Abstract
Background
Although immunotherapy shows tremendous potential in the treatment of bladder cancer (BLCA), the overall prognosis and response rates to immunotherapy in BLCA remain suboptimal.
Methods
We performed an extensive evaluation of glycosyltransferase expression patterns in BLCA patients by analyzing 210 glycosyltransferase-related genes. Subsequently, we established correlations between these glycosyltransferase patterns, prognosis, and tumor microenvironment (TME) phenotypes. To offer personalized patient assessments, we developed a glycosyltransferase risk score that accurately predicts prognosis, TME phenotypes, and molecular subtypes. Importantly, we developed a RNA-seq cohort, named Xiangya cohort, to validate our results.
Results
Two distinct patterns of glycosyltransferase expression were identified, corresponding to inflamed and noninflamed TME phenotypes, and demonstrated the potential to predict prognosis. We developed and validated a comprehensive risk score that accurately predicted individual patient prognosis in the TCGA-BLCA cohort. Additionally, we constructed a nomogram that integrated the risk score with several key clinical factors. Importantly, this risk score was successfully validated in external cohorts, including the Xiangya cohort and GSE48075. Furthermore, we discovered a positive correlation between this risk score and tumor-infiltrating lymphocytes in both the TCGA-BLCA and Xiangya cohorts, suggesting that patients with a higher risk score exhibited an inflamed TME phenotype and were more responsive to immunotherapy. Finally, we observed that the high and low risk score groups were consistent with the luminal and basal subtypes of BLCA, respectively, providing further validation of the risk score’s role in the TME in terms of molecular subtypes.
Conclusions
Glycosyltransferase patterns exhibit distinct TME phenotypes in BLCA. Our comprehensive risk score provides a promising approach for prognostic prediction and assessment of immunotherapy efficacy, offering valuable guidance for precision medicine.
Similar content being viewed by others
Introduction
Bladder cancer (BLCA), is one of the most common malignant tumors of the urinary system, with over 550,000 new cases diagnosed each year and over 200,000 deaths worldwide [1]. In the field of BLCA therapeutics, progress had been stagnant for many years until the introduction and utilization of immune checkpoint blockade (ICB) treatment. This groundbreaking treatment has revolutionized the limited options available and offer immense hope, particularly for patients with locally advanced or metastatic BLCA who experience intolerance or resistance to conventional first-line therapies. The advent and application of immunotherapy has opened new avenues for treatment and hold great promise in improving outcomes for these patients [2]. However, the benefits of ICB treatment are restricted to a specific group of patients, and the overall prognosis remains unfavorable [3]. Therefore, it is crucial to investigate and identify reliable immunotherapy indicators and prognostic biomarkers to determine which BLCA patients will benefit from ICB treatment and to aid in risk stratification.
Glycosylation, a ubiquitous modification of proteins and lipids, plays a pivotal role in regulating cellular and molecular interactions [4]. In the context of cancer, glycosylation undergoes significant alterations [5, 6], and a multitude of clinical and experimental studies have provided substantial evidence supporting the involvement of specific carbohydrate structures in determining the malignancy of cancer. Alterations in glycosyltransferase expression, availability of glycosylation cofactors or substrates, changes in glycosyltransferase subcellular localization, and mutations in genes encoding glycosyltransferases have been observed in cancer samples. These changes contribute to the development of aberrant glycosylation signatures, which are associated with tumorigenesis, metastasis, and even treatment resistance [7, 8]. In cancer immunology, the N-glycosylation of PD-L1 is crucial for maintaining its stability and facilitating interactions, allowing breast cancer cells to progressively evade immune surveillance [9, 10]. Furthermore, glycosylation can impact the recognition of tumors by the immune system and trigger immunosuppressive responses by binding to glycan receptors, thus aiding cancer cells in evading immune surveillance [11, 12]. Nevertheless, there is currently a lack of comprehensive analysis regarding glycosyltransferase-related genes, prognosis, and phenotypes of the tumor microenvironment (TME) in BLCA. This study represents the first comprehensive linkage of glycosyltransferase-related genes to molecular subtypes in BLCA. Additionally, a novel risk score was developed and validated, enabling the prediction of prognosis and TME phenotypes in BLCA.
Materials and methods
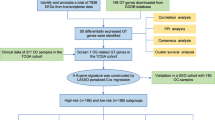
Data sources
We downloaded the TCGA-BLCA data from UCSC Xena (https://xenabrowser.net/), which includes Fragments Per Kilobase Million (FPKM), counts, and clinical data. The FPKM values were converted to Transcripts Per Kilobase Million (TPM) values. We excluded duplicate patients and those without matched RNA sequencing and survival data. In the end, we selected 411 tumor samples from TCGA-BLCA for further analysis. The Xiangya cohort (GSE188715) was established as detailed in our earlier studies [13, 14]. This cohort comprises tumor samples from 56 patients, accompanied by comprehensive survival outcomes. Detailed clinical characteristics of these patients are available in the supplementary material of our previous study [13]. Additionally, we utilized the ‘getGEO’ function from the ‘GEOquery’ R package to download the dataset GSE48075 from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). This dataset comprises tumor samples from 142 patients, but our study included only the 73 patients who had complete survival outcome data.
