Abstract
Background
Hepatocellular carcinoma (HCC), a prevalent primary malignant tumor, is notorious for its high mortality rate. Despite advancements in HCC treatment, patient outcomes remain suboptimal. This study endeavors to assess the potential prognostic significance of POLH-AS1 in HCC.
Methods
In this research, we gathered RNA-Seq information from individuals with HCC in The Cancer Genome Atlas (TCGA). We analyzed the levels of POLH-AS1 expression in both HCC cells and tissues using statistical tests. Additionally, we examined various prognostic factors in HCC using advanced methodologies. Furthermore, we employed Spearman’s rank correlation analysis to examine the association between POLH-AS1 expression and the tumor’s immune microenvironment. Finally, the functional roles of POLH-AS1 in HCC were validated in two HCC cell lines (HEP3B and HEPG2).
Results
Our analysis revealed elevated POLH-AS1 expression across various cancers, including HCC, with heightened expression correlating with HCC progression. Notably, POLH-AS1 expression emerged as a potential biomarker for HCC patient survival and prognosis. Mechanistically, we identified the involvement of POLH-AS1 in tumorigenesis pathways such as herpes simplex virus 1 infection, interactions with neuroactive receptors, and the cAMP signaling pathway. Lastly, inhibition of POLH-AS1 was discovered to hinder the proliferation, invasion and migration of HEP3B and HEPG2 HCC cells.
Conclusions
POLH-AS1 emerges as a promising prognostic biomarker and therapeutic target for HCC, offering potential avenues for enhanced patient management and treatment strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Hepatocellular carcinoma (HCC) is a significant malignant tumor originating from liver cells and ranks as one of the most lethal cancers globally [1, 2]. In 2020, approximately 906,000 individuals were diagnosed with HCC worldwide, making it the third leading cause of cancer-related deaths. The prognosis remains grim, with a five-year relative survival rate of around 18% [3]. Currently, early surgical resection remains the foremost therapeutic strategy, aiming to curtail mortality rates associated with HCC [4]. Despite advancements in medical science, the anticipated therapeutic efficacy for patients with HCC has not yet achieved optimal levels. The challenges are attributed to the highly invasive, heterogeneous, and drug-resistant nature of HCC, resulting in a poor prognosis [4,5,6]. Understanding the molecular mechanisms underlying HCC initiation and progression is crucial for improving clinical interventions and patient outcomes. Therefore, a deeper exploration of these molecular processes is essential to develop more effective treatment strategies and enhance survival rates in HCC patients.
Long non-coding RNAs (lncRNAs) have emerged as critical players in cancer biology, influencing six key characteristics: cell growth, motility, immortality, angiogenesis, and survival [7]. Various studies have highlighted the dualistic role of lncRNAs, acting as either tumor promoters or suppressors within the tumor microenvironment [8]. Their significant role in cancer-related pathways suggests their potential as biomarkers for tumor diagnosis, treatment, and prognosis [9]. Consequently, the exploration of lncRNAs as biomarkers has become a prominent area of research in oncology.
POLH antisense RNA1 (POLH-AS1) is a long non-coding RNA derived from the reverse transcription of the POLH gene. Recent studies have illuminated its pivotal role as a master regulator in HCC, significantly impacting patient prognosis by modulating various emerging cell death pathways, including necroptosis, ferroptosis, and cuproptosis [10,11,12]. Furthermore, a set of necrosis-associated lncRNAs, including POLH-AS1, has been proposed to guide the prognosis of HCC and inform immunotherapeutic approaches [13]. However, the precise regulatory mechanisms through which POLH-AS1 affects HCC progression remain largely unexplored.
In this study, we spotlight a promising lncRNA, POLH-AS1, demonstrating its potential to forecast prognosis and guide the choice of immunotherapy for HCC patients. Furthermore, we tentatively examined the expression levels of POLH-AS1 across HCC cell lines and human normal liver cell lines. Finally, we delved into the functional relevance of POLH-AS1 in HCC progression, unveiling that its inhibition resulted in attenuated cell proliferation, migration, and invasion. In aggregate, our findings underscore POLH-AS1 as a noteworthy prognostic biomarker and a viable target for tailored HCC treatment.
