Abstract
Background
Previous research has found associations between various non-genetic factors and breast cancer (BrCa) risk. This study summarises and appraises the credibility of the available evidence on the association between non-genetic factors and BrCa risk.
Methods
We conducted an umbrella review of meta-analyses. Medline, Scopus, and the Cochrane databases were systematically searched for meta-analyses examining non-genetic factors and BrCa incidence or mortality. The strength of the evidence was graded in four categories (i.e., weak, suggestive, highly suggestive, convincing).
Results
A total of 781 meta-analyses from 280 publications were evaluated and graded. We included exposures related to anthropometric measurements, biomarkers, breast characteristics and diseases, diet and supplements, environment, exogenous hormones, lifestyle and social factors, medical history, medication, reproductive history, and pregnancy. The largest number of examined associations was found for the category of diet and supplements and for exposures such as aspirin use and active smoking. The statistically significant (P-value < 0.05) meta-analyses were 382 (49%), of which 204 (53.4%) reported factors associated with increased BrCa risk. Most of the statistically significant evidence (n = 224, 58.6%) was graded as weak. Convincing harmful associations with heightened BrCa risk were found for increased body mass index (BMI), BMI and weight gain in postmenopausal women, oral contraceptive use in premenopausal women, increased androstenedione, estradiol, estrone, and testosterone concentrations, high Breast Imaging Reporting and Data System (BIRADS) classification, and increased breast density. Convincing protective factors associated with lower BrCa risk included high fiber intake and high sex hormone binding globulin (SHBG) levels while highly suggestive protective factors included high 25 hydroxy vitamin D [25(OH)D] levels, adherence to healthy lifestyle, and moderate-vigorous physical activity.
Conclusions
Our findings suggest some highly modifiable factors that protect from BrCa. Interestingly, while diet was the most studied exposure category, the related associations failed to reach higher levels of evidence, indicating the methodological limitations in the field. To improve the validity of these associations, future research should utilise more robust study designs and better exposure assessment techniques. Overall, our study provides knowledge that supports the development of evidence-based BrCa prevention recommendations and guidance, both at an individual level and for public health initiatives.
Trial registration
PROSPERO CRD42022370675.
Similar content being viewed by others
Background
Breast cancer (BrCa) is the most commonly diagnosed cancer worldwide, with an estimated 2.3 million cases and 685,000 deaths in 2020 [1]. Incidence and death rates of female BrCa remain high in developed countries [1] and rapidly increase in transitioning ones (countries with lower Human Development Index). The latter could be attributed to the fact that countries with growing economies have been experiencing significant changes of lifestyle and sociocultural patterns, which, along with the increasing involvement of women in the industrial workforce, have resulted in changes of the prevalence of BrCa risk factors [1, 2].
Approximately 10% of all female BrCa cases are familial and linked to specific highly penetrant gene mutations (e.g., BRCA1, BRCA2) [3]. However, the highest proportion of cases are attributed to both low penetrant genetic and non-genetic factors [3]. For example, menopausal status is an important non-genetic factor that determines BrCa risk [4]. Variations in premenopausal and postmenopausal BrCa incidence and mortality across different countries are associated with income differences as well as with the differential distribution of distinct molecular features and risk factors in each of the two menopausal statuses [4]. In addition, BrCa is classified into molecular subtypes based on whether BrCa cells grow in response to female hormones (i.e., estrogen, progesterone) or growth factors [5]. Stratification of women based on non-genetic risk factors for BrCa is of paramount importance for developing more effective risk reduction strategies as well as for targeted risk- stratified BrCa screening programmes [6].
There is a large number of systematic reviews and meta-analyses on non-genetic factors (including obesity, hormone levels, alcohol consumption, and smoking) and their association with BrCa risk and mortality [7,8,9,10]. However, the results are often contradictory and subject to biases. A few umbrella reviews (i.e., reviews of systematic reviews and meta-analyses), which examined certain types of non-genetic exposures, also included BrCa as one of the studied outcomes [11,12,13,14,15,16]. However, to our knowledge, there has been no systematic effort to summarise and evaluate the robustness of evidence on non-genetic risk factors for BrCa.
Therefore, in view of the large, and often contradictory, amount of published evidence on non-genetic risk factors for BrCa incidence and mortality, we aimed to summarise and evaluate the findings of systematic reviews and meta-analyses in this field, following an umbrella review methodology. The added value of the present umbrella review is that it offers a comprehensive and deep understanding of the aetiology of BrCa by integrating findings from various systematic reviews and meta-analyses, thereby providing a thorough and reliable assessment of the evidence regarding non-genetic factors and risk of BrCa.
Methods
A standardised methodology based on a predefined internal protocol was registered in PROSPERO (CRD42022370675). The findings are reported according to the PRIOR [17] (Preferred Reporting Items for Overviews of Reviews) recommendations (Additional file 1 – PRIOR Checklist).
Search strategy
We identified relevant systematic reviews and meta-analyses investigating the association of any non-genetic factor and BrCa incidence and/or mortality. We searched Medline (via PubMed), Scopus, and the Cochrane database for systematic reviews from inception to October 31st, 2022. The following search algorithm was used: ((Breast OR mammary) AND (cancer* OR neoplasm* OR malignant* OR tumour* OR tumor* OR carcinoma* OR adenocarcinoma*)) AND (meta-analysis OR "systematic review" OR systematic review). The full strategy can be found in the supplement (Additional file 1 – Search strategy).
Eligibility criteria
We included systematic reviews and meta-analyses published in English that studied the association of any non-genetic exposure with female BrCa incidence or mortality due to BrCa as the primary cause of death (when mortality was reported as proxy for incidence in primary studies) among healthy individuals at risk for BrCa. Studies involving women with pre-existing breast cancer investigating survival outcomes following cancer diagnosis were excluded. There were no restrictions depending on publication status such as preprints. However, certain types of publication (e.g., books, commentary, letters) were not evaluated as they were considered unlikely to provide sufficient data for inclusion in our analysis. We only included papers that had performed a systematic literature search; meta-analysis papers without a systematic search of the literature were excluded. We considered meta-analyses if they included at least two independent primary studies. Sub-analyses in a meta-analysis that included only one study were excluded. Finally, we excluded any (otherwise eligible) publications when they did not provide effect estimates and their corresponding confidence intervals (CIs) or some other measure, such as standard errors or P-values, for the individual studies in the meta-analyses, or enough data to reproduce them. Systematic reviews focusing on the association between genes or genetic markers and BrCa risk or on the survival of BrCa cases were not considered. The exclusion criteria are presented in the supplement (Additional file 1 – Exclusion criteria).
