥ 10% are summarized in Table 3. In general, F-627 was well tolerated in patients with breast cancer, and the safety profile of F-627 was similar to that of filgrastim.
Most patients in both groups experienced adverse events (99.2% in the F-627 group, 100% in the filgrastim group), however, as expected, most of adverse events were associated with EC chemotherapy regimen or primary disease. 57 of 120 subjects in the F-627 group (47.5%) and 66 of 119 subjects in the filgrastim group (55.5%) reported at least one adverse event that was considered by the investigator to be related to the study drug. No deaths occurred during the study. 5.0% subjects in the F-627 group and 6.7% subjects in the filgrastim group experienced serious adverse events (SAEs). The incidence of SAEs was low in both groups and no SAEs were related to study drugs, as judged by investigators, and were resolved during the study.
The most frequently reported adverse events considered to be related to the study drug by the investigator were bone pain and back pain. The incidences of bone pain and back pain in the F-627 group were lower than those in the filgrastim group but the difference was not statistically significant. All these adverse events were grade 1 or 2 in severity, and most could be recovered without any treatment. There were no safety concerns in either group.
Immunogenicity
Immunogenicity was only evaluated in 120 patients who received F-627. Five patients (9.2%) were anti-drug antibody (ADA)-positive before receiving F-627 in cycle 1. After the first dose in cycle 1, only 2 patients tested as ADA-positive in baseline had ADA-positive results at other time points of each cycle, however, the titers at each time point of the other cycles did not change from baseline. No neutralizing antibodies were detected. Overall, no treatment emergent ADA-positive samples were identified and all ADA-positive samples were prior to start of treatment and none of the patients who were ADA-positive tested positive for neutralizing antibodies.
Discussion
This phase III study was designed to further evaluate the efficacy and safety of a fixed dose of 20 mg of F-627 as compared with filgrastim in a standard EC chemotherapy regimen. The current study demonstrated that a single fixed dose injection of F-627 per cycle can be as safe and effective in treating chemotherapy-induced neutropenia in Chinese breast cancer patients receiving EC regimen as daily injection of filgrastim. This finding was consistent with the results of other phase II and Phase III studies in F-627’s clinical development program [14,15,16,17,18].
Risk of infection is well correlated with the duration and severity of neutropenia (DSN) [3], therefore, DSN can be used as a sensitive surrogate endpoint for the exploratory and confirmatory trials of G-CSF [19]. As the primary endpoint of this study, the duration of grade 3 or 4 neutropenia in the F-627 group was not inferior to that in the filgrastim group in cycle 1. The Hodges-Lehmann estimate of the between group median difference (F-627 − Filgrastim) was 0 day and the upper limit of the one-sided 97.5% CI was 0 day, which was within the prespecified non-inferiority margin of 1 day. Thus it was concluded that the efficacy of F-627 in reducing duration of grade 3 or 4 neutropenia was non-inferior to filgrastim. Secondary endpoints (the incidence of grade 3 or 4 and grade 4 neutropenia, the duration of grade 4 neutropenia, the incidence of FN) were comparable between F-627 and filgrastim groups in cycles 1–4.
In China, AT/ET and AC/EC chemotherapy regimens are commonly used in clinical practice and are also recommended in Chinese treatment guidelines for breast cancer patients. Compared with doxorubicin, epirubicin has fewer adverse reactions and is generally adopted by Chinese clinicians [20]. Previous studies have shown that 100 mg/m2 epirubicin may be the optimal dose for patients with breast cancer [21]. The results of this study provided new evidence that a fixed dose of 20 mg F-627 can give enough neutrophil support to patients receiving standard EC chemotherapy. In addition, it is worth noting that the ANC nadir in the F-627 group was significantly higher than that in the filgrastim group in each cycle. Furthermore, the incidence of grade 3 or 4 and grade 4 neutropenia tended to be lower in the F-627 group than those in the filgrastim group. This phenomenon was observed in other studies [17, 18]. Also supported by MOA and PK profile [16]. The findings suggest that F-627 therapy may be more beneficial for patients undergoing chemotherapy regimens that have higher myelosuppressive toxicity compared to filgrastim. A superiority trial is needed to confirm the potential benefit.
The incidence and severity of adverse events in patients treated with F-627 were indistinguishable from those in patients treated with filgrastim, and no unexpected AEs occurred in patients received F-627. The types of AEs observed in breast cancer patients undergoing cytotoxic chemotherapy was in line with the symptoms of myelosuppression. F-627-related adverse reactions were primarily characterized by bone pain and back pain, which is similar to other products of the same kind [7, 10,11,12]; but in this study, it was observed that the incidences of bone pain and back pain in the F-627 group were slightly lower than those in the filgrastim group, which was consistent with the trends observed in data from a previous phase II trial. The reason for this finding is unclear and merits further investigation in future clinical trials.