Unsupervised clustering
We gathered a total of 210 glycosyltransferase-related genes from the research conducted by Ashkani et al. [15]. To perform consensus clustering, we utilized the ‘ConsensusClusterPlus’ R package, repeating the process 1000 times. The parameters used were as follows: maxK = 4, reps = 1000, pItem = 0.8, distance=‘manhattan’, and clusterAlg=‘pam’.
Analysis of enriched pathways
Differential gene expression analysis was performed using the empirical Bayesian algorithm (‘limma’ R package). Gene Ontology (GO), HALLMARK, and Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets were obtained from MSigDB (http://www.gsea-msigdb.org/gsea/index.jsp) [16]. Subsequently, GSEA analysis was conducted using the ‘clusterProfiler’ R package. We collected a total of 25 signatures for our study. Among them, three signatures (including: IMmotion150_Teff, JAVELIN, and Tumor Inflammation Score) were related to immune response [17], one signature was conducted based on immune checkpoint inhibitors (ICIs) [18] and 21 signatures were associated with the effectiveness of immune checkpoint blockade (ICB) therapy [19]. Furthermore, we incorporated signatures related to the molecular subtypes of BLCA from Kamoun’s study [20]. To assess the enrichment of these signatures at the sample level, we utilized the single sample gene set enrichment analysis (ssGSEA) method, which was implemented using the ‘GSVA’ R package.
Visualization of the tumor immune microenvironment (TIME)
The anti-cancer immunity cycle is a conceptual model that outlines a seven-step process for activating immune cells against tumors. In our previous study, we obtained data on the levels of each step in this cycle from the tracking tumor immunophenotype (TIP) database (http://biocc.hrbmu.edu.cn/TIP/) [14]. Additionally, we calculated the relative abundances of 28 immune cell types in the TCGA-BLCA and Xiangya cohorts using the ssGSEA algorithm, which is based on gene sets reported in Charoentong’s study [21].
Development and validation of glycosyltransferase risk score
We employed the univariate Cox regression model to identify prognostic genes from 210 glycosyltransferase-related genes. Furthermore, the narrowed down prognostic genes were selected using the least absolute shrinkage and selector operation (LASSO) technique with ten-fold cross-validation. Then, the TCGA-BLCA cohort was randomly divided into a training set and a validation set in a 7:3 ratio. Afterwards, glycosyltransferase risk score was developed using the Cox proportional hazards regression algorithm, implemented with the ‘glmnet’ R package:
risk score = Σβі*RNAi.
We further validated the glycosyltransferase risk score algorithm in multiple datasets, including the TCGA validation set, the Xiangya cohort and GSE48075. Additionally, we investigated the correlation between the glycosyltransferase risk score and tumor grade and stage. To identify independent prognostic factors, we performed multivariate Cox analyses, considering variables such as age, gender, tumor grade, tumor stage, and glycosyltransferase risk score. Furthermore, we developed a comprehensive nomogram incorporating the clinicopathological factors with independent prognostic values. To assess its statistical performance, we validated the nomogram using clinical decision curves.
Evaluation of molecular subtypes in BLCA
The ‘ConsensusMIBC’ and ‘BLCAsubtyping’ R packages were employed to classify BLCA patients into distinct molecular subtypes. Subsequently, we categorized these subtypes into two main groups, namely ‘basal’ and ‘luminal’ subtypes, based on the consensus subtype [20].
Statistical analysis
If the continuous variables followed a normal distribution, the differences were compared using an unpaired t-test. Otherwise, the Wilcoxon rank-sum test was used. Categorical values were compared using the χ2 or Fisher’s exact test. The Kaplan-Meier method and log-rank tests, implemented using the ‘survminer’ R package, were used to generate survival curves and compare survival differences between two groups. Pearson correlation coefficients were calculated to perform correlation analyses. The predictive accuracy was assessed using time-dependent receiver operating characteristic (ROC) analysis, with the ‘timeROC’ function in the ‘tROC’ R package. A two-tailed p-value less than 0.05 was considered statistically significant, and all analyses were conducted using R 4.2.2.
Results
Glycosyltransferase-associated patterns in BLCA
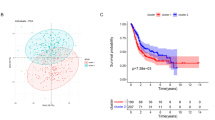
We performed unsupervised clustering analysis using a set of 210 glycosyltransferase-related genes. The analysis revealed distinct clustering patterns among TCGA-BLCA patients, resulting in the identification of two clusters termed glycosyltransferase cluster1 and cluster2 (Fig. 1A, Figure S1A-C). Notably, patients belonging to glycosyltransferase cluster1 exhibited significantly better overall survival outcomes compared to those in cluster2 (Fig. 1B). The GSEA analysis revealed that several pro-cancer pathways, including epithelial-mesenchymal transition and mTOR, were suppressed in glycosyltransferase cluster1 within the hallmark pathways (Figure S1D, Table S1), uncovering potential molecular mechanisms underlying the better prognosis in cluster1. In addition, we found that several inflammatory-related pathways, like interferon α and γ response and inflammatory response were also suppressed in glycosyltransferase cluster1 (Figure S1D, Table S1). We further compare the differences in GO and KEGG pathway enrichment between cluster1 and cluster2. As depicted in Fig. 1C and D, patients in glycosyltransferase cluster1 exhibited a downregulation of both GO and KEGG pathways associated with antigen presentation and cytokine and chemokine secretion. Additionally, patients in cluster1 displayed a noteworthy decrease in T or natural killer (NK) cell proliferation and activation pathways (Fig. 1D-E, Table S1). Further ssGSEA analysis revealed that most tumor-infiltrating lymphocytes (TILs), such as activated CD4 T cell, activated dendritic cell (DC), central memory CD8 T cell, and NK cell, were significantly decreased in glycosyltransferase cluster1 (Fig. 1F). These results support the notion that glycosyltransferase cluster1 represents a non-inflammatory TME phenotype, which is insensitive to ICB treatment, while cluster 2 represents an inflammatory phenotype, which may be sensitive to ICB treatment.