Methods
Data acquisition and processing
Data on hepatocellular carcinoma and transcriptome data obtained from RNA sequencing were extracted from The Cancer Genome Atlas (TCGA) database. 374 HCC samples and 50 samples of normal tissue were included in the research after individuals with missing clinical data were eliminated. Following this, research was carried out to explore the relationship between the levels of POLH-AS1 expression and the survival rate of individuals diagnosed with HCC.
Tumor samples collection
Between Oct 2021 and Mar 2022, 8 HCC tissue samples and 8 normal liver tissues were gathered from patients at the First Affiliated Hospital of Zhengzhou University, Henan, China. The clinicopathological characteristics of the patients with HCC were summarized in supplementary table S1. HCC tissue samples were stored with liquid nitrogen after resection, and mRNA expression levels were evaluated using quantitative reverse transcription polymerase chain reaction (RT-qPCR).
Prognostic model development and evaluation
Univariate and multivariate Cox regression analyses were carried out to evaluate the potential of POLH-AS1 as an independent prognostic indicator at a significance level of p < 0.05. The analyses incorporated clinicopathological variables such as age, gender, histologic grade, histologic type, pathologic stage and alpha-fetoprotein (AFP). Time-dependent receiver operating characteristic (ROC) curve analysis was performed using the survivor ROC software [14]. Further, we developed the nomogram for the prediction of clinical outcomes for HCC patients [15].
Functional enrichment analysis
Functional enrichment analysis was performed as described in our previous study [16, 17]. All HCC samples in the TCGA dataset were categorized into high and low expression groups based on the median expression level of POLH-AS1 as the cutoff value. The ‘edgeR’ software was utilized to detect differentially expressed genes (DEGs) in HCC tissue with low and high POLH-AS1 expression levels, meeting the adjusted criteria (p < 0.05 and |log2fold-change (FC)| > 1). Next, the DEGs underwent Gene Ontology (GO) analyses to identify the most significantly enriched biological functions. To determine the enriched signaling pathways, the DEGs underwent Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis [16].
Immune infiltration and immune checkpoint analysis
Immune infiltration and immune checkpoint analysis were conducted as described previously [16, 17]. The Gene Set Enrichment Analysis (GSEA) was utilized to evaluate the immune infiltration cells linked to POLH-AS1. A study on immunity revealed marker genes for 24 distinct types of immune cells [18]. Using Spearman’s rank correlation, the relationships between POLH-AS1 and these 24 cell types were investigated [17]. Subsequent analysis of the relationship between POLH-AS1 and immunological checkpoints produced a statistically significant result (p < 0.05).
Cell culture
For the cell culture, we used a previously published protocol [16, 17]. The Chinese Academy of Sciences (Shanghai, China) provided the HEP3B and HEPG2 human HCC cell lines, along with normal human liver cells (NCs). These cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine at 37 °C in an incubator with 5% CO2.
RNA extraction and RT-qPCR
The RNA extraction and RT-qPCR were administered as described previously [16, 17]. RNA extraction from the specified cell lines and tissue samples was conducted using the RNA easy mini kit (QIAGEN, USA). GAPDH mRNA levels were utilized for data normalization, and the 2^(-ΔΔCT) method was employed for outcome quantification. The primer sequences are shown in table S2.
Cell transfection
Small interfering RNA (siRNA) plasmid of POLH-AS1 and the negative control (si-NC) were packed from GenePharma (Shanghai, China). The full length of POLH-AS1 synthesized by GenePharma was subcloned into a lentivirus vector. HEP3B and HEPG2 cells were transfected using Lipofectamine® 3000 (Invitrogen, USA) according to our previously published article and the manufacturer’s protocol [16]. The sequences of siRNA are shown in table S3.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was performed to detect cell proliferation as previously reported [16, 19]. In 96-well plates, the HCC cells were planted at a density of 3 × 103 cells per well. Next, 10 µL of CCK-8 solution was applied to the medium at 0, 24, 48, and 72 h, and it was then incubated for two hours. At 450 nm in wavelength, the optical density (OD) was measured with a SpectraMax i3x device. Then, a proliferation curve was produced using the absorbance values that were discovered at the 72-hour mark.