Title, abstract, and full text screening was performed in duplicate by 9 authors (AP, AG, AH, ME, KL, EK, CK, MC, MT). Conflicts were resolved by discussion with other team members (AY, KP, GM, GKN) until consensus was reached. In case there were multiple overlapping meta-analyses, we chose only one for our umbrella review, based on the following algorithm: First, we selected the most recent systematic review and meta-analysis. If another meta-analysis had been conducted within 5 years from the date of publication of the most recent one, we chose the one with the largest number of individual studies and largest number of participants, and the most comprehensive one (i.e., the one evaluating the largest number of different comparisons for the risk factor in question). Quality was assessed using the AMSTAR tool [18], which also served as an additional selection criterion if the preceding criteria were comparable.
Data extraction
Data extraction was performed by 7 authors (AP, CK, EK, KL, KP, MC, MT) using a predefined extraction form in Excel. The validity of data extraction was evaluated by another 4 independent authors (AG, AH, GM, ME). The extracted information from each eligible publication included the first author’s last name, year of publication, BrCa types with respect to hormone receptors and human epidermal growth factor receptor 2 (HER2), BrCa stage, examined risk factors, number of studies and estimates included in meta-analyses, characteristics of the study populations (e.g., origin, menopausal status, other characteristics), meta-analysis metric (odds ratio, risk ratio, hazard ratio, etc.; if the meta-analysis metric was not clear from the original publication we used the summary metric as reported in the meta-analysis), meta-analysis method (fixed- or random-effects), summary effect estimates and 95% CIs, and the level of control for potential confounders performed in the studies included in the meta-analysis (adjusted, not adjusted). Our umbrella review described in detail and graded only meta-analyses that were based on studies with adjusted estimates. Meta-analyses of primary studies with crude summary effect estimates (either in totality, dubbed as unadjusted, or partially, dubbed as mixed) are included in the Additional files 1 and 2 to allow for a comprehensive review of the non-genetic risks examined in the literature. However, they were not graded to avoid grading associations at high risk of bias. Within each of the studied associations we extracted data on the first author of the primary study included in the meta-analysis along with the year of publication, study design, and effect estimate with corresponding 95% CI (or any other measure of variation of the effect estimate reported), number of cases and population size (in cohort studies) or the numbers of cases and controls (in case–control studies).
Statistical analysis
This study adopted the methodological approach used in umbrella reviews [19, 20]. Briefly, for each association included in this umbrella review we calculated the summary effect estimate and the corresponding 95% CI using the inverse variance weighted random-effects model [21] due to the expected clinical and methodological heterogeneity across primary studies included in the meta-analytical associations and for consistency in the application of the evidence grading criteria. We assessed the proportion of total variability in effect estimates due to between-study heterogeneity of each meta-analysis using the I2 metric of inconsistency [22] and we also calculated the 95% prediction intervals, which show the range in which the effect estimate of a new study in the future is expected to lie [23]. The possibility of small study effects was assessed using the Egger’s regression asymmetry test [24] (with a significance threshold of 0.10), and based on whether the summary estimate was larger in magnitude than the effect estimate of the largest (i.e., most precise; smallest standard error) primary study included in that meta-analysis. Finally, we used the excess significance test to evaluate whether the observed number of studies in the meta-analysis that presented a nominally significant result (P-value < 0.05) was different from the expected number of studies with significant results [22]. The expected number of statistically significant studies was estimated based on the sum of the statistical power of each individual study, which is a function of the number cases and the total sample size. For meta-analyses in which this information was missing for at least 20% of the primary studies, the excess significance test was not performed.
Quality assessment
The quality of the eligible systematic reviews/meta-analyses was evaluated using the AMSTAR tool [18]. AMSTAR critically appraises the quality of systematic reviews and meta-analyses using 11 items and focusing on key methodological issues. Due to the large number of meta-analyses and primary studies included in this umbrella review, the risk of bias was not assessed individually for each primary study considered in each meta-analysis.
Grading of the evidence
The certainty of the evidence (i.e., the confidence in the effect estimate) was graded in a four-point scale (i.e., weak, suggestive, highly suggestive, and convincing evidence) using certain statistical criteria [19, 20] (Table 1) in accordance with previous umbrella reviews [12, 25, 26]. Associations that did not present at least a statistically significant result (P-value < 0.05) in the random-effects model were “non-significant” and, thus, they were not graded.
Results
Literature search
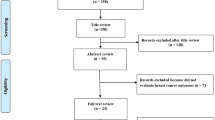
The search algorithm yielded a total of 20,646 unique citations across the three databases (Fig. 1), of which 1,278 were deemed potentially eligible. After excluding 998 publications in the full-text screening phase (Additional file 2—Table S1), 280 publications [7,8,9,10, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302] were included in our review, presenting a total of 895 meta-analytic associations of non-genetic factors with BrCa (Additional file 2—Tables S2 and S3). Of these, 781 were meta-analyses of studies with adjusted estimates while 114 meta-analyses included (either in totality or partially) primary studies with crude summary effect estimates. The publication dates ranged between 1995 and 2022.
Quality assessment
Methodological quality, as assessed using AMSTAR, varied across the 280 publications considered in our umbrella review (Additional file 2—Table S4). The median score was 8 (interquartile range: 6 to 9). Common flaws were the absence of reference to a published protocol (n = 178, 63.6%), the use of publication status as an inclusion criterion (n = 213, 76.1%), and the use of methodological quality in formulating conclusions and recommendations (n = 165, 5.89%). In about 25% (n = 69) of the publications there was no reference to a comprehensive literature search.
Description of the results
In the following sections, only the 781 meta-analyses with adjusted estimates are considered. A brief description of the 114 meta-analyses including (either in totality or partially) primary studies with crude summary effect estimates is presented in the Additional file 1 and the Additional file 2—Table S3.
The median number of included studies in the meta-analyses was 7 (range 2 to 80). Six-hundred-and-thirty-nine (81.8%) meta-analytic estimates pertained to overall BrCa incidence or mortality (with 7 estimates being specific to BrCa related mortality), while 131 (16.8%) focused on BrCa molecular subtypes, i.e., estrogen (ER), progesterone (PR), HER2, luminal A and B, and triple-negative, and 11 (1.4%) specifically to the locoregional spread, i.e., in-situ, invasive, localised, non-localised. Most associations (n = 568, 72.7%) pertained to the general population, while 176 (22.5%) associations pertained to menopausal status and 37 (4.8%) to specific populations (i.e., country-, race-, mutation-, parity-, or hormone replacement therapy-specific).
Overview of the available evidence
The identified non-genetic factors were classified in 11 categories (anthropometric measurements, biomarkers, breast characteristics, diet and dietary supplements, environment, exogenous hormones, lifestyle and social factors, medical history, medication, reproductive history, and pregnancy; Fig. 2). All meta-analyses in the family history–consanguinity category were based on unadjusted estimates; thus, this category was not further considered in the evidence assessment.