Exploring glycosyltransferase-related patterns in bladder cancer (BLCA). (A) Unsupervised clustering of 210 glycosyltransferase-associated genes yielded two distinct clusters. (B) Kaplan-Meier survival curves for the two identified glycosyltransferase-related patterns are displayed. The blue curve represents Cluster 1, while the red curve signifies Cluster 2. (C-E) Gene Set Enrichment Analysis (GSEA) was conducted to compare GO and KEGG pathways between Cluster 1 and Cluster 2 of glycosyltransferase-associated patterns. (F) Variations in immune cell infiltration into the Tumor Microenvironment (TME) are observed between the two glycosyltransferase-related patterns. Cluster 1 is indicated in red, and Cluster 2 in blue. *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001. ns, not significant
Development and internal validation of glycosyltransferase risk score based on the TCGA-BLCA cohort
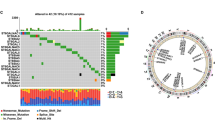
We conducted a comprehensive analysis by performing univariable Cox regression analysis on a panel of 210 glycosyltransferase-related genes. Through this analysis, we identified 56 genes that demonstrated significant prognostic value (Table S2). To further refine our gene selection, we employed LASSO and ten-fold cross-validation techniques (Fig. 2A-B). Ultimately, we identified 19 genes including ALG11, ALG3, ALG8, B3GALT2, B3GNT3, B4GALNT2, CERCAM, DAD1, DPAGT1, EXTL2, ENGASE, MAN2A1, OGT, PIGV, ST3GAL5, ST6GALNAC3, UGT2B11, UGT2B4 and UGT2B7 to construct a risk score. As shown in Figure S2, the univariable prognostic values of these genes indicate that only the expression levels of UGT2B4, UGT2B11, ENGASE, OGT, ST3GAL5, B3GNT3, and UGT2B7 were positively related to the survival outcomes of BLCA. We observed that patients with higher risk scores displayed significantly poorer survival outcomes in the TCGA-BLCA train cohort (Fig. 2C). Importantly, our risk score demonstrated satisfactory predictive accuracy, with an area under the curve (AUC) around 0.75 (Fig. 2D). Also, these results could be well validated in TCGA-BLCA test cohort (Fig. 2E), with AUC reaching 0.80 (Fig. 2F). Consistent with the prognostic significance of the glycosyltransferase risk score, we observed a significant increase in the risk score among patients with higher tumor grade and stage (Fig. 2G-H).
Development and internal validation of a glycosyltransferase-based risk score. (A-B) LASSO regression was applied to 56 genes with prognostic significance to construct the risk score model. (C-D) In the TCGA-BLCA training cohort, Kaplan-Meier survival analysis (C) and time-dependent Receiver Operating Characteristic (ROC) curves (D) were used to evaluate the predictive accuracy of the risk score. (E-F) Kaplan-Meier survival analysis (E) and time-dependent ROC curves (F) were conducted in the TCGA-BLCA testing cohort to validate the risk score model. (G-H) Boxplots illustrate the distribution of risk scores across different tumor grades (G) and stages (H) within the entire TCGA-BLCA cohort
Relationship between the glycosyltransferase risk score and clinicopathological characteristics
We conducted a multivariate Cox analysis and found that the glycosyltransferase risk score, age, and tumor stage were independent prognostic factors (Fig. 3A). These findings suggest that the glycosyltransferase risk score has the potential to serve as a reliable predictive marker for the prognosis of BLCA patients. To facilitate the clinical application of the glycosyltransferase risk score, we developed a comprehensive nomogram that incorporates the glycosyltransferase risk score and clinicopathological characteristics with independent prognostic value, including age and tumor stage (Fig. 3B). The nomogram demonstrated good predictive accuracy for 1-, 3-, and 5-year overall survival rates, with AUC values of 0.755, 0.768, and 0.7662, respectively (Fig. 3C). Furthermore, the calibration curves (Fig. 3D) showed a high level of agreement between the predicted and actual overall survival rates, underscoring the clinical significance of this integrated nomogram.
