Abstract
Background and purpose
In the context of the widespread availability of magnetic resonance imaging (MRI) and aggressive salvage irradiation techniques, there has been controversy surrounding the use of prophylactic cranial irradiation (PCI) for small-cell lung cancer (SCLC) patients. This study aimed to explore whether regular brain MRI plus salvage brain irradiation (SBI) is not inferior to PCI in patients with limited-stage SCLC (LS-SCLC).
Methods
This real-world multicenter study, which was conducted between January 2014 and September 2020 at three general hospitals, involved patients with LS-SCLC who had a good response to initial chemoradiotherapy and no brain metastasis confirmed by MRI. Overall survival (OS) was compared between patients who did not receive PCI for various reasons but chose regular MRI surveillance and followed salvage brain irradiation (SBI) when brain metastasis was detected and patients who received PCI.
Results
120 patients met the inclusion criteria. 55 patients received regular brain MRI plus SBI (SBI group) and 65 patients received PCI (PCI group). There was no statistically significant difference in median OS between the two groups (27.14 versus 33.00 months; P = 0.18). In the SBI group, 32 patients underwent whole brain radiotherapy and 23 patients underwent whole brain radiotherapy + simultaneous integrated boost. On multivariate analysis, only extracranial metastasis was independently associated with poor OS in the SBI group.
Conclusion
The results of this real-world study showed that MRI surveillance plus SBI is not inferior to PCI in OS for LS-SCLC patients who had a good response to initial chemoradiotherapy.
Similar content being viewed by others
Introduction
Currently, prophylactic cranial irradiation (PCI) is a category 1 recommendation for limited-stage SCLC (LS-SCLC) patients who have a good response to chemoradiotherapy (CRT), according to guidelines such as the National Comprehensive Cancer Network [1]. These recommendations have been implemented in clinical practice for decades and are based primarily on the meta-analysis of LS-SCLC trials indicating that PCI improves overall survival (OS) by 5.4% [2]. However, within the above meta-analysis, the detection of brain metastases (BM) was primarily achieved by brain computed tomography (CT) or even by plain X-rays of the brain. Theoretically, patients with occult BM may be included in this population, which may have exaggerated the actual benefits of PCI. The RTOG 0212 study demonstrated that the preferred dose for PCI is 25 Gy in 10 daily fractions; patients receiving a higher dose of 36 Gy in 18 daily fractions had higher mortality and higher chronic neurotoxicity [3].
With the rapid development and widespread application of MRI technologies and the publication of a Japanese study showing PCI was not superior to MRI follow-up for extensive SCLC, the role of PCI for LS-SCLC has become more contentious [4]. In the real world, many patients did not undergo PCI due to various concerns such as neurotoxicity [5, 6].
In the MRI era, no prospective study has demonstrated the benefit of PCI in LS-SCLC. We conducted this real-world study to investigate whether MRI surveillance plus salvage brain irradiation (SBI) is not inferior to PCI in terms of OS for patients with LS-SCLC and to further explore prognostic factors.
Materials and methods
Study design and patient population
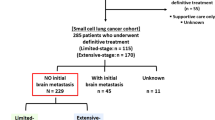
Patients’ clinicopathological characteristics, cancer treatment history, and outcomes were reviewed retrospectively. The inclusion criteria were as follows: (i) LS-SCLC with pathological or cytological confirmation, according to the Tumor-Node-Metastasis (TNM) staging system of the American Joint Committee on Cancer (eighth edition), as well as the Veterans Administration Lung Study Group staging system; (ii) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; (iii) brain MRI was carried out at diagnosis and after chemoradiotherapy to rule out BM; and (iv) had a complete or partial response to initial concurrent/sequential CRT. The main exclusion criteria included: (i) combined with other malignancies; (ii) chemotherapy cycles < 4; (iii) the disease progressed during CRT; and (iv) incomplete medical records (such as no ECOG PS, prescription medication information, etc.) or imaging data. The primary end point was OS in all patients included in the study. The secondary endpoint was survival after BM (SABM) in patients who underwent SBI.