Most of the 781 meta-analyses with adjusted estimates (Additional file 2—Table S2) that examined the association of non-genetic factors with BrCa risk were classified in the diet and supplements category (n = 240, 30.7%; Fig. 2). Biomarkers were examined by 18.7% (n = 146) of the meta-analyses with adjusted estimates, and lifestyle and social factors by 10.4% (n = 81) of them. A large number of meta-analyses were found for aspirin use (n = 22, 2.8%), body mass index (BMI) in adulthood or childhood (n = 17, 2.2%), night shift work (n = 16, 2.1%), weight gain (n = 16, 2.1%), Mediterranean dietary pattern (n = 13, 1.7%), body weight (n = 12, 1.5%), and breastfeeding¸ bisphosphonates use, and oral contraceptives (OC) use (each n = 11, 1.4%).
About half (n = 382; 49%) of the 781 meta-analyses were statistically significant (random-effects P-value < 0.05). Of these, 178 (46.6%) associations indicated a decreased risk of BrCa, and 204 (53.4%) an increased risk of BrCa. At a P-value threshold of 10–3, 166 (21.3%) meta-analyses were significant (of these, 103 [62%] indicated an increased risk), whereas for a P-value threshold of 10–6, 81 (10.4%) meta-analyses remained significant (n = 59, 72.8% indicated an increased risk).
High heterogeneity (I2 ≥ 50%) was found in 346 (44.3%) meta-analyses, and in 181 (52.3%) among those with statistically significant results (P-value < 0.05). The 95% prediction intervals excluded the null value (i.e., 1 for binary outcomes) in 69 (8.9%) associations. Evidence of small study effects was observed in 121 (15.5%) meta-analyses. Evidence of excess significance bias was observed in 83 (10.6%) meta-analyses. However, for almost half of the meta-analyses (n = 370; 47.4%), excess significance bias was not estimated, as in these meta-analyses, information was missing for at least 20% of the primary studies.
Evidence for non-genetic factors and BrCa risk
A comprehensive description of the evidence for the association between the non-genetic factors and BrCa risk from meta-analyses with adjusted estimates is shown in the Additional file 1.
Strength of epidemiological evidence
Figure 2 illustrates the classification of non-genetic factors examined in the 781 meta-analyses and the distribution of the grading for their association with BrCa. Tables 2 and 3 summarise the confidence in the effect estimates for protective and harmful non-genetic factors for BrCa (Table S2) and for BrCa receptor-related outcomes [estrogen receptor positive/negative (ER ±), progesterone receptor positive/negative (PR ±), human epidermal growth factor receptor 2 (HER2), luminal, triple negative], reaching at least weak evidence (Table S3).
Seventeen associations (4.4% of 382 meta-analyses with significant results; 2.2% of 781 meta-analyses with adjusted estimates) were graded as convincing. These included three protective associations [older age at menarche (BrCa, ER + /PR + BrCa) [60], higher sex hormone binding globulin (SHBG) [182], and higher total fiber [134]] and 15 associations supporting an increased risk of BrCa [alcohol consumption [64], higher BMI (PR + BrCa) [7], BMI gain [7] and weight gain in postmenopausal women [286], Breast Imaging Reporting and Data System (BIRADS) classification for breast density (D versus B) [215], breast density (25%-49% and 50%-74% vs < 25%; ER- BrCa) [247], higher levels of sex hormones including androstenedione, estradiol, estrone, and testosterone in the general population and in postmenopausal women [182], and oral contraceptive (OC) use in premenopausal women [71].
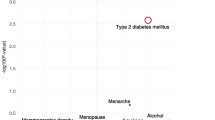
Highly suggestive epidemiological evidence was found for 26 associations (6.8% of 382 meta-analyses with significant results; 3.3% of 781 meta-analyses with adjusted estimates). Of these, an increased risk of BrCa was found for higher BMI in postmenopausal women (BrCa, ER + and ER + /PR + BrCa) [7], body weight in postmenopausal women (ER + /PR + BrCa) [137], height [300], weight gain in postmenopausal women [7, 179], estradiol levels [182], Wolfe grade (P1, P2, Dy versus N1) [49], breast density (≥ 75% vs < 25%; ER + BrCa) [247], estrogen-progestin therapy [85] and digoxin use (BrCa, ER + BrCa) [65], ever active smoking [8], higher educational level [116], and diabetes mellitus [242]. In contrast, higher early adult BMI in postmenopausal women [7], 25 hydroxy vitamin D [25(OH)D] levels [123], adherence to the World Cancer Research Fund/American Institute for Cancer Research Recommendations (WCRF/AICR) score [130], and moderate-vigorous recreational physical activity (PA) [54] had a protective role.
Sixty-eight associations (17.8% of 382 meta-analyses with significant results; 8.7% of 781 meta-analyses with adjusted estimates) were graded as suggestive, while 224 (58.6% of the 382 significant; 28.7% of the 781 total) statistically significant meta-analyses were graded as weak. Finally, 47 (12.3% of the 382 significant; 6% of the 781 total) nominally significant meta-analyses that did not provide the necessary data for grading (number of cases and excess significance bias) were not considered (Additional file 2—Table S2).
Discussion
Principal findings
This large umbrella review of meta-analyses systematically summarised and critically appraised the epidemiological evidence for the association between non-genetic risk factors and female BrCa. Overall, 895 associations (781 meta-analyses of studies with adjusted estimates) were considered, reporting exposures related to anthropometric measurements, biomarkers, breast characteristics and diseases, diet and supplements, environmental parameters, exogenous hormones, factors associated with pregnancy or birth, lifestyle and social factors, medical history, medication, and reproductive history. The highest number of examined associations was found for the category of diet and supplements and for exposures such as aspirin use and active smoking.
Most of the examined associations were either non-significant (51%) or were supported by weak evidence (28.7%). Only about 5.5% of the associations (11.3% of those with statistically significant results) were graded as convincing or highly suggestive. These meta-analyses supported that alcohol consumption, high BMI (BrCa and ER + , PR + , ER + /PR + BrCa), high body weight (BrCa, ER + /PR + BrCa) and body weight gain in postmenopausal women, high height, P1/ P2/ DY Wolfe grade and high BIRADS/ Breast density classification (BrCa and ER-, ER + BrCa), OC use in premenopausal women, ever active smoking, high androstenedione, estradiol, estrone, and testosterone levels, estrogen-progestin therapy use, high educational level, diabetes mellitus, and digoxin use (BrCa, ER + BrCa) were associated with increased BrCa risk. On the other hand, high BMI at ages 18–30 years in premenopausal women, adherence to the WCRF/AICR score, high moderate-vigorous recreational physical activity in postmenopausal women, menarche at an older age (BrCa, ER + /PR + BrCa), increased total fiber intake, increased blood levels of 25(OH)D, and high levels of SHBG were found to prevent from BrCa. Of note, the associations of body weight and breast density with BrCa, despite reaching high levels of evidence, had a low score in the AMSTAR quality assessment.