Development of a comprehensive nomogram incorporating glycosyltransferase risk score and additional clinicopathological parameters. (A) Results from multivariable regression analysis, evaluating the impact of the risk score alongside other clinicopathological factors. (B) The nomogram was formulated by amalgamating the risk score with age and tumor stage variables. (C) Receiver Operating Characteristic (ROC) curves representing the predictive accuracy of our nomogram at 12, 36, and 60-month intervals. (D) Calibration curves validating the performance of our nomogram over 12, 36, and 60-month time frames
External validation of the glycosyltransferase risk score
We further investigated the prognostic value of the glycosyltransferase risk score in the Xiangya cohort and GSE48075 dataset. Consistent with our expectations, patients in the high-risk score group exhibited worse overall survival (OS) compared to those in the low-risk score group in the Xiangya cohort (Fig. 4A). The glycosyltransferase risk score demonstrated moderate predictive accuracy for 1-, 3-, and 5-year OS, with AUC values of 0.63, 0.65, and 0.67, respectively (Fig. 4B). Similarly, in the GSE48075 dataset, a higher risk score was associated with poorer OS (Fig. 4C). The predictive accuracy for 1-, 3-, and 5-year OS in this dataset were 0.66, 0.60, and 0.63, respectively (Fig. 4D). These findings further support the potential of the glycosyltransferase risk score as a prognostic indicator in BLCA.
External validation of the glycosyltransferase risk score across different cohorts. (A-B) In the Xiangya cohort, Kaplan-Meier survival analysis (A) and time-dependent Receiver Operating Characteristic (ROC) curves for the risk score (B) are presented. (C-D) For the GSE48075 cohort, Kaplan-Meier survival analysis (C) and time-dependent ROC curves evaluating the risk score (D) are depicted
The significance of evaluating immune cell infiltration in individual patients for glycosyltransferase risk score assessment
As anticipated, there was a significant positive correlation observed between the glycosyltransferase risk score and various cancer immune cycles in both the TCGA-BLCA and Xiangya cohorts. These immune cycles included the release of cancer antigens, recruitment of T cells, recruitment of CD8 T cells, and recruitment of macrophages (Fig. 5A, Table S3). Furthermore, the infiltration of tumor-infiltrating lymphocytes (TILs), as calculated using the ssGSEA algorithm, showed a generally positive correlation with the risk score in both the TCGA-BLCA cohort (Fig. 5B, Table S3) and the Xiangya cohort (Fig. 5C, Table S3). This correlation was observed for various immune cell types, such as NK T cells, central memory CD8 T cells, type 1 T helper (Th1) cells, and activated CD8 T cells. Consistent with our previous studies, we found that the effector genes of CD8 T cells, DCs, macrophages, NK cells, and Th1 cells were upregulated in the group with a higher glycosyltransferase risk score (Fig. 5D) [14]. These results indicate that patients with higher risk scores exhibit an inflammatory phenotype, which may make them more sensitive to ICI therapy. Consistently, our risk score showed significant positive correlations with the IMmotion150 Teff signature (Fig. 5E, R = 0.22, p < 0.001), JAVELIN signature (Fig. 5F, R = 0.17, p < 0.001), TIS (Fig. 5G, R = 0.23, p < 0.001), and ICI signature (Fig. 5H, R = 0.24, p < 0.001), all of which are associated with a higher immune therapy response [17, 18]. Furthermore, all 21 pathways associated with ICI treatment efficacy were significantly upregulated in the high-risk score group (Fig. 5I) [19].
Assessing the interplay between glycosyltransferase risk score and tumor microenvironment (TME) phenotypes. (A) Examines the relationship between glycosyltransferase risk scores and cancer immunity cycles in both the TCGA-BLCA and Xiangya cohorts. (B-C) Investigate the correlation of glycosyltransferase risk scores with immune cell infiltration in the TCGA-BLCA (B) and Xiangya (C) cohorts. (D) Presents a heatmap illustrating the impact of genes on CD8+ T cells, dendritic cells (DCs), macrophages, natural killer (NK) cells, and type 1 T helper (Th1) cells among patients with varying glycosyltransferase risk scores. (E-H) Explore the associations between glycosyltransferase risk scores and various immune signatures, including IMmotion 150 Teff (E), JAVELIN (F), Tumor Inflammation (G), and Immune Checkpoint Inhibitor (H) signatures. (I) Highlights the enrichment of immunotherapy-related pathways among patients with high and low glycosyltransferase risk scores. *P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001. ns, not significant
Glycosyltransferase risk score for predicting molecular subtypes of BLCA
For BLCA, different molecular subtypes have been identified with varying response rates to immunotherapy treatment. The basal subtypes, for example, have shown poorer survival outcomes but higher response rates to immunotherapy compared to the luminal subtypes [20]. Consistent with previous findings, patients with a high-risk score were found to belong to the basal subtype, which was characterized by basal differentiation, EMT differentiation, and immune differentiation pathways. On the other hand, patients with a low-risk score belonged to the luminal subtype, which was enriched for urothelial differentiation and the Ta pathway in the TCGA-BLCA cohort (Fig. 6A). Additionally, the risk score demonstrated excellent predictive accuracy for distinguishing basal and luminal subtypes, with all AUC values reaching 0.75 (Fig. 6B). These findings were further validated in the Xiangya cohort (Fig. 6C-D). Overall, these results indicate that our risk score has the potential to predict TILs infiltration and immunotherapy efficacy in terms of molecular subtypes of BLCA.