Transwell migration and invasion assays
Transwell assays were used to evaluate the migratory and invasive potential of HCC cells, as previously described [16, 19]. The bottom chambers of the migration experiment were filled with 500 µL of culture media that contained 10% FBS. In the top chamber, 3 × 104 HCC cells were seeded per well using 250 µL of serum-free media. Swabs were used to extract the cells from the top compartment after 48 h. Using an Olympus microscope (Tokyo, Japan), the remaining cells were preserved with 95% ethanol, dyed with a 0.5% crystal violet solution, photographed, and the number of moving cells was counted. Before cell seeding, the filter for the invasion experiment was pre-coated with Matrigel (BD Biosciences, San Jose, CA, USA). The next steps were the same as for the migration test.
Wound healing assay
An assay of wound healing was conducted based on previous studies [16]. In 6-well plates, cells were seeded and cultured until reaching confluence. A wound was then made in the center of the plate using pipette tips, followed by a switch to a serum-free medium. After 48 h, images were captured, and the closure of the wound was subsequently assessed.
Statistical analysis
Statistical data analysis was conducted using R software version 3.6.3. A comparison of the variations in POLH-AS1 levels among the two groups was performed utilizing Fisher’s exact test, Mann-Whitney test, and Chi-square test. The Wilcox or Kruskal test was utilized to evaluate the relationship between POLH-AS1 levels in patients with HCC and their clinical information. The Kaplan-Meier technique was used to analyze survival. p < 0.05 was deemed as the statistically significant threshold.
Results
Features of HCC patients
The TCGA databases provided 374 RNAseq data sets of HCC patients with clinical resources including their age, histologic grade, histological type, pathologic stage, AFP, and vascular invasion. Clinical data is displayed in Table 1.
The high expression level of POLH-AS1 in HCC tissues
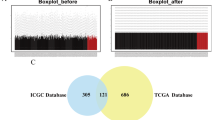
Initially, we examined the expression levels of POLH-AS1 in various tumor tissues, utilizing data from TCGA. The analysis revealed that POLH-AS1 expression was elevated in several malignancies, with HCC showing particularly high levels compared to corresponding normal tissues (Fig. 1A). As shown in Fig. 1B and C, the RNA levels of POLH-AS1 in HCC tissues were consistently higher than those in normal liver tissues. Furthermore, we performed RT-qPCR on POLH-AS1 levels in eight individual HCC cases and their corresponding normal liver tissues. The qPCR results confirmed that POLH-AS1 expression was significantly higher in HCC tissues than in the matched normal tissues (Fig. 1D).
The expression level of POLH-AS1 in different types of tumors. (A) The expression of POLH-AS1 between normal tissues and cancer samples in TCGA database. (B) The expression of POLH-AS1 in normal tissues and HCC tissues in TCGA database. (C) The expression of POLH-AS1 in 50 pairs of HCC tissues and non-cancerous adjacent tissues in TCGA database. (D) The expression of POLH-AS1 was assessed in 8 HCC tissues and 8 normal liver tissues by RT‑qPCR assay. *p < 0.05, **p < 0.01, ***p < 0.001
The expression of POLH-AS1 correlates with clinicopathological characteristics of HCC
Utilizing data from the TCGA cohort, we explored the association between POLH-AS1 levels and various clinical factors, including histologic grade, histologic type, pathological stage, and AFP concentration. The analysis revealed that POLH-AS1 expression was significantly elevated in G3&G4 HCC compared to G1&G2 HCC (Fig. 2A). Additionally, POLH-AS1 expression was higher in hepatocholangio carcinoma (mixed type) than in fibrolamellar carcinoma and hepatocellular carcinoma (Fig. 2B). Furthermore, elevated expression of POLH-AS1 was observed in Stage III&IV compared to Stage I&II (Fig. 2C). A positive correlation between POLH-AS1 expression and AFP concentration was also established, with higher POLH-AS1 expression observed in patients with elevated serum AFP levels (Fig. 2D).
The correlations between POLH-AS1 expression and clinicopathological characteristics of HCC. (A)-(D) The relationship between the POLH-AS1 expression and the histological grade (A), histological type (B), pathological stage (C), AFP (D). (E) ROC analysis of POLH-AS1 expression shows promising discrimination power between normal samples and HCC tissues. (F) Time-dependent ROC curves and AUC values for 1-year, 3-year, and 5-year OS prediction. *p < 0.05, ***p < 0.001
Moreover, ROC curve analysis was performed to evaluate the diagnostic potential of POLH-AS1 in HCC patients. The findings demonstrated that POLH-AS1 possesses significant diagnostic value for HCC, as evidenced by an AUC value of 0.864 (95% CI = 0.823–0.906) (Fig. 2E). Additionally, time-dependent ROC curve analysis revealed AUC values of 0.677, 0.624, and 0.608 for predicting 1-year, 3-year, and 5-year survival rates in HCC patients, respectively (Fig. 2F), indicating that POLH-AS1 serves as a reliable prognostic marker for HCC survival.