Strengths and weaknesses in relation to other studies
In the current era of abundant scientific research, umbrella reviews have emerged as a crucial tool to consolidate and synthesize evidence across entire research domains. It is expected that a few associations covered in our extensive analysis might have already been partially addressed in existing umbrella reviews [12, 14, 303,304,305,306,307]. Nonetheless, our review stands out as the most comprehensive to date, offering a thorough mapping and assessment of all non-genetic risk factors for BrCa. Of note, the association between human papillomavirus infection and BrCa that was graded as convincing in a recent umbrella [305] review was not assessed in ours because it was based on unadjusted estimates. We considered this type of meta-analysis to have a high likelihood of bias.
Our study findings align significantly with existing evidence, reinforcing associations previously acknowledged as robust or reaching high evidence levels, such as alcohol [14], BMI [12], physical activity [11], dietary uptake of fiber [308], diabetes [13], sex hormones, and age of menarche [309]. Additionally, our research highlights new associations, including those for digoxin [65], 25(OH)D, breast density, and healthy lifestyle measured as a WCRF/AICR score. However, we did observe a limited number of associations for which our evidence level conflicted with that from previous studies. For example, coffee consumption, one of the most studied exposures in other umbrella reviews [14, 310, 311], reached “probable” levels of evidence in one of them [310]. However, there was no statistically significant association in our umbrella review similarly to the rest of the reviews in this topic [14, 311]. That review, which found “probable” levels of evidence for coffee consumption, followed a grading approach that allowed for higher levels of evidence to be reached although the included meta-analytical association was not statistically significant [310].
Biological plausibility
The biological mechanisms of the association between BrCa risk and height, obesity, physical activity, diabetes, and sex hormones are related. Height is related to the onset of puberty, which is affected by endogenous estrogens, whose role on BrCa has been very well documented [312,313,314]. On the other hand, there might be a causal association between height and BrCa, in which various genetic and non-genetic factors affect height and, subsequently, BrCa risk through a shared biological pathway [300, 315]. As an example, insulin-like growth factor 1 (IGF-I) has been proven to play a pivotal role in cell proliferation enhancement and apoptosis suppression, while it is also considered to be a major determinant of growth and height [214, 316]. In postmenopausal women, synthesis of estrogens takes place in the adipose tissue, whereas in premenopausal women the major source of estrogens are the ovaries. Obesity in postmenopausal women leads to increased conversion of androgens to estrogens, and, as result, to the promotion of cell proliferation and the inhibition of apoptosis. Furthermore, obesity has been associated with insulin resistance and hyperinsulinemia, which downregulates sex hormone binding globulin production, and, thus, results in increased levels of circulating estradiol. On the other hand, it has been reported that more frequent anovulatory cycles among obese premenopausal women [317, 318], and faster clearance rate of free estrogen in the liver among obese compared to lean women [319] may lead to lower levels of both estrogen and progesterone [320]. The protective effect of high moderate-vigorous physical activity against BrCa in postmenopausal women is most probably explained by the fact that exercise helps to prevent obesity.
The association of BrCa with diabetes could be explained through similar pathways such as the activation of the insulin pathway, the activation of insulin- like growth factor pathway, as well as the regulation of sex hormones. Moreover, hyperglycemia has been associated with increased levels of IGF-I and inflammatory cytokines, resulting in direct and indirect effects on cancer cells proliferation, apoptosis, and metastasis. Insulin promotes the expression of insulin receptors in BrCa cells and thus leads to the malignant transformation of breast epithelial cells. Increased insulin resistance on the other hand could cause higher levels of insulin and, as a result, increased androgen synthesis and decreased estrogen production. High SHBG levels have been shown to have a protective role against BrCa. Apart from the apparent function of the regulation of free sex hormones levels, SHBG seems to act as a direct mediator for cell-surface signaling, cellular delivery, and the biologic action of sex hormones, which results in the regulation of the bioavailable fraction of circulating estradiol [321, 322]. Through these unique features, SHBG reduces BrCa cell growth and proliferation [323, 324].
Alcohol is classified as a Group 1 human carcinogen by the International Agency for Research on Cancer [325] and is acknowledged by the World Health Organization as one of the major modifiable risk factors for breast cancer [326]. Alcohol consumption may contribute to BrCa development through various pathways, including hormonal modulation, DNA damage, oxidative stress, immune system impairment, disruption of normal liver function, folate and other nutrients malabsorption, and induction of inflammation [327].
Vitamin D is a steroid hormone with an established role in mammary gland development through the actions of its main mediator, vitamin D receptor (VDR). Through VDR, vitamin D is known to exhibit an anti-proliferative, pro-differentiating, and pro-apoptotic effect. The active form of vitamin D, 1,25(OH)2D, is responsible for the activation of VDR, therefore, circulating 25(OH)D could potentially have an inverse association with breast cancer risk [102]. Nevertheless, these results should be cautiously interpreted in the light of the consistently null associations of genetically predicted circulating 25(OH)D and breast cancer observed in Mendelian randomisation studies [328,329,330].
There are multiple mechanisms through which fiber uptake could have a protective role against breast cancer development [235]. It has been suggested that fiber delays gastric emptying and increases small intestine transit time, which result in reduced glucose absorption and insulin secretion. Furthermore, fiber could reduce circulating estrogens by promoting their fecal excretion during the enterohepatic circulation. Moreover, fiber seems to reduce reabsorption of estrogens through a reverse effect in intestinal β-glucuronidase activity, which is an essential step for the absorption of hydrolysed conjugated estrogens [235].
The mechanisms explaining the association of breast density with increased BrCa risk have not been clearly determined [331]. Increased breast density reflects an increased proportion of fibroglandular tissue, which could also depict an increased number of epithelial cells more susceptible to carcinogenesis and proliferation. Moreover, known determinants of breast density, such as late menopause, low parity, and use of estrogens, have been found to have a clear role on BrCa risk. Dense breast tissue is believed to exhibit a greater aromatase activity, thus resulting in hormonal sensitive tumors [49].
OC use has been found to be carcinogenic particularly when used before first childbirth. A full-term pregnancy contributes to a natural mature process of the breast epithelial cells in 2 stages, an early growth phase and a later phase of lobular differentiation. The nulliparous breast with its undifferentiated structures is more prone to the carcinogenic effects of OC use [71]. Considering estrogen-progestin therapy use, the progestin upregulates the expression of epidermal growth factor (EGF) and IGF receptors [332]. Progesterone and EGF significantly increase cell proliferation [333]. Non-steroidal anti-inflammatory drugs (NSAIDS) use appears to be protective through their effect on prostaglandin E2, which has been shown to up-regulate aromatase expression in adipose tissue fibroblasts by promoting binding of various transcription factors to aromatase promoters I.3 and II [334]. The structural similarity of digoxin and other cardiac glycosides to digitalis compounds like estradiol could explain the observed positive association with BrCa [65].