Assessing the interplay between glycosyltransferase risk score and molecular subtypes of BLCA. (A-B) In TCGA-BLCA, the distribution of molecular subtypes and bladder cancer-related pathways among patients with high and low glycosyltransferase risk score (A), and the ROC curves of glycosyltransferase risk score for predicting molecular subtypes (B). (C-D) In Xiangya cohort, the distribution of molecular subtypes and bladder cancer-related pathways among patients with high and low glycosyltransferase risk score (C), and the ROC curves of glycosyltransferase risk score for predicting molecular subtypes (D)
Discussion
BLCA is the most common type of carcinoma worldwide, posing a significant threat to both human health and the economy. Non-muscle invasive bladder cancer (NMIBC) is characterized by a high recurrence rate and the potential for progression, requiring long-term invasive surveillance. In contrast, muscle-invasive bladder cancer (MIBC) has a high potential for metastasis, leading to a higher disease-specific mortality rate [22]. Additionally, approximately 20% of NMIBC cases will eventually develop into MIBC, which is associated with even worse disease outcomes and poorer response to neoadjuvant chemotherapy (NAC) compared to primary MIBC cases [3, 23, 24]. Due to the high tumor mutational burden (TMB) observed in BLCA, immune checkpoint inhibitors (ICIs) have shown durable efficacy in a subset of patients. In fact, the US Food and Drug Administration (FDA) has already approved five ICIs for the treatment of BLCA [3]. However, it is important to note that not all patients exhibited a positive response to ICIs treatment. This highlights the pressing need to identify biomarkers that can accurately predict the efficacy of ICIs.
Immunotherapy resistance is a complex phenomenon that is influenced by genetic and epigenetic mechanisms. These mechanisms play a crucial role in the interaction between cancer cells and the immune cells in TME. In tumors that are immunologically ‘cold’ or non-inflamed, there is a lack or limited presence of TILs. This can be attributed to factors such as low tumor mutational burden (TMB), impaired antigen presentation, or physical barriers that hinder the migration of lymphocytes. Consequently, these tumors show minimal response to immunotherapy. On the other hand, immunologically ‘hot’ or inflamed tumors typically exhibit high TMB, indicating a greater number of neoantigens, as well as increased expression of programmed cell death ligand 1 (PD-L1). As a result, these tumors tend to have a higher presence of TILs and are more responsive to immunotherapy [25]. Consequently, the transformation of cold tumors into hot tumors or the ability to distinguish between them is crucial in enhancing the effectiveness of ICI [26]. In this study, we developed and validated a novel glycosyltransferase risk score. We found that there was a positive correlation between this risk score and TILs, suggesting that patients with a higher risk score exhibited an inflamed TME phenotype and were more responsive to immunotherapy. This is vital for precision medicine in BLCA.
Glycosylation, a widespread modification of proteins and lipids, plays a crucial role in controlling cellular and molecular interactions [4]. In the context of cancer, glycosylation undergoes significant alterations, as evidenced by numerous clinical and experimental studies. These studies provide substantial evidence that specific carbohydrate structures play a crucial role in determining the malignancy of cancer [5, 6]. Tan et al. revealed the upregulation of the glycosyltransferase C1GALT1, which promotes migration and proliferation, and provided preliminary data on the mechanism of dysregulated C1GALT1 through the miR-1-3p/cHP1BP3 axis, uncovering abnormal glycosylation phenomena in BLCA [27]. Kvorjak et al. found that M2-like macrophages could induce the upregulation of ST6GALNAC1, a glycosyltransferase responsible for adding sialic acid to O-linked GalNAc residues. This enzymatic activity promotes the formation of tumor-associated sialyl-Tn O-glycans, thereby contributing to the pathogenesis of ulcerative colitis and colitis-associated colon cancer [28]. Also, it was reported that the upregulated expression and altered glycosylation of PD-L2 in tumors play a significant role in the development of resistance to cetuximab therapy. Consequently, combining PD-L2 glycosylation inhibition with cetuximab represents a promising therapeutic approach for the treatment of cancer [29]. However, most of these studies have focused on individual glycosyltransferase related genes. To the best of our knowledge, this study is the first to systematically associate 210 glycosyltransferase related genes with the prognosis of bladder cancer and their correlation with the TME.