Treatment and follow up
Chemotherapy consisted of at least four cycles of platinum plus etoposide every 3–4 weeks. Intensity-modulated radiation therapy (IMRT) was used to deliver a total dose of 60 to 70 Gy in single daily fractions of 2 Gy for thoracic radiotherapy (TRT). When TRT was administered concurrently, it began on the first or second chemotherapy cycle, when administered sequentially, it began after the last chemotherapy cycle. It is recommended that lung CT be reexamined after 4 to 5 weeks following TRT in order to compare lesions before and after treatment. If the size of the tumor decreased notably, repositioning was performed to outline the target area and irradiate with a reduced field depending on each patient’s physical condition.
PCI was delivered using a CT-based treatment plan to patients who had a response to initial therapy at the discretion of the treating physician. For those patients who did not undergo PCI, the treatment protocol was for surveillance brain MRI with use of whole brain radiation therapy (WBRT) or WBRT + simultaneous integrated boost (SIB) at the time of BM. Monitoring of intracranial status with a thin slice (3–5 mm) plain scan and enhanced MRI was conducted at least once every two to three months for all patients, regardless of whether PCI was performed.
The decision regarding systemic treatments or post-progression treatments for two groups was made by the treating physicians based on the guidelines, patients’ willingness, and general health status. All patients were followed up by the outpatient clinic and telephone calls with an interval of 1–3 months.
Efficacy assessments
The Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) criteria was used for efficacy assessment every six weeks during protocol treatment, every three months after treatment for a period of two years, and every six months thereafter. If clinically indicated, additional CT, MRI, bone scans, etc., may be performed between scheduled scans. Using the pathological diagnosis date as the index date, all enrolled patients were followed until death or censored at the date of last follow-up (May 23, 2022). OS was defined as the interval between the date of pathological diagnosis and the date of death or the last date known to be alive. The SABM was defined as the time from the diagnosis of BM until death or last follow-up.
Statistical analysis
To determine whether there were differences in basic clinical characteristics between groups, continuous variables were compared using a Student t-test, and categorical variables were compared by the chi-square test. Survival distributions were estimated by the Kaplan-Meier method and compared using the log-rank test. Stratification by initial disease status was undertaken in an attempt to reduce the influence of potential confounders. Multivariate and univariate survival analyses were conducted using the Cox proportional hazards regression model. If univariate analysis indicated possible association with the outcome (P < 0.10), the variables were included in the multivariate analysis. Interactions between variables and interaction with time were tested. Statistical analyses were completed using SPSS software (version 22.0, IBM SPSS) and R version 4.2.0.
Ethical aspects
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Retrospective data were retrieved from electronic medical records upon patient informed consent. If the patients died, informed consent was obtained from the patient’s family.
Results
Patient and treatment characteristics
The information of 143 patients was initially recorded for analysis in the study. However, 23 patients were excluded due to combined with other malignancies (n = 2), chemotherapy cycles < 4 (n = 5), disease progression during CRT (n = 11), incomplete medical records (n = 3), and incomplete imaging data (n = 2). Finally, 120 patients with LS-SCLC who were treated within our centers between January 2014 and September 2020 met inclusion criteria for our analysis. Of these, 55 patients received SBI (WBRT or WBRT + SIB). According to the linear quadratic model, the equivalent dose in 2 Gy per fraction of WBRT was calculated to be 32.5–50 Gy. For brain metastatic lesions, the cumulative SIB dose was 50–60 Gy in 2–4 Gy per fraction per day, five days a week. PCI was performed on 65 patients using 25 Gy in 10 fractions. The baseline clinicopathological characteristics of patients are summarized in Table 1. There were no baseline differences for sex, ECOG PS and smoker between the two groups. The median ages for the SBI and PCI groups were 63 and 58 years, respectively (P < 0.01). In the SBI group, patients with TNM stage I/II (12.73% vs. 29.23%, P = 0.04), patients receiving concurrent CRT (10.91% vs. 32.31%, P < 0.01), and patients with complete response efficacy (12.73% vs. 29.23%, P = 0.04) were all significantly lower than those in the PCI group. The median follow-up duration period in the two groups (24.21 vs. 28.12 months, P = 0.15) was similar.