The mechanisms by which higher education level was associated with increased risk of BrCa remains unclear, although it is likely that this association is driven by other factors. One theory could be that women of a higher educational level usually have their first childbirth at a later age and, also, have fewer children. Other explanation might be that higher educational level has been associated with later menopause, higher alcohol use, and higher prevalence of hormonal treatments. Menarche at an older age shows a protective role particularly among Luminal tumors. Although there seems to be a hormonal mechanism supporting this association, evidence shows that when estrogen receptor positive (ER +) progenitor cells are exposed to estrogen, they produce paracrine signals that cause neighboring populations of ER- cells to proliferate [335].
Strengths and weaknesses of the study
Certain limitations should be considered with respect to the findings of this umbrella review. The analysis focused on meta-analyses of observational studies missing probably the latest evidence of primary observational studies not considered yet in any evidence synthesis. However, given the large amount of included evidence it seems unlikely that single primary studies would affect the evidence grading to a modest degree. The methodological quality of the included publications was moderate as several publications failed to report or apply critical items of the AMSTAR tool, such as the comprehensive literature search, the use of publication status as an inclusion criterion, and the use of the scientific quality of the included studies for drawing conclusions. A relatively high number of meta-analyses included less than 10 primary studies; hence, the excess significance and small study effects tests could be underpowered. Furthermore, the necessary information for the calculation of the excess statistical significance test was often absent in several meta-analyses, resulting in about one-eighth of the included meta-analyses being non-evaluable, thus likely underestimating the number of convincing associations. Although we graded the certainty of evidence according to prespecified criteria, association does not equal causation, which is difficult to demonstrate in non-randomised studies. While we prioritised meta-analyses of prospective cohort studies providing adjusted estimates, most meta-analyses also included case–control designs further accommodating our evidence interpretation. While case–control studies, especially those with suboptimal designs, are more likely to be subject to epidemiological biases, we did not restrict our analyses to cohort designs to ensure maximal comprehensiveness in the included studies. While we focused our grading only on analyses of adjusted estimates, residual or unmeasured confounding may be present. Furthermore, reverse causation cannot be excluded and the retrospective studies included in certain meta-analyses may be vulnerable to recall bias. Therefore, considering these weaknesses, we advise caution in any interpretation of the results presented in our review. Nevertheless, this umbrella review provides the most comprehensive assessment of the published epidemiological literature of non-genetic factors and BrCa risk. A vast amount of data was considered, and robust methodological approaches were used to assess the evidence. Overall, although the constraints of this umbrella review probably would make our assessment somewhat more lenient, their effect on the associations supported by convincing evidence is expected to be trivial.
Implications for future research
Our findings suggest some highly modifiable protective factors for BrCa. Interestingly, while diet was the most studied exposure category, associations failed to reach higher levels of evidence, indicating the methodological limitations in the field. To improve the validity of these associations future research should focus: i) on more robust study designs, such as high quality randomised controlled trials or Mendelian randomisation studies that have the potential to minimise biases common in observational epidemiological designs, and ii) on better exposure assessment techniques including objective measures and uitilising large scale omics technology to bolster our understanding of the mechanistic evidence underlying these associations. Overall, our study provides knowledge that supports the development of BrCa prevention recommendations and guidance, both at an individual level and for public health initiatives.
Conclusions
As the incidence of BrCa increases in many countries worldwide, the identification of modifiable risk factors is imperative for health care professionals to provide both individualised and public health guidance for BrCa prevention. Our study summarised a large number of publications describing associations between non-genetic factors and BrCa risk with varying methodological quality and varying strength and validity of the associated evidence. The validity of several well-established risk factors was reaffirmed and several risk factors with potentially higher levels of evidence strength were highlighted. These results reinforce the existing guidelines and recommendations advocating for women to maintain a healthy weight, engage in regular physical activity, and adopt a nutritious, high-fiber diet to mitigate the risk of developing BrCa. Moreover, our findings underscore the importance of regular screening, particularly for high-risk groups, i.e., women over 50 years old with increased breast density, poor lifestyle, and prior use of OC, further emphasising the proactive measures that can significantly contribute to breast cancer prevention. However, it is important to note that many associations did not reach higher levels of evidence. Studies following consistent standardisation definitions and procedures could improve the quality of publications and the level of the evidence.
Availability of data and materials
Most of the data and the list of all meta-analyses not selected for data extraction are provided in the supplementary material. The data extracted from the primary studies can be made available upon a reasonable request.
Abbreviations
- BIRADS:
-
Breast Imaging Reporting and Data System
- BMI:
-
Body mass index
- BrCa:
-
Breast cancer
- EGF:
-
Epidermal growth factor
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- IGF:
-
Insulin-like growth factor
- NSAIDS:
-
Non-steroidal anti-inflammatory drugs
- OC:
-
Oral contraceptive
- PRIOR:
-
Preferred Reporting Items for Overviews of Reviews
- SHBG:
-
Sex hormone binding globulin
- VDR:
-
Vitamin D receptor
- WCRF/AICR:
-
World Cancer Research Fund/American Institute for Cancer Research Recommendations
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Joko-Fru WY, Jedy-Agba E, Korir A, Ogunbiyi O, Dzamalala CP, Chokunonga E, et al. The evolving epidemic of breast cancer in sub-Saharan Africa: results from the African cancer registry network. Int J Cancer. 2020;147:2131–41.
Arpino G, Pensabene M, Condello C, Ruocco R, Cerillo I, Lauria R, et al. Tumor characteristics and prognosis in familial breast cancer. BMC Cancer. 2016;16:924.
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Heal. 2020;8:e1027–37.
Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602.
Mavaddat N, Pharoah PDP, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. Prediction of breast cancer risk based on profiling with common genetic variants. JNCI J Natl Cancer Inst. 2015;107:djv036.
Chan DSM, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, et al. World Cancer Research Fund International: Continuous Update Project—systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30:1183–200.
Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2015;154:213–24.
Suzuki R, Orsini N, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status - a meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–41.
Yoon YS, Kwon AR, Lee YK, Oh SW. Circulating adipokines and risk of obesity related cancers: a systematic review and meta-analysis. Obes Res Clin Pract. 2019;13:329–39.
de Rezende LFM, de Sá TH, Markozannes G, Rey-López JP, Lee IM, Tsilidis KK, et al. Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. Br J Sports Med. 2018;52:826–33.