High POLH-AS1 expression indicated poor prognosis in HCC patients
The prognostic value of POLH-AS1 in HCC was assessed using Kaplan-Meier survival analysis with RNA-seq data from TCGA. The findings revealed a significant inverse correlation between POLH-AS1 expression levels and overall survival (p = 0.005), disease-specific survival (DSS, p = 0.011), and progression-free interval (PFI, p = 0.015) (Fig. 3A-C). Further univariate and multivariate analyses confirmed that POLH-AS1 upregulation is an independent prognostic factor in HCC (Fig. S1A-B and table S4).
The relationship between POLH-AS1 and the survival of patients with HCC. (A)-(C) K-M survival analysis showing the effect of POLH-AS1 expression level on OS (A), DSS (B), and PFI (C) in patients with HCC in TCGA cohort. (D) Nomogram for predicting the probability of 1-, 3-, and 5-year OS for patients with HCC. (E) The calibration curves showing the concordance between the prediction by nomogram and actual survival
A nomogram model incorporating clinical characteristics was developed to predict overall survival rates for HCC patients at 1, 3, and 5 years (Fig. 3D). Calibration curves were then used to evaluate the accuracy of the nomogram’s predictions, showing a strong concordance between the predicted survival rates and actual outcomes (Fig. 3E).
Identification of DEGs and functional enrichment analysis
To explore the potential mechanisms of POLH-AS1 in HCC, we categorized HCC patients into high- and low-POLH-AS1 expression groups based on the median expression level of POLH-AS1. We identified 2,183 upregulated and 682 downregulated genes, applying the criteria of |log2FC| > 1 and adjusted p < 0.05 (Fig. 4A). The relative expression levels of the top 30 DEGs between the two groups are depicted in Fig. 4B.
Identification of DEGs and functional enrichment analysis of POLH-AS1 in HCC. (A) Volcano plot of differentially expressed genes. Red and green indicated up-regulated and down-regulated genes, respectively (|log2 fold change (FC)| > 1 and p < 0.05). (B) Heatmap showing the top 30 co-expressed differential genes in the POLH-AS1 low and high expression groups. (C) The bubble plot showing the GO functional enrichment analysis results (BP, biological process; CC, cellular component; MF, molecular function). (D) The bubble plot showing the results of KEGG enrichment analysis
To assess the functional significance of these DEGs in HCC, we conducted KEGG and GO enrichment analyses. GO analysis highlighted significant enrichment in processes such as organelle fission, ion channel complex formation, and passive transmembrane transporter activity (Fig. 4C). KEGG analysis revealed enrichment in pathways linked to carcinogenesis, including herpes simplex virus 1 infection, neuroactive ligand-receptor interaction, cAMP signaling pathway, and proteoglycans in cancer (Fig. 4D). These findings strongly suggest the involvement of these DEGs in the development and progression of HCC.
Examination of the relationship between POLH-AS1 and immune infiltration in HCC
Immune infiltration plays a crucial role in HCC progression and provides valuable insights for potential immunotherapies [20]. Utilizing the ssGSEA technique, we explored the correlation between POLH-AS1 levels and the presence of 24 unique immune cell populations within HCC. The results demonstrated that POLH-AS1 expression was significantly positively correlated with Th2 cells (R = 0.276, p < 0.001) and T helper cells (R = 0.192, p < 0.001). Conversely, POLH-AS1 expression was negatively associated with DCs (R = − 0.383, p < 0.001), neutrophils (R = -0.300, p < 0.001), pDCs (R = − 0.286, p < 0.001), and cytotoxic cells (R = -0.285, p < 0.001) (Fig. 5A-B and Fig. S2A-F). Additionally, we explored the relationship between POLH-AS1 and immune checkpoints. The analysis revealed a significant positive correlation between POLH-AS1 expression and several immune checkpoint molecules, including CD276 (R = 0.4, p < 0.001), TNFSF4 (R = 0.37, p < 0.001), TNFSF15 (R = 0.3, p < 0.001), and NRP1 (R = 0.27, p < 0.001) (Fig. 5C and Fig. S2G). Collectively, these findings suggested that POLH-AS1 expression is closely related to the immune microenvironment in HCC, potentially influencing tumor immune cell infiltration and the expression of immune checkpoints, which could hold significant implications for immunotherapy strategies in HCC.