Survival outcomes and prognostic analysis
Among the entire cohort, the median OS was 29.18 months (95% confidence interval [CI] 23.04–35.31). The Kaplan-Meier survival curves for OS are displayed in Fig. 1. In the SBI and PCI groups, the median OS times were 27.14 (95% CI 21.08‒33.20) and 33.00 (95% CI 25.79‒40.18) months, respectively. The 2-year OS rates were 58% and 66% in the SBI and PCI groups, respectively (hazard ratio: 1.36, 95% CI: 0.86–2.15, P = 0.18). There were no significant interactions between treatment group and any prespecified subgroups including age, sex, ECOG PS, smoker, TNM stage, CRT sequence, and response to initial treatment (Fig. 2).
The cumulative 1-year SABM rates in the WBRT vs. WBRT + SIB groups were 54% vs. 48%, respectively (Fig. 3). The median SABM times in the WBRT and WBRT + SIB groups were 15.67 and 12.85 months, respectively. The difference in survival rate was not significant (P = 0.97). Predictors of OS on univariate and multivariate analyses in patients with MRI surveillance plus aggressive SBI are shown in Table 2. On univariate Cox regression analysis, where the primary outcome was mortality from any cause, factors associated with increased overall mortality were female and with extracranial metastasis. Only extracranial metastasis was independent predictor for OS on multivariate analysis.
Discussion
Given improvements in technology and a higher accessibility to MRI, it is uncertain whether PCI remains beneficial for patients with LS-SCLC when compared to MRI surveillance plus SBI, as this has not yet been evaluated in prospective trials [7]. In this multi-institutional study with 120 consecutive MRI staged patients with LS-SCLC diagnosed between 2014 and 2020, we investigated the effects of management strategies for brain radiotherapy on OS.
There was no significant difference in OS between the SBI and PCI groups (P = 0.18), although younger age and higher ratios of early TNM stage, CR, and concurrent CRT were advantageous for the PCI group. No significant interaction was observed between treatment assignment and subgroups. However, the subgroup analysis may be underpowered and should be considered exploratory. This study reported a longer median OS (29.18 months; 95%CI 23.04–35.31) than what has been reported in the randomized phase III CONVERT trial, which used 66 Gy in single daily fractions of 2 Gy (25 months; 95%CI 21–31) [8]. Perhaps the best explanation of these results is that brain MRI and IMRT in the CONVERT trial were not mandated; in this study, all patients received brain MRI monitoring and IMRT.
Several non-randomized retrospective studies conducted after 1999 have reported a significant difference in OS between patients with LS-SCLC who did or did not receive PCI [9,10,11], but this could not be confirmed by other studies [12,13,14]. Novel to this paper is its emphasis on the optimal management strategies of brain radiotherapy (SBI for BM detected early by MRI compared to PCI). As MRI screening has become widespread, brain MRI is routinely performed for patients with LS-SCLC. Meanwhile, the long-term side effects of PCI are concerning. There has been evidence from several phase III trials that PCI is associated with a deterioration in cognitive and neuropsychological function [5, 15, 16]. Receiving memantine orally and hippocampal avoidance using IMRT may be considered potential strategies to prevent cognitive dysfunction [17,18,19]. However, their role in PCI remains controversial. There is a paucity of evidence regarding the efficacy of memantine in PCI in randomized trials [20]. Phase III trials evaluating neurocognitive function after hippocampal avoidance-PCI versus PCI have shown conflicting results [19, 21]. The investigators hypothesized that regular brain MRI surveillance plus aggressive SBI would be an appropriate treatment model rather than PCI for LS-SCLC. In this study, the probability of oligometastases in the brain was higher than that of multimetastases in the SBI group (65.45% vs. 34.55%); the median OS of the SBI and PCI groups did not differ significantly. These results support this alternative strategy.