Kyrgiou M, Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ. 2017;356:1–10.
Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, et al. Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. 2021;30:1218–28.
Papadimitriou N, Markozannes G, Kanellopoulou A, Critselis E, Alhardan S, Karafousia V, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12:4579.
Makvandi M, Zhou X, Li C, Deng Q. A field investigation on adaptive thermal comfort in an urban environment considering individuals’ psychological and physiological behaviors in a cold-winter of Wuhan. Sustain. 2021;13:678.
Hermelink R, Leitzmann MF, Markozannes G, Tsilidis K, Pukrop T, Berger F, et al. Sedentary behavior and cancer–an umbrella review and meta-analysis. Eur J Epidemiol. 2022;37:447–60.
Gates M, Gates A, Pieper D, Fernandes RM, Tricco AC, Moher D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378:e070849.
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10.
Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21:95–100.
Papatheodorou SI, Evangelou E. Umbrella reviews: what they are and why we need them. Methods Mol Biol. 2022;2345:135–46.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trails. 1986;7:177–88.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59.
Egger M, Smith GD, Schneider M, Minder C, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Bellou V, Belbasis L, Tzoulaki I, Evangelou E. Risk factors for type 2 diabetes mellitus: an exposure-wide umbrella review of meta-analyses. PLoS One. 2018;13:e0194127.
Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72:30–43.
Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer. 2004;111:584–91.
Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, et al. Breastfeeding and breast cancer risk by receptor status-a systematic review and meta-analysis. Ann Oncol. 2015;26:2398–407.
Balk EM, Earley A, Avendano EA, Raman G. Long-term health outcomes in women with silicone gel breast implants. Ann Intern Med. 2016;164:164–75.
Zhang J, Huang Y, Wang X, Lin K, Wu K. Environmental polychlorinated biphenyl exposure and breast cancer risk: a meta-analysis of observational studies. PLoS One. 2015;10:e0142513.
Cao Y, Hou L, Wang W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: a meta-analysis of prospective cohort studies. Int J Cancer. 2016;138:1894–904.
Zhang Y, Lai J, Ruan G, Chen C, Wang DW. Meta-analysis of extremely low frequency electromagnetic fields and cancer risk: a pooled analysis of epidemiologic studies. Environ Int. 2016;88:36–43.
Xia H, Ma S, Wang S, Sun G. Meta-analysis of saturated fatty acid intake and breast cancer risk. Med. 2015;94:e2391.
Leng L, Li J, Luo XM, Kim JY, Li YM, Guo XM, et al. Polychlorinated biphenyls and breast cancer: a congener-specific meta-analysis. Environ Int. 2016;88:133–41.
Zang J, Shen M, Du S, Chen T, Zou S. The association between dairy intake and breast cancer in western and Asian populations: a systematic review and meta-analysis. J Breast Cancer. 2015;18:313–22.
Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, et al. Selenium exposure and cancer risk: an updated meta-analysis and meta-regression. Sci Rep. 2016;6:19213.
Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57:3640–9.
Takkouche B, Etminan M, Montes-Martínez A. Personal use of hair dyes and risk of cancer: a meta-analysis. J Am Med Assoc. 2005;293:2516–25.
Zhou Y, Li W, Herath C, Xia J, Hu B, Song F, et al. Off-hour admission and mortality risk for 28 specific diseases: a systematic review and meta-analysis of 251 cohorts. J Am Heart Assoc. 2016;5:e003102.
Chen JY, Zhu HC, Guo Q, Shu Z, Bao XH, Sun F, et al. Dose-dependent associations between wine drinking and breast cancer risk - meta-analysis findings. Asian Pacific J Cancer Prev. 2016;17:1221–33.
Hidayat K, Chen GC, Zhang R, Du X, Zou SY, Shi BM, et al. Calcium intake and breast cancer risk: Meta-analysis of prospective cohort studies. Br J Nutr. 2016;116:158–66.
Lundqvist A, Andersson E, Ahlberg I, Nilbert M, Gerdtham U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe - a systematic review and meta-analysis. Eur J Public Health. 2016;26:804–13.
Li C, Yang L, Zhang D, Jiang W. Systematic review and meta-analysis suggest that dietary cholesterol intake increases risk of breast cancer. Nutr Res. 2016;36:627–35.
Zhihui W, Weihua Y, Zupei W, Jinlin H. Fish consumption and risk of breast cancer: meta-analysis of 27 observational studies. Nutr Hosp. 2016;33:703–12.
Lambertini M, Santoro L, Del Mastro L, Nguyen B, Livraghi L, Ugolini D, et al. Reproductive behaviors and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis of epidemiological studies. Cancer Treat Rev. 2016;49:65–76.
Lee PN, Hamling JS. Environmental tobacco smoke exposure and risk of breast cancer in nonsmoking women. An updated review and meta-analysis. Inhal Toxicol. 2016;28:431–54.
Wulaningsih W, Sagoo HK, Hamza M, Melvin J, Holmberg L, Garmo H, et al. Serum calcium and the risk of breast cancer: findings from the Swedish AMORIS study and a meta-analysis of prospective studies. Int J Mol Sci. 2016;17:1487.
Kolahdouz Mohammadi R, Bagheri M, Kolahdouz Mohammadi M, Shidfar F. Ruminant trans-fatty acids and risk of breast cancer: a systematic review and meta-analysis of observational studies. Minerva Endocrinol. 2017;42:385–96.
McCormack VA, Dos Santos SI. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159–69.
Elands RJJ, Simons CCJM, Van Dongen M, Schouten LJ, Verhage BJ, Van Den Brandt PA, et al. A systematic literature review and meta-regression analysis on early-life energy restriction and cancer risk in humans. PLoS One. 2016;11:e0158003.
Shobeiri F, Jenabi E. The association between polycystic ovary syndrome and breast cancer: a meta-analysis. Obstet Gynecol Sci. 2016;59:367–72.
Godos J, Bella F, Sciacca S, Galvano F, Grosso G. Vegetarianism and breast, colorectal and prostate cancer risk: an overview and meta-analysis of cohort studies. J Hum Nutr Diet. 2017;30:349–59.
Allam MF. Breast cancer and deodorants/ antiperspirants: a systematic review. Cent Eur J Public Health. 2016;24:245–7.
Neilson HK, Farris MS, Stone CR, Vaska MM, Brenner DR, Friedenreich CM. Moderate-vigorous recreational physical activity and breast cancer risk, stratified by menopause status: a systematic review and meta-analysis. Menopause. 2016;24:322–44.
Chen S, Chen Y, Ma S, Zheng R, Zhao P, Zhang L, et al. Dietary fibre intake and risk of breast cancer: a systematic review and meta-analysis of epidemiological studies. Oncotarget. 2016;7:80980–9.