The association between POLH-AS1 expression and immune infiltration in HCC. (A) The infiltrating levels of 24 subtypes immune cells in high and low POLH-AS1 expression groups. (B) The correlation between the 24 subtypes immune cells and POLH-AS1 expression level. (C) The correlation between POLH-AS1 and immune checkpoint genes. *p < 0.05, **p < 0.01, ***p < 0.001
Inhibition of POLH-AS1 impeded cell proliferation in HCC
To further investigate the role of POLH-AS1 in the initiation and progression of HCC, we examined its expression in HEP3B and HEPG2 cell lines. RT-qPCR analysis revealed a significantly elevated expression of POLH-AS1 in both HEP3B and HEPG2 cell lines compared to the negative control (NC) cells (Fig. 6A). Subsequently, POLH-AS1 expression in HEP3B and HEPG2 cells was downregulated using small interfering RNA (siRNA), effectively silencing POLH-AS1, as confirmed by RT-qPCR analysis (Fig. 6B-C). The results of CCK8 assays demonstrated that knockdown of POLH-AS1 significantly inhibited the proliferation of HEP3B and HEPG2 cells (Fig. 6D-E), whereas overexpression of POLH-AS1 markedly promoted these cellular behaviors (Fig. S3A-C).
Inhibition of POLH-AS1 impeded cell proliferation in HCC. (A) RT-qPCR analysis showing the expression of POLH-AS1 in two HCC cell lines (HEP3B and HEPG2) and a normal liver cell (NC). (B)-(C) RT-qPCR analysis showing the efficiency of si-POLH-AS1 transfection in HEP3B and HEPG2 cells. (D)-(E) CCK8 assays showing proliferation of HEP3B (D) and HEPG2 (E) cells transfected with control (si-NC) or si-POLH-AS1. Data are presented as the mean ± SDs. ***p < 0.001
Suppression of POLH-AS1 hindered migration and invasion of HCC
Our study extended beyond the effects of POLH-AS1 on HCC cell growth to examine its role in cell migration and invasion. Transwell assays demonstrated that knockdown of POLH-AS1 significantly impaired the migration and invasion capabilities of HEP3B and HEPG2 cells (Fig. 7A-D), while overexpression of POLH-AS1 markedly enhanced these cellular behaviors (Fig. S3D-G). Additionally, wound healing assays revealed that knockdown of POLH-AS1 significantly reduced the wound closure rate in HCC cells (Fig. 7E-H). Collectively, these findings provide compelling evidence that suppression of POLH-AS1 hinders the migration and invasion of HCC cells, suggesting that targeting POLH-AS1 may offer therapeutic benefits for HCC.
Knockdown of POLH-AS1 inhibited cell migration and invasion in HCC. (A)-(D) Transwell migration and invasion assays showing the migratory and invasive ability of POLH-AS1-deficient HEP3B (A, B) and HEPG2 (C, D) cells. Scales bar, 100 µM. The data are the means ± SDs. (E)-(H) Wound healing migration assays showing HEP3B (E, F) and HEPG2 (G, H) cell migration of control cells compared to POLH-AS1-depleted cells. Scales bar, 100 µM. Data are presented as the mean ± SDs. ***p < 0.001
Discussion
HCC is a common yet highly aggressive malignant tumor, often progressing silently and resulting in a grim prognosis for patients. Despite the availability of various treatment modalities, including radiation, chemotherapy, and surgery, each approach has its inherent limitations. Although multiple therapeutic options exist for HCC, the overall prognosis remains poor, with a 5-year survival rate of just 18% [21]. The urgent need for novel therapeutic strategies is underscored by the unfavorable prognosis, emerging drug resistance, and significant side effects associated with current treatments.