Qi et al. identified a glycosyltransferase-related signature for glioma survival and tumor-associated macrophage (TAM) phenotype prediction based on TCGA and CGGA cohorts. They observed that patients with higher risk scores had poorer survival outcomes and increased M2 TAM infiltration [30]. Similarly, Wu et al. established a risk score based on six glycosyltransferase-related genes for prognosis and immune cell infiltration in uterine corpus endometrial carcinoma [31]. Another study developed and validated a risk score based on five glycosyltransferase-related genes, linking it systematically with the TME in head and neck squamous cell carcinoma [32]. In lung adenocarcinoma, Bian et al. created a glycosyltransferase-related lncRNA signature for prognosis, though it lacked external validation [33]. Our study presents the first glycosyltransferase-related signature for BLCA, thoroughly validated and systematically associated with immune cell infiltration. We incorporated the expression of ALG11, ALG3, ALG8, B3GALT2, B3GNT3, B4GALNT2, CERCAM, DAD1, DPAGT1, EXTL2, ENGASE, MAN2A1, OGT, PIGV, ST3GAL5, ST6GALNAC3, UGT2B11, UGT2B4, and UGT2B7 into our risk score. Many of these genes play vital roles in cancer development. For instance, Sun et al. reported that ALG3 could promote cancer stemness and radioresistance by contributing to the glycosylation of TGFBR2 in breast cancer [34]. ALG3 also acts as an oncogene in ovarian cancer, lung cancer, liver cancer, and other types [35,36,37]. Li et al. found that B3GNT3 plays a key role in breast cancer and cancer immunity, and targeting B3GNT3 could enhance CD8+ T cell-mediated anti-tumor immunity [38]. Additionally, recent studies suggest that targeting the DAD1 gene in intravesical therapy could serve as a novel treatment for BLCA [39]. Other genes, including DPAGT1, MAN2A1, OGT, ST3GAL5, and UGT2B7, have been found to play crucial roles in various cancers, such as breast cancer, oral cancer, liver cancer, ovarian cancer, and colorectal cancer [40,41,42,43,44,45,46].
There are certain limitations to our study that should be acknowledged. Firstly, our findings were all derived from retrospective data, and it is crucial to conduct further validation through prospective studies. Secondly, the prognostic power of our risk score was moderate in external validation. To enhance its accuracy, future studies will require larger and more diverse cohorts. Thirdly, while we have successfully validated the significance of the glycosyltransferase risk score in the TME and molecular subtypes, the underlying mechanisms involved require additional investigation both in vitro and in vivo.
Conclusion
Glycosyltransferase patterns exhibit distinct TME phenotypes in BLCA. Our comprehensive risk score provides a promising approach for prognostic prediction and assessment of immunotherapy efficacy, offering valuable guidance for precision medicine.
Data availability
TCGA-BLCA: https://portal.gdc.cancer.gov/; GEO database: https://www.ncbi.nlm.nih.gov/geo/.
Abbreviations
- BLCA:
-
Bladder carcinoma
- TME:
-
Tumor microenvironment
- ICB:
-
Immune checkpoint blockade
- FPKM:
-
Fragments Per Kilobase Million
- TPM:
-
Transcripts Per Kilobase Million
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- ICI:
-
Immune checkpoint inhibitor
- ssGSEA:
-
Single sample gene set enrichment analysis
- TIME:
-
Tumor immune microenvironment
- TIP:
-
Tracking tumor immunophenotype
- LASSO:
-
Least absolute shrinkage and selector operation
- ROC:
-
Receiver operating characteristic
- NK:
-
Natural killer
- TIL:
-
Tumor-infiltrating lymphocyte
- DC:
-
Dendritic cell
- AUC:
-
Area under the curve
- OS:
-
Overall survival
- Th1:
-
Type 1 T helper
- NMIBC:
-
Non-muscle invasive bladder cancer
- MIBC:
-
Muscle invasive bladder cancer
- NAC:
-
Neoadjuvant chemotherapy
- TMB:
-
Tumor mutational burden
- FDA:
-
Food and Drug Administration
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Wu Z, Liu J, Dai R, Wu S. Current status and future perspectives of immunotherapy in bladder cancer treatment. Sci China Life Sci. 2021;64(4):512–33.
Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. Cancer J Clin. 2020;70(5):404–23.
Varki A. Biological roles of glycans. Glycobiology. 2017;27(1):3–49.
Dall’Olio F, Malagolini N, Trinchera M, Chiricolo M. Mechanisms of cancer-associated glycosylation changes. Front Bioscience (Landmark Edition). 2012;17(2):670–99.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15(9):540–55.
Fernández LP, Sánchez-Martínez R, Vargas T, Herranz J, Martín-Hernández R, Mendiola M, Hardisson D, Reglero G, Feliu J, Redondo A, et al. The role of glycosyltransferase enzyme GCNT3 in colon and ovarian cancer prognosis and chemoresistance. Sci Rep. 2018;8(1):8485.
Fernández-Ponce C, Geribaldi-Doldán N, Sánchez-Gomar I, Quiroz RN, Ibarra LA, Escorcia LG, Fernández-Cisnal R, Martinez GA, García-Cózar F, Quiroz EN. The role of glycosyltransferases in Colorectal Cancer. Int J Mol Sci 2021, 22(11).
Li CW, Lim SO, Xia W, Lee HH, Chan LC, Kuo CW, Khoo KH, Chang SS, Cha JH, Kim T, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity. Nat Commun. 2016;7:12632.
Lu RM, Hwang YC, Liu IJ, Lee CC, Tsai HZ, Li HJ, Wu HC. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1):1.
Cadena AP, Cushman TR, Welsh JW. Glycosylation and Antitumor Immunity. Int Rev cell Mol Biology. 2019;343:111–27.
Läubli H, Borsig L. Altered cell adhesion and glycosylation promote Cancer Immune suppression and metastasis. Front Immunol. 2019;10:2120.
Li H, Liu S, Li C, Xiao Z, Hu J, Zhao C. TNF family-based signature predicts prognosis, Tumor Microenvironment, and molecular subtypes in bladder carcinoma. Front cell Dev Biology. 2021;9:800967.