ANC-time profiles in the F-627 and filgrastim groups were similar during days 1–11 of the same cycle. The difference in ANC-time profiles between the two groups in days 11–21 may be explained by the different half-lives of the study drug. In the filgrastim group, the drug was started at 48 h after chemotherapy (day 3 of chemotherapy), administered once daily for ≤ 2 weeks, or until the patient’s ANC recovered from the nadir to 5 × 109/L, and was discontinued as per the package insert labeling. The median number of actual dosing was 8 days. Because F-627 has a longer half-life compared to filgrastim, F-627 continued to produce pharmacological effects after day 11, while filgrastim was quickly cleared and no additional effect was achieved. F-627 might have the potential to provide continuous efficacy on raising neutrophils compared with filgrastim. The difference in the ANC-time profile changes between the two groups was not clinically significant, consistent with the observation that the types and incidences of AEs were similar in the two groups. No significant effect on platelet formation or erythropoiesis was observed. The F-627 is approved for marketing currently in China, the EU and the United States, and adverse reactions will be monitored for as required by regulations in all patients using it, including those with hematologic diseases.
In clinical practice, a fixed-dose regimen is generally preferred for administration, but this can give rise to two potential issues: a fixed dose may not provide full clinical benefits for over-weighted patients and could result in a less favorable safety profile for under-weighted patients. To address these concerns, a post-hoc subgroup sensitivity analysis was performed, and the findings suggested that a fixed dose F-627 was equally effective for underweight and overweight patients [16]. Moreover, there were no differences in incidence or severity of adverse events across any weight groups when compared to patients who received filgrastim.
Conclusions
This phase III study demonstrated that a single fixed dose of 20 mg of F-627 can be as safe and efficacious in treating chemotherapy induced neutropenia in Chinese breast cancer patients as daily dose of filgrastim. F-627 was well tolerated, and the overall safety profile of F-627 was not different from that of filgrastim. As F-627 is administered once per cycle, it is expected to offer significant advantages in terms of patient compliance and convenience, leading to improved overall benefits. The study has provided new evidence supporting the efficacy of F-627, a novel long-acting rhG-CSF, which can serve as a potential alternative for oncologists in China to simplify the management of chemotherapy-induced neutropenia.
Availability of data and materials
The datasets from the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADA:
-
Anti-drug antibody
- AE:
-
Adverse event
- ANC:
-
Absolute neutrophil counts
- BSA:
-
Body surface area
- CI:
-
Confidence interval
- EC:
-
Epirubicin + cyclophosphamide
- ECOG:
-
Eastern Cooperative Oncology Group
- FAS:
-
Full analysis set
- FN:
-
Febrile neutropenia
- G-CSF:
-
Granulocyte-colony stimulating factor
- ICF:
-
Informed Consent Form
- PPS:
-
Per Protocol Set
- SAE:
-
Serious adverse event
- SC:
-
Subcutaneous
- SD:
-
Standard deviation
- TA:
-
Docetaxel + Cyclophosphamide
References
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–70.
Morstyn G, Campbell L, Souza LM, Alton NK, Keech J, Green M, et al. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988;1(8587):667–72.
Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–40.
Ba Y, Shi Y, Jiang W, et al. Current management of chemotherapy-induced neutropenia in adults: key points and new challenges: Committee of Neoplastic Supportive-Care (CONS), China anti-cancer association committee of clinical chemotherapy, China anti-cancer association. Cancer Biol Med. 2020;17(4):896–909.
Lyman GH, Michels SL, Reynolds MW, Barron R, Tomic KS, Yu J. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116(23):5555–63.
Welte K, Gabrilove J, Bronchud MH, Platzer E, Morstyn G. Filgrastim (r-metHuG-CSF): the first 10 years. Blood. 1996;88(6):1907–29.
Crawford J, Becker PS, Armitage JO, Blayney DW, Chavez J, Curtin P, et al. Myeloid growth factors, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(12):1520–41.
Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–212.
Biganzoli L, Untch M, Skacel T, Pico JL. Neulasta (pegfilgrastim): a once-per-cycle option for the management of chemotherapy-induced neutropenia. Semin Oncol. 2004;31(3 Suppl 8):27–34.
Green MD, Koelbl H, Baselga J, Galid A, Guillem V, Gascon P, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29–35.