Given the high rate of BM in SCLC, WBRT rather than stereotactic radiotherapy alone is still preferred in patients who develop BM [7, 22]. Several studies have shown that the use of WBRT + SIB is superior to WBRT alone, and the application of SIB-IMRT for the treatment of BM is growing [23,24,25], but its real prognostic value is unclear, particularly in the context of lung cancer. So, we further compared the effects of WBRT and SIB on survival. It should be noted, however, that SABM did not differ significantly between the WBRT + SIB and the WBRT groups. There is no agreement on the optimal hypofractionation schedule and each institute makes its decision based on clinical judgment and experience. This may explain our findings.
As immune checkpoint inhibitors have demonstrated success in the extensive-stage, this immunotherapeutic strategy is now being implemented in the potentially curative, limited stage [26,27,28]. As a result, it is imperative to reassess the therapeutic effectiveness of PCI in SCLC patients in the era of immunotherapy.
Despite its strengths, the study has certain limitations. Although the eligibility and exclusion criteria were fairly strict, due to the retrospective nature of this study, selection bias and heterogeneity were inevitable among enrolled patients. Secondly, due to the limited sample size in our study, a lack of sufficient statistical power may be accounting for the absence of benefit observed. Lastly, since this project was conducted retrospectively, data on cognitive outcomes were rarely available for analysis.
Conclusion
In conclusion, this study suggests that MRI surveillance plus SBI might be an appropriate alternative to PCI for patients with LS-SCLC. Multicenter and prospective randomized phase III clinical trials are warranted.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D’Amico TA, et al. Small cell Lung Cancer, Version 2.2022, NCCN Clinical Practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(12):1441–64.
Aupérin A, Arriagada R, Pignon JP, Le Péchoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview Collaborative Group. N Engl J Med. 1999;341(7):476–84.
Le Péchoux C, Dunant A, Senan S, Wolfson A, Quoix E, Faivre-Finn C, et al. Standard-dose versus higher-dose prophylactic cranial irradiation (PCI) in patients with limited-stage small-cell lung cancer in complete remission after chemotherapy and thoracic radiotherapy (PCI 99 – 01, EORTC 22003 – 08004, RTOG 0212, and IFCT 99 – 01): a randomised clinical trial. Lancet Oncol. 2009;10(5):467–74.
Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(5):663–71.
Le Péchoux C, Laplanche A, Faivre-Finn C, Ciuleanu T, Wanders R, Lerouge D, et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003 – 08004, RTOG 0212 and IFCT 99 – 01). Annals Oncology: Official J Eur Soc Med Oncol. 2011;22(5):1154–63.
Sun A, Bae K, Gore EM, Movsas B, Wong SJ, Meyers CA, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncology: Official J Am Soc Clin Oncol. 2011;29(3):279–86.
Seute T, Leffers P, ten Velde GP, Twijnstra A. Detection of brain metastases from small cell lung cancer: consequences of changing imaging techniques (CT versus MRI). Cancer. 2008;112(8):1827–34.
Faivre-Finn C, Snee M, Ashcroft L, Appel W, Barlesi F, Bhatnagar A, et al. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18(8):1116–25.
Patel S, Macdonald OK, Suntharalingam M. Evaluation of the use of prophylactic cranial irradiation in small cell lung cancer. Cancer. 2009;115(4):842–50.
Farooqi AS, Holliday EB, Allen PK, Wei X, Cox JD, Komaki R. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: do all patients benefit? Radiotherapy Oncology: J Eur Soc Therapeutic Radiol Oncol. 2017;122(2):307–12.
Lim YJ, Song C, Kim HJ. Survival impact of prophylactic cranial irradiation in small-cell lung cancer in the modern era of magnetic resonance imaging staging. Radiation Oncol (London England). 2022;17(1):26.
Pezzi TA, Fang P, Gjyshi O, Feng L, Liu S, Komaki R, et al. Rates of overall survival and Intracranial Control in the magnetic resonance imaging era for patients with Limited-Stage Small Cell Lung Cancer with and without prophylactic cranial irradiation. JAMA Netw open. 2020;3(4):e201929.