Li H, Chen J, Li Z, Chen M, Ou Z, Mo M, Wang R, Tong S, Liu P, Cai Z et al. S100A5 Attenuates Efficiency of Anti-PD-L1/PD-1 Immunotherapy by Inhibiting CD8(+) T Cell-Mediated Anti-Cancer Immunity in Bladder Carcinoma. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2023:e2300110.
Ashkani J, Naidoo KJ. Glycosyltransferase gene expression profiles classify Cancer types and propose prognostic subtypes. Sci Rep. 2016;6:26451.
Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular signatures database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1(6):417–25.
Braun DA, Hou Y, Bakouny Z, Ficial M, Sant’ Angelo M, Forman J, Ross-Macdonald P, Berger AC, Jegede OA, Elagina L, et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat Med. 2020;26(6):909–18.
Auslander N, Zhang G, Lee JS, Frederick DT, Miao B, Moll T, Tian T, Wei Z, Madan S, Sullivan RJ, et al. Robust prediction of response to immune checkpoint blockade therapy in metastatic melanoma. Nat Med. 2018;24(10):1545–9.
Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–8.
Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, Hoadley KA, Groeneveld CS, Al-Ahmadie H, Choi W, et al. A Consensus Molecular classification of muscle-invasive bladder Cancer. Eur Urol. 2020;77(4):420–33.
Charoentong P, Finotello F, Angelova M, Mayer C, Efremova M, Rieder D, Hackl H, Trajanoski Z. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–62.
Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: a review. JAMA. 2020;324(19):1980–91.
Ge P, Wang L, Lu M, Mao L, Li W, Wen R, Lin J, Wang J, Chen J. Oncological outcome of primary and secondary muscle-invasive bladder Cancer: a systematic review and Meta-analysis. Sci Rep. 2018;8(1):7543.
Pietzak EJ, Zabor EC, Bagrodia A, Armenia J, Hu W, Zehir A, Funt S, Audenet F, Barron D, Maamouri N, et al. Genomic differences between primary and secondary muscle-invasive bladder Cancer as a basis for disparate outcomes to cisplatin-based Neoadjuvant Chemotherapy. Eur Urol. 2019;75(2):231–9.
Indini A, Massi D, Pirro M, Roila F, Grossi F, Sahebkar A, Glodde N, Bald T, Mandalà M. Targeting inflamed and non-inflamed melanomas: biological background and clinical challenges. Sem Cancer Biol. 2022;86(Pt 2):477–90.
Duan Q, Zhang H, Zheng J, Zhang L. Turning Cold into Hot: firing up the Tumor Microenvironment. Trends cancer. 2020;6(7):605–18.
Tan Z, Jiang Y, Liang L, Wu J, Cao L, Zhou X, Song Z, Ye Z, Zhao Z, Feng H, et al. Dysregulation and prometastatic function of glycosyltransferase C1GALT1 modulated by cHP1BP3/ mir-1-3p axis in bladder cancer. J Experimental Clin cancer Research: CR. 2022;41(1):228.
Kvorjak M, Ahmed Y, Miller ML, Sriram R, Coronnello C, Hashash JG, Hartman DJ, Telmer CA, Miskov-Zivanov N, Finn OJ, et al. Cross-talk between Colon Cells and macrophages increases ST6GALNAC1 and MUC1-sTn expression in Ulcerative Colitis and Colitis-Associated Colon cancer. Cancer Immunol Res. 2020;8(2):167–78.
Xu Y, Gao Z, Hu R, Wang Y, Wang Y, Su Z, Zhang X, Yang J, Mei M, Ren Y et al. PD-L2 glycosylation promotes immune evasion and predicts anti-EGFR efficacy. J Immunother Cancer 2021, 9(10).
Qi Y, Lv W, Liu X, Wang Q, Xing B, Jiang Q, Wang Z, Huang Y, Shu K, Lei T. Comprehensive Analysis Identified Glycosyltransferase Signature to Predict Glioma Prognosis and TAM Phenotype. BioMed research international 2023;2023:6082635.
Wu J, Zhou X, Ren J, Zhang Z, Ju H, Diao X, Jiang S, Zhang J. Glycosyltransferase-related prognostic and diagnostic biomarkers of uterine corpus endometrial carcinoma. Comput Biol Med. 2023;163:107164.
Wu H, Zhao X, Zhu T, Rong D, Wang Y, Leng D, Wu D. A glycosyltransferase-related signature for Predicting overall survival in Head and Neck squamous cell carcinoma. Front Genet. 2022;13:856671.
Bian C, Sun X, Huang J, Zhang W, Mu G, Wei K, Chen L, Xia Y, Wang J. A novel glycosyltransferase-related lncRNA signature correlates with lung adenocarcinoma prognosis. Front Oncol. 2022;12:950783.
Sun X, He Z, Guo L, Wang C, Lin C, Ye L, Wang X, Li Y, Yang M, Liu S, et al. ALG3 contributes to stemness and radioresistance through regulating glycosylation of TGF-β receptor II in breast cancer. J Experimental Clin cancer Research: CR. 2021;40(1):149.