Holmes FA, O’Shaughnessy JA, Vukelja S, Jones SE, Shogan J, Savin M, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol. 2002;20(3):727–31.
Holmes FA, Jones SE, O’Shaughnessy J, Vukelja S, George T, Savin M, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–9.
Yan XQ, Hodsman PG, Huang ZH, Sun RY, Sun NC, Huang YL. An open-label, single ascending dose phase I study of F-627, a G-CSF dimer. In Healthy Male Subjects Blood. 2010;116(21):4722–822.
Glaspy J, Bondarenko I, Krasnozhon D, et al. Efbemalenograstim alfa not inferior to pegfilgrastim in providing neutrophil support in women with breast cancer undergoing myelotoxic chemotherapy: results of a phase 2 randomized, multicenter, open-label trial. Support Care Cancer. 2024;32:91.
Glaspy J, Bondarenko I, Burdaeva O, et al. Efbemalenograstim alfa, an Fc fusion protein, long-acting granulocyte-colony stimulating factor for reducing the risk of febrile neutropenia following chemotherapy: results of a phase III trial. Support Care Cancer. 2023;32(1):34.
The U.S. Food and Drug Administration. INTEGRATED REVIEW. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/761134Orig1s000IntegratedR.pdf.
Li SXM, Hou QS, Wang SF, Chen JM, Yao W, Fu YY, et al. Efbemalenograstim alfa, a long-acting granulocyte colony-stimulating factor, a novel dimeric G-CSF Fc fusion protein for reducing the risk of febrile neutropenia following chemotherapy. J Clin Exp Hematol. 2023;2(1):10–2.
Blair HA. Efbemalenograstim Alfa: First Approval. Drugs. 2023;83(12):1125–30.
European Medicines Agency. Guideline on similar biological medicinal products containing recombinant granulocyte-colony stimulating factor (rG-CSF). 26 July 2018. https://www.ema.europa.eu/documents/scientific-guideline/draft-guideline-similar-biological-medicinal-products-containing-recombinant-granulocyte-colony_en.pdf.
Bontenbal M, Andersson M, Wildiers J, Cocconi G, Jassem J, Paridaens R, et al. Doxorubicin vs epirubicin, report of a second-line randomized phase II/III study in advanced breast cancer. EORTC breast cancer cooperative group. Br J Cancer. 1998;77(12):2257–63.
Bonneterre J, Roche H, Kerbrat P, Bremond A, Fumoleau P, Namer M, et al. Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French adjuvant study group 05 randomized trial. J Clin Oncol. 2005;23(12):2686–93.
Acknowledgements
We thank the participating patients and staffs at each of the study centers. Qingsong Hou, MD and Simon Li, MD assisted with the medical writing of the article.
Funding
This study was funded by Evive Biotechnology (Shanghai) Ltd and supported by National Science and Technology Major Projects for Major New Drugs Innovation and Development (2018ZX09733001-001–001).
Author information
Authors and Affiliations
Contributions
Zhimin Shao was the coordinating principal investigator of the study and was involved in the development of study protocol, study conduct and evaluation of the study results. Qingyuan Zhang, Hongsheng Li, Changsheng Ye, Tao Sun, Hongjian Yang, Zhendong Chen, Zhihong Wang, Xiaoan Liu, Cuizhi Geng, Xingrui Li, Jin Zhang, Zhonghua Wang, and Hong Zheng were involved in the study design, preparation, coordination and evaluation of the trial. Shufang Wang, Gaochong Zhang and Wei Yao were involved in drafting the manuscript. All authors were involved in the review and approval of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review boards and ethics committees at Fudan university Shanghai cancer center, Tianjin medical university cancer Institute & Hospital, the fourth hospital of Hebei medical university, Huazhong university of science and technology, Nanfang hospital of southern medical university, cancer center of Guangzhou medical university, Jiangsu province hospital, Zhejiang cancer hospital, Beijing cancer hospital, the second hospital of Anhui medical university, west China hospital of Sichuan university, Liaoning cancer Hospital & Institute, Harbin medical university cancer hospital and Guizhou cancer hospital. The study was conducted in accordance with Declaration of Helsinki and the Chinese Good Clinical Practice Guidelines. Informed consent was obtained from all patients before any study-related procedure was performed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Q., Wang, Z., Yao, W. et al. A randomized, multicenter phase III Study of once-per-cycle administration of efbemalenograstim alfa (F-627), a novel long-acting rhG-CSF, for prophylaxis of chemotherapy-induced neutropenia in patients with breast cancer. BMC Cancer 24, 1143 (2024). https://doi.org/10.1186/s12885-024-12892-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12892-5