Recent research has revealed a significant correlation between altered lncRNA expression levels and poor prognosis in HCC, underscoring the potential of these biomarkers in predicting both diagnosis and prognosis [11]. These pioneering discoveries offer renewed hope for advanced HCC patients by opening new avenues for treatment. LncRNAs are crucial regulatory elements that influence cancer aggressiveness by modulating its progression, particularly in HCC. The depletion of oncogenic lncRNAs has been shown to induce apoptotic cell death and cause cell cycle arrest in HCC [22], whereas their overexpression substantially increases the proliferation of cancer cells [23]. For instance, researchers exploring immunotherapy for HCC have identified several lncRNAs that can predict patient prognosis [24, 25]. From a selection of potentially significant lncRNAs, we have focused on POLH-AS1 to investigate its relationship with HCC and to assess its potential utility in predicting outcomes and guiding treatment strategies for HCC patients.
In our research, we found that POLH-AS1 was highly expressed in HCC and that this elevated expression was associated with more advanced clinicopathological features. Further investigation into the relationship between POLH-AS1 expression and the prognosis of HCC patients revealed that increased POLH-AS1 expression may be linked to poorer outcomes. Additionally, the results from univariate and multivariate analyses, ROC curve analysis, and Kaplan-Meier survival analysis all support the notion that POLH-AS1 can serve as an independent prognostic marker in HCC. Finally, we explored the functional relevance of POLH-AS1 in HCC progression, unveiling that its inhibition resulted in attenuated cell proliferation, migration, and invasion. In aggregate, our finding suggested that POLH-AS1 might be used as a potential prognostic factor that affected the prognosis of patients with HCC. However, the mechanism by which POLH-AS1 leads to poor prognosis of HCC is unclear and needs further investigation.
To explore the role of POLH-AS1 in the malignant progression of HCC, we performed a GSEA using RNA-Seq data from TCGA. GSEA is widely used to reliably uncover potential molecular mechanisms underlying specific genes involved in disease pathology [26]. Our analysis revealed significant enrichment of POLH-AS1 in pathways related to herpes simplex virus 1 infection, neuroactive ligand-receptor interaction, and cAMP signaling, all of which are known to impact the prognosis and treatment of HCC patients. Emerging evidence suggests that these pathways are critically involved in HCC tumorigenesis and progression [27,28,29]. For example, Lam et al. identified an efficient and safe herpes simplex virus type 1 amplicon vector for transcriptionally targeted therapy in human hepatocellular carcinomas [28]. Similarly, neuroactive ligand-receptor interaction has been shown to play a pivotal role in HCC cell proliferation and invasion [29]. Moreover, vasoactive intestinal peptide was found to induce apoptosis in hepatocellular carcinoma by inhibiting the cAMP/Bcl-xL signaling pathway [27]. These findings collectively indicate that POLH-AS1 may influence the prognosis of HCC through its involvement in these cancer-related signaling pathways.
A growing body of research has highlighted that immune infiltration, a key component of the tumor microenvironment, plays a crucial role in oncogenesis and tumor progression, as well as influencing the response to immunotherapy [30]. However, no studies had previously reported a correlation between POLH-AS1 and immune infiltration in HCC. In our study, we identified a negative association between POLH-AS1 expression and dendritic cells (DCs) in HCC. Previous research has shown that local ablation of hepatocellular carcinoma can activate dendritic cells, thereby inducing sustained anti-tumor immune responses and ultimately reducing tumor progression and recurrence [31, 32]. This suggests that POLH-AS1 may promote HCC progression by impairing DC function. Moreover, we explored the relationship between POLH-AS1 expression and immune checkpoints, including CD276, TNFSF4, TNFSF15, and NRP1, discovering a significant positive co-expression correlation between POLH-AS1 and these immune checkpoints. Immune checkpoints are known as a class of immunosuppressive molecules that enhance the immune response against HCC [30]. These findings suggest that POLH-AS1 plays a role in the tumor immune microenvironment primarily by regulating DCs function and immune checkpoints, and that POLH-AS1 may influence patient prognosis by modulating the immune microenvironment in HCC.
Nevertheless, our study has certain limitations. Firstly, the sample data were exclusively sourced from TCGA databases, with no clinical information from external cohorts to validate the findings. Additionally, the molecular mechanisms through which POLH-AS1 affects HCC growth, migration, and invasion remain inadequately elucidated. Further investigation into the regulatory mechanisms of POLH-AS1 will be pursued both in vivo and in vitro.
Conclusion
Our investigation showcased the potential of POLH-AS1 as both a prognostic determinant and a viable target for therapeutic intervention in HCC patients. A deeper comprehension of its impact on cell growth regulation could pave the way for clinical innovations aimed at enhancing the prognostic outlook for individuals with HCC.