Cui X, Pei X, Wang H, Feng P, Qin H, Liu S, Yan Q, Liu J. ALG3 Promotes Peritoneal Metastasis of Ovarian Cancer through Increasing Interaction of α1,3-mannosylated uPAR and ADAM8. Cells 2022;11(19).
Ke SB, Qiu H, Chen JM, Shi W, Han C, Gong Y, Chen YS. ALG3 contributes to the malignancy of non-small cell lung cancer and is negatively regulated by MiR-98-5p. Pathol Res Pract. 2020;216(3):152761.
Zhao Z, Zheng Z, Huang J, Wang J, Peng T, Lin Y, Jian Z. Expression of ALG3 in Hepatocellular Carcinoma and its clinical implication. Front Mol Biosci. 2022;9:816102.
Li CW, Lim SO, Chung EM, Kim YS, Park AH, Yao J, Cha JH, Xia W, Chan LC, Kim T, et al. Eradication of Triple-negative breast Cancer cells by targeting glycosylated PD-L1. Cancer Cell. 2018;33(2):187–e201110.
Tang D, Yan Y, Li Y, Li Y, Tian J, Yang L, Ding H, Bashir G, Zhou H, Ding Q, et al. Targeting DAD1 gene with CRISPR-Cas9 system transmucosally delivered by fluorinated polylysine nanoparticles for bladder cancer intravesical gene therapy. Theranostics. 2024;14(1):203–19.
Yang M, Li Y, Kong L, Huang S, He L, Liu P, Mo S, Lu X, Lin X, Xiao Y et al. Inhibition of DPAGT1 suppresses HER2 shedding and trastuzumab resistance in human breast cancer. J Clin Investig 2023, 133(14).
Nita-Lazar M, Noonan V, Rebustini I, Walker J, Menko AS, Kukuruzinska MA. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009;69(14):5673–80.
Chen ZH, Yu YP, Tao J, Liu S, Tseng G, Nalesnik M, Hamilton R, Bhargava R, Nelson JB, Pennathur A, et al. MAN2A1-FER Fusion Gene is expressed by Human Liver and other Tumor types and has oncogenic activity in mice. Gastroenterology. 2017;153(4):1120–e11321115.
Shi Q, Shen Q, Liu Y, Shi Y, Huang W, Wang X, Li Z, Chai Y, Wang H, Hu X, et al. Increased glucose metabolism in TAMs fuels O-GlcNAcylation of lysosomal cathepsin B to promote cancer metastasis and chemoresistance. Cancer Cell. 2022;40(10):1207–e12221210.
Zhou F, Yang X, Zhao H, Liu Y, Feng Y, An R, Lv X, Li J, Chen B. Down-regulation of OGT promotes cisplatin resistance by inducing autophagy in ovarian cancer. Theranostics. 2018;8(19):5200–12.
van der Haar Àvila I, Zhang T, Lorrain V, de Bruin F, Spreij T, Nakayama H, Iwabuchi K, García-Vallejo JJ, Wuhrer M, van Kooyk Y et al. Limited impact of cancer-derived gangliosides on anti-tumor immunity in colorectal cancer. Glycobiology 2024, 34(7).
Shen ML, Xiao A, Yin SJ, Wang P, Lin XQ, Yu CB, He GH. Associations between UGT2B7 polymorphisms and cancer susceptibility: a meta-analysis. Gene. 2019;706:115–23.
Acknowledgements
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (No.82202866) and Natural Science Foundation of Hunan Province (2023JJ40991).
Funding
This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (No.82202866) and Natural Science Foundation of Hunan Province (2023JJ40991).
Author information
Authors and Affiliations
Contributions
LRY, YT, HJY, XZC, LJH performed analyses and drafted the manuscript. LRY, YT, and LZH searched and downloaded the original datasets from TCGA and GEO. LRY, YT, HJY and TSY contributed to statistical analyses. LRY, HJY, LZH and TSY edited the pictures. LZH and TSY conceived and supervised the study. All authors contributed to writing the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Xiangya Hospital of Central South University. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
: Figure S1. (A-C) Unsupervised clustering of 210 glycosyltransferase related genes in the TCGA-BLCA cohort. (D) Gene Set Enrichment Analysis (GSEA) was conducted to compare Hallmark pathways between Cluster 1 and Cluster 2 of glycosyltransferase-associated patterns.
Supplementary Material 2
: Figure S2. Univariate results of selected genes for constructing risk score.
Supplementary Material 3
: Table S1. Gene Set Enrichment Analysis (GSEA) was conducted to compare GO, KEGG and Hallmark pathways between Cluster 1 and Cluster 2 of glycosyltransferase-associated patterns.
Supplementary Material 4
: Table S2. Results of univariate cox regression analysis.
Supplementary Material 5
: Table S3. The correlation of glycosyltransferase risk scores with cancer immune cycles and immune cell infiltration in the TCGA-BLCA and Xiangya cohorts respectively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it.The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, R., Yang, T., Huang, J. et al. Results from a real-world study: a novel glycosyltransferase risk score for prognosis, tumor microenvironment phenotypes and immunotherapy in bladder cancer. BMC Cancer 24, 947 (2024). https://doi.org/10.1186/s12885-024-12712-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12712-w