Karasneh RA, Murray LJ, Cardwell CR. Cardiac glycosides and breast cancer risk: a systematic review and meta-analysis of observational studies. Int J cancer. 2017;140:1035–41.
Wu J, Zeng R, Huang J, Li X, Zhang J, Ho JCM, et al. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. 2016;8:730.
Grosso G, Godos J, Lamuela-Raventos R, Ray S, Micek A, Pajak A, et al. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res. 2017;61:2–12.
Bae J, Kim E. Breast density and risk of breast cancer in asian women: a meta-analysis of observational studies. J Prev Med Public Heal. 2016;49:367–75.
Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8:R43.
Zhang C, Xie SH, Xu B, Lu S, Liu P. Digitalis use and the risk of breast cancer: a systematic review and meta-analysis. Drug Saf. 2017;40:285–92.
Unar-Munguía M, Torres-Mejía G, Colchero MA, de Cosío González T. Breastfeeding mode and risk of breast cancer: a dose-response meta-analysis. J Hum Lact. 2017;33:422–34.
Lu L, Shi L, Zeng J, Wen Z. Aspirin as a potential modality for the chemoprevention of breast cancer: a dose-response meta-analysis of cohort studies from 857,831 participants. Oncotarget. 2017;8:40389–401.
Choi YJ, Myung SK, Lee JH. Light alcohol drinking and risk of cancer: a meta-analysis of cohort studies. Cancer Res Treat. 2018;50:474–87.
Osman MH, Farrag E, Selim M, Osman MS, Hasanine A, Selim A. Cardiac glycosides use and the risk and mortality of cancer; systematic review and meta-analysis of observational studies. PLoS One. 2017;12:e0178611.
Li L, Zhong Y, Zhang H, Yu H, Huang Y, Li Z, et al. Association between oral contraceptive use as a risk factor and triple-negative breast cancer: a systematic review and meta-analysis. Mol Clin Oncol. 2017;7:76–80.
Xu J, Huang L, Sun GP. Urinary 6-sulfatoxymelatonin level and breast cancer risk: systematic review and meta-analysis. Sci Rep. 2017;7:5353.
Sun M, Fan Y, Hou Y, Fan Y. Preeclampsia and maternal risk of breast cancer: a meta-analysis of cohort studies. J Matern Fetal Neonatal Med. 2018;31:2484–91.
Fu Z, Zhao F, Chen K, Xu J, Li P, Xia D, et al. Association between urinary phthalate metabolites and risk of breast cancer and uterine leiomyoma. Reprod Toxicol. 2017;74:134–42.
Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis. Nutrients. 2017;9:1561–86.
Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc. 2006;81:1290–302.
Schlesinger S, Chan DSM, Vingeliene S, Vieira AR, Abar L, Polemiti E, et al. Carbohydrates, glycemic index, glycemic load, and breast cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Nutr Rev. 2017;75:420–41.
Rienks J, Barbaresko J, Nöthlings U. Association of isoflavone biomarkers with risk of chronic disease and mortality: a systematic review and meta-analysis of observational studies. Nutr Rev. 2017;75:616–41.
Ni H, Rui Q, Zhu X, Yu Z, Gao R, Liu H. Antihypertensive drug use and breast cancer risk: a metaanalysis of observational studies. Oncotarget. 2017;8:62545–60.
Du R, Lin L, Cheng D, Xu Y, Xu M, Chen Y, et al. Thiazolidinedione therapy and breast cancer risk in diabetic women: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2018;34:9–11.
Chen Y, Liu L, Zhou Q, Imam MU, Cai J, Wang Y, et al. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: a dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health. 2017;17:936.
Hardefeldt PJ, Penninkilampi R, Edirimanne S, Eslick GD. Physical activity and weight loss reduce the risk of breast cancer: a meta-analysis of 139 prospective and retrospective studies. Clin Breast Cancer. 2018;18:e601–12.
Zhao TT, Jin F, Li JG, Xu YY, Dong HT, Liu Q, et al. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: a meta-analysis of prospective cohort studies. Clin Nutr. 2019;38:136–45.
Nindrea RD, Aryandono T, Lazuardi L. Breast cancer risk from modifiable and non-modifiable risk factors among women in Southeast Asia: a meta-analysis. Asian Pacific J Cancer Prev. 2017;18:3201–6.
Deng Y, Xu H, Zeng X, Tarantino G. Induced abortion and breast cancer: an updated meta-analysis. Med. 2018;97:e9613.
Overbeek JA, Bakker M, van der Heijden AAWA, van Herk-Sukel MPP, Herings RMC, Nijpels G. Risk of dipeptidyl peptidase-4 (DPP-4) inhibitors on site-specific cancer: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2018;34:e3004.
Qin LQ, Xu JY, Wang PY, Hoshi K. Soyfood intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J Nutr Sci Vitaminol (Tokyo). 2006;52:428–36.
Wild JB, Hwang MJ, Jones G. A meta-analysis of consanguinity and breast cancer. Ir J Med Sci. 2018;187:895–9.
Tang GH, Satkunam M, Pond GR, Steinberg GR, Blandino G, Schunemann HJ, et al. Association of metformin with breast cancer incidence and mortality in patients with type ii diabetes: a GRADE-assessed systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2018;27:627–35.
Kim S, Ko Y, Lee HJ, Lim JE. Menopausal hormone therapy and the risk of breast cancer by histological type and race: a meta-analysis of randomized controlled trials and cohort studies. Breast Cancer Res Treat. 2018;170:667–75.
Estébanez N, Gómez-Acebo I, Palazuelos C, Llorca J, Dierssen-Sotos T. Vitamin D exposure and risk of breast cancer: a meta-analysis. Sci Rep. 2018;8:9039.
Yang B, Ren XL, Wang ZY, Wang L, Zhao F, Guo XJ, et al. Biomarker of long-chain n-3 fatty acid intake and breast cancer: accumulative evidence from an updated meta-analysis of epidemiological studies. Crit Rev Food Sci Nutr. 2019;59:3152–64.
Salamat F, Niakan B, Keshtkar A, Rafiei E, Zendehdel M. Subtypes of benign breast disease as a risk factor of breast cancer: a systematic review and meta analyses. Iran J Med Sci. 2018;43:355–64.
Farvid MS, Stern MC, Norat T, Sasazuki S, Vineis P, Weijenberg MP, et al. Consumption of red and processed meat and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Int J Cancer. 2018;143:2787–99.
Bond-Smith D, Stone J. Methodological challenges and updated findings from a meta-analysis of the association between mammographic density and breast cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:22–31.
Chen H, Shao F, Zhang F, Miao Q. Association between dietary carrot intake and breast cancer: a meta-analysis. Med. 2018;97:e12164.
Xiao Y, Ke Y, Wu S, Huang S, Li S, Lv Z, et al. Association between whole grain intake and breast cancer risk: a systematic review and meta-analysis of observational studies. Nutr J. 2018;17:87.
Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–100.
Namazi N, Irandoost P, Heshmati J, Larijani B, Azadbakht L. The association between fat mass and the risk of breast cancer: a systematic review and meta-analysis. Clin Nutr. 2019;38:1496–503.
Song L, Wang Y, Zhang J, Song N, Xu X, Lu Y. The risks of cancer development in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Arthritis Res Ther. 2018;20:270.
Gào X, Wilsgaard T, Jansen EHJM, Holleczek B, Zhang Y, Xuan Y, et al. Pre-diagnostic derivatives of reactive oxygen metabolites and the occurrence of lung, colorectal, breast and prostate cancer: An individual participant data meta-analysis of two large population-based studies. Int J Cancer. 2019;145:49–57.
Shao J, Wu L, Leng WD, Fang C, Zhu YJ, Jin YH, et al. Periodontal disease and breast cancer: a meta-analysis of 1,73,162 participants. Front Oncol. 2018;8:601.
Xiao Y, Xia J, Li L, Ke Y, Cheng J, Xie Y, et al. Associations between dietary patterns and the risk of breast cancer: a systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019;21:16.
Chen JH, Yuan Q, Ma YN, Zhen SH, Wen DL. Relationship between bone mineral density and the risk of breast cancer: a systematic review and dose–response meta-analysis of ten cohort studies. Cancer Manag Res. 2019;11:1453–64.
Li N, Huang Z, Zhang Y, Sun H, Wang J, Zhao J. Increased cancer risk after myocardial infarction: factor fiction? A systemic review and meta-analysis. Cancer Manag Res. 2019;11:1959–68.
Chen L, Li M, Li H. Milk and yogurt intake and breast cancer risk: a meta-analysis. Medicine (Baltimore). 2019;98:e14900.
Hossain S, Beydoun MA, Beydoun HA, Chen X, Zonderman AB, Wood RJ. Vitamin D and breast cancer: a systematic review and meta-analysis of observational studies. Clin Nutr ESPEN. 2019;30:170–84.
Anjom-Shoae J, Sadeghi O, Larijani B, Esmaillzadeh A. Dietary intake and serum levels of trans fatty acids and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Clin Nutr. 2020;39:755–64.
Ambrosone CB, Kropp S, Yang J, Yao S, Shields PG, Chang-Claude J. Cigarette smoking, N-acetyltransferase 2 genotypes, and breast cancer risk: pooled analysis and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:15–26.
Bahri N, Fathi Najafi T, Homaei Shandiz F, Tohidinik HR, Khajavi A. The relation between stressful life events and breast cancer: a systematic review and meta-analysis of cohort studies. Breast Cancer Res Treat. 2019;176:53–61.
Vishwakarma G, Ndetan H, Das DN, Gupta G, Suryavanshi M, Mehta A, Singh KP, et al. Reproductive factors and breast cancer risk: a meta-analysis of case–control studies in Indian women. South Asian J cancer. 2019;08:080–4.
Marsh GM, Keeton KA, Riordan AS, Best EA, Benson SM. Ethylene oxide and risk of lympho-hematopoietic cancer and breast cancer: a systematic literature review and meta-analysis. Int Arch Occup Environ Health. 2019;92:919–39.
Nindrea RD, Aryandono T, Lazuardi L, Dwiprahasto I. Association of dietary intake ratio of n-3/n-6 polyunsaturated fatty acids with breast cancer risk in Western and Asian countries: a meta-analysis. Asian Pacific J Cancer Prev. 2019;20:1321–7.
Chang VC, Cotterchio M, Khoo E. Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer. 2019;19:543.
Catalá-López F, Forés-Martos J, Driver JA, Page MJ, Hutton B, Ridao M, et al. Association of anorexia nervosa with risk of cancer: a systematic review and meta-analysis. JAMA Netw open. 2019;2:e195313.
Seretis A, Cividini S, Markozannes G, Tseretopoulou X, Lopez DS, Ntzani EE, et al. Association between blood pressure and risk of cancer development: a systematic review and meta-analysis of observational studies. Sci Rep. 2019;9:1974.
Farahmand M, Monavari SH, Shoja Z, Ghaffari H, Tavakoli M, Tavakoli A. Epstein-Barr virus and risk of breast cancer: a systematic review and meta-analysis. Futur Oncol. 2019;15:2873–85.
Hidayat K, Zhou HJ, Shi BM. Influence of physical activity at a young age and lifetime physical activity on the risks of 3 obesity-related cancers: Systematic review and meta-analysis of observational studies. Nutr Rev. 2020;78:1–18.
Liu Y, Zhang X, Sun H, Zhao S, Zhang Y, Li D, et al. Bisphosphonates and primary breast cancer risk: an updated systematic review and meta-analysis involving 963,995 women. Clin Epidemiol. 2019;11:593–603.
Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70.
Dong JY, Qin LQ. Education level and breast cancer incidence: a meta-analysis of cohort studies. Menopause. 2020;27:113–8.
Ji LW, Jing CX, Zhuang SL, Pan WC, Hu XP. Effect of age at first use of oral contraceptives on breast cancer risk: an updated meta-analysis. Med. 2019;98:e15719.
Zhang P, Liu B. Association between Parkinson’s disease and risk of cancer: a PRISMA-compliant meta-analysis. ACS Chem Neurosci. 2019;10:4430–9.
Ren C, Zeng K, Wu C, Mu L, Huang J, Wang M. Human papillomavirus infection increases the risk of breast carcinoma: a large-scale systemic review and meta-analysis of case-control studies. Gland Surg. 2019;8:486–500.
Wei Y, Lv J, Guo Y, Bian Z, Gao M, Du H, et al. Soy intake and breast cancer risk: a prospective study of 300,000 Chinese women and a dose–response meta-analysis. Eur J Epidemiol. 2020;35:567–78.
Guo M, Liu T, Li P, Wang T, Zeng C, Yang M, et al. Association between metabolic syndrome and breast cancer risk: an updated meta-analysis of follow-up studies. Front Oncol. 2019;9:1290.
Zhang Z, Yan W, Chen Q, Zhou N, Xu Y. The relationship between exposure to particulate matter and breast cancer incidence and mortality: a meta-analysis. Med (United States). 2019;98:e18349.
Song D, Deng Y, Liu K, Zhou L, Li N, Zheng Y, et al. Vitamin D intake, blood vitamin D levels, and the risk of breast cancer: a dose-response meta-analysis of observational studies. Aging (Albany NY). 2019;11:12708–32.
Hiller TWR, O’sullivan DE, Brenner DR, Peters CE, King WD. Solar ultraviolet radiation and breast