Data availability
Data information from this research is available in the TCGA repositories (http://cancergenome.nih.gov) and UCSC Xena (http://xenabrowser.net/datapages/) platform.
References
Ma H, Kang Z, Foo TK, Shen Z, Xia B. Disrupted BRCA1-PALB2 interaction induces tumor immunosuppression and T-lymphocyte infiltration in HCC through cGAS-STING pathway. Hepatology. 2023;77(1):33–47.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62.
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17(3):139–52.
Xu LX, He MH, Dai ZH, Yu J, Wang JG, Li XC, Jiang BB, Ke ZF, Su TH, Peng ZW, et al. Genomic and transcriptional heterogeneity of multifocal hepatocellular carcinoma. Ann Oncol. 2019;30(6):990–7.
Schmitt AM, Chang HY. Long noncoding RNAs in Cancer pathways. Cancer Cell. 2016;29(4):452–63.
Zhang Y, Dong X, Guo X, Li C, Fan Y, Liu P, Yuan D, Ma X, Wang J, Zheng J, et al. LncRNA-BC069792 suppresses tumor progression by targeting KCNQ4 in breast cancer. Mol Cancer. 2023;22(1):41.
Pan Y, Zhang Q, Zhang H, Kong F. Prognostic and immune microenvironment analysis of cuproptosis-related LncRNAs in breast cancer. Funct Integr Genomics. 2023;23(1):38.
Wang W, Wang L, Song C, Mu T, Hu J, Feng H. Prognostic signature constructed of seven ferroptosis-related lncRNAs predicts the prognosis of HBV-Related HCC. J Gastrointest Cancer 2023.
Hashemi M, Mirzaei S, Zandieh MA, Rezaei S, Amirabbas K, Dehghanpour A, Esmaeili N, Ghahremanzade A, Saebfar H, Heidari H, et al. Long non-coding RNAs (lncRNAs) in hepatocellular carcinoma progression: Biological functions and new therapeutic targets. Prog Biophys Mol Biol. 2023;177:207–28.
Liu X, Cheng W, Li H, Song Y. Identification and validation of cuproptosis-related LncRNA signatures as a novel prognostic model for head and neck squamous cell cancer. Cancer Cell Int. 2022;22(1):345.
Wang W, Ye Y, Zhang X, Ye X, Liu C, Bao L. Construction of a necroptosis-Associated Long non-coding RNA signature to Predict Prognosis and Immune Response in Hepatocellular Carcinoma. Front Mol Biosci. 2022;9:937979.
Song J, Wang L, Ng NN, Zhao M, Shi J, Wu N, Li W, Liu Z, Yeom KW, Tian J. Development and validation of a machine learning model to explore tyrosine kinase inhibitor response in patients with stage IV EGFR variant-positive Non-small Cell Lung Cancer. JAMA Netw Open. 2020;3(12):e2030442.
Wang W, Ye Y, Zhang X, Sun W, Bao L. An angiogenesis-related three-long non-coding ribonucleic acid signature predicts the immune landscape and prognosis in hepatocellular carcinoma. Heliyon. 2023;9(3):e13989.
Xu L, Chen S, Li Q, Chen X, Xu Y, Zhou Y, Li J, Guo Z, Xing J, Chen D. Integrating bioinformatics and experimental validation to unveil disulfidptosis-related lncRNAs as prognostic biomarker and therapeutic target in hepatocellular carcinoma. Cancer Cell Int. 2024;24(1):30.
Zhu X, Chen D, Sun Y, Yang S, Wang W, Liu B, Gao P, Li X, Wu L, Ma S, et al. LncRNA WEE2-AS1 is a diagnostic biomarker that predicts poor prognoses in patients with glioma. BMC Cancer. 2023;23(1):120.
Wang L, Cao Y, Guo W, Xu J. High expression of cuproptosis-related gene FDX1 in relation to good prognosis and immune cells infiltration in colon adenocarcinoma (COAD). J Cancer Res Clin Oncol. 2023;149(1):15–24.
Chen D, Xu Y, Gao X, Zhu X, Liu X, Yan D. A novel signature of cuproptosis-related lncRNAs predicts prognosis in glioma: evidence from bioinformatic analysis and experiments. Front Pharmacol. 2023;14:1158723.