Mamesaya N, Wakuda K, Omae K, Miyawaki E, Kotake M, Fujiwara T, et al. Efficacy of prophylactic cranial irradiation in patients with limited-disease small-cell lung cancer who were confirmed to have no brain metastasis via magnetic resonance imaging after initial chemoradiotherapy. Oncotarget. 2018;9(25):17664–74.
Qi C, Li W, Li H, Wen F, Zhou L, Sun X, et al. Benefits of prophylactic cranial irradiation in the MRI era for patients with Limited Stage Small Cell Lung Cancer. Front Oncol. 2022;12:833478.
Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77–84.
Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013;86(4):656–64.
Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal avoidance during whole-brain Radiotherapy Plus Memantine for patients with brain metastases: Phase III Trial NRG oncology CC001. J Clin Oncol. 2020;38(10):1019–29.
Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–37.
Schunn F, Koerber S. [Prophylactic cranial irradiation with or without hippocampal avoidance for small-cell lung cancer (PREMER)- a randomized phase III trial]. Strahlenther Onkol. 2022;198(3):319–21.
Robin TP, Rusthoven CG. Strategies to preserve cognition in patients with brain metastases: a review. Front Oncol. 2018;8:415.
Belderbos JSA, De Ruysscher DKM, De Jaeger K, Koppe F, Lambrecht MLF, Lievens YN, et al. Phase 3 Randomized Trial of Prophylactic Cranial Irradiation with or without Hippocampus Avoidance in SCLC (NCT01780675). J Thorac Oncol. 2021;16(5):840–9.
Wang B, Yang J. New technologies and machines for stereotactic radiation therapy. Precision Radiation Oncol. 2022;6(4):321–7.
Rodrigues G, Zindler J, Warner A, Bauman G, Senan S, Lagerwaard F. Propensity-score matched pair comparison of whole brain with simultaneous in-field boost radiotherapy and stereotactic radiosurgery. Radiotherapy Oncology: J Eur Soc Therapeutic Radiol Oncol. 2013;106(2):206–9.
Zhong J, Waldman AD, Kandula S, Eaton BR, Prabhu RS, Huff SB, et al. Outcomes of whole-brain radiation with simultaneous in-field boost (SIB) for the treatment of brain metastases. J Neurooncol. 2020;147(1):117–23.
Dobi Á, Fodor E, Maráz A, Együd Z, Cserháti A, Tiszlavicz L, et al. Boost Irradiation Integrated to Whole Brain Radiotherapy in the management of Brain metastases. Pathol Oncol Research: POR. 2020;26(1):149–57.
Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell Lung Cancer. N Engl J Med. 2018;379(23):2220–9.
Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39.
Scott SC, Hann CL. Immunotherapy for small cell lung cancer: established applications and novel approaches. Clin Adv Hematol Oncol. 2021;19(10):654–63.
Acknowledgements
We thank all the patients, their families, and investigators involved in this study.
Funding
The authors declare that this study was not funded.
Author information
Authors and Affiliations
Contributions
NY: Conceptualization, data curation, formal analysis, writing–original draft, and writing–review and editing. ZQ: Conceptualization, formal analysis, and writing–review and editing. MC: Data curation, formal analysis, and writing–review and ed-iting. LH: Data curation and writing–review and editing. JM: Data curation and writing–review and editing. JL: Data curation and writing–review and editing. ST: Formal analysis and writing–review and editing. NL: Formal analysis and writing–review and editing. YY: Conceptualization, methodology, writing–original draft, and writing–review and editing.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local ethics committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participate
Written informed consent was waived given the nature of the study.
Conflict of interest for all authors
There are no conflicts of interest.
Consent for publication
This manuscript contains no individual person’s data.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yao, N., Qin, Z., Chen, M. et al. Effects of brain radiotherapy strategies on survival in the era of MRI for patients with limited stage small cell lung cancer. BMC Cancer 24, 953 (2024). https://doi.org/10.1186/s12885-024-12739-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12739-z