Bekric D, Ocker M, Mayr C, Stintzing S, Ritter M, Kiesslich T, Neureiter D. Ferroptosis in Hepatocellular Carcinoma: mechanisms, drug targets and approaches to clinical translation. Cancers (Basel) 2022, 14(7).
Paskeh MDA, Asadi A, Mirzaei S, Hashemi M, Entezari M, Raesi R, Hushmandi K, Zarrabi A, Ertas YN, Aref AR, et al. Targeting AMPK signaling in ischemic/reperfusion injury: from molecular mechanism to pharmacological interventions. Cell Signal. 2022;94:110323.
Zhong X, Huang S, Liu D, Jiang Z, Jin Q, Li C, Da L, Yao Q, Wang D. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer Med. 2020;9(15):5546–57.
Luo Y, Lin J, Zhang J, Song Z, Zheng D, Chen F, Zhuang X, Li A, Liu X. LncRNA SNHG17 Contributes to Proliferation, Migration, and Poor Prognosis of Hepatocellular Carcinoma. Can J Gastroenterol Hepatol 2021, 2021:9990338.
Fang C, Liu S, Feng K, Huang C, Zhang Y, Wang J, Lin H, Wang J, Zhong C. Ferroptosis-related lncRNA signature predicts the prognosis and immune microenvironment of hepatocellular carcinoma. Sci Rep. 2022;12(1):6642.
Zhang Z, Zhang W, Wang Y, Wan T, Hu B, Li C, Ge X, Lu S. Construction and validation of a ferroptosis-related lncRNA signature as a Novel Biomarker for Prognosis, Immunotherapy and targeted therapy in Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:792676.
Liu T, Yang K, Chen J, Qi L, Zhou X, Wang P. Comprehensive Pan-cancer Analysis of KIF18A as a marker for prognosis and immunity. Biomolecules 2023, 13(2).
Hara M, Takeba Y, Iiri T, Ohta Y, Ootaki M, Watanabe M, Watanabe D, Koizumi S, Otsubo T, Matsumoto N. Vasoactive intestinal peptide increases apoptosis of hepatocellular carcinoma by inhibiting the cAMP/Bcl-xL pathway. Cancer Sci. 2019;110(1):235–44.
Lam PY, Sia KC, Khong JH, De Geest B, Lim KS, Ho IA, Wang GY, Miao LV, Huynh H, Hui KM. An efficient and safe herpes simplex virus type 1 amplicon vector for transcriptionally targeted therapy of human hepatocellular carcinomas. Mol Ther. 2007;15(6):1129–36.
Li C, Jia Y, Li N, Zhou Q, Liu R, Wang Q. DNA methylation-mediated high expression of CCDC50 correlates with poor prognosis and hepatocellular carcinoma progression. Aging. 2023;15(15):7424–39.
Liu B, Liu Z, Wang Y, Lian X, Han Z, Cheng X, Zhu Y, Liu R, Zhao Y, Gao Y. Overexpression of GINS4 is associated with poor prognosis and survival in glioma patients. Mol Med. 2021;27(1):117.
Ali MY, Grimm CF, Ritter M, Mohr L, Allgaier HP, Weth R, Bocher WO, Endrulat K, Blum HE, Geissler M. Activation of dendritic cells by local ablation of hepatocellular carcinoma. J Hepatol. 2005;43(5):817–22.
Cabillic F, Toutirais O, Lavoué V, de La Pintière CT, Daniel P, Rioux-Leclerc N, Turlin B, Mönkkönen H, Mönkkönen J, Boudjema K, et al. Aminobisphosphonate-pretreated dendritic cells trigger successful Vgamma9Vdelta2 T cell amplification for immunotherapy in advanced cancer patients. Cancer Immunol Immunother. 2010;59(11):1611–9.
Acknowledgements
Not applicable.
Funding
This work was funded by the Henan Medical Science and Technology Joint Building Program (no. LHGJ20190255).
Author information
Authors and Affiliations
Contributions
LXX, YD, and XYC were responsible for designing the project and writing the manuscript. SY, DC and XPG downloaded and analyzed the data. YLF and LYW collected samples and processed the data. The final manuscript was reviewed and approved by all writers.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Approval for the study was granted by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University, in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, Y., Chen, X., Yang, S. et al. Comprehensive analysis of POLH-AS1 as a prognostic biomarker in hepatocellular carcinoma. BMC Cancer 24, 1112 (2024). https://doi.org/10.1186/s12885-024-12857-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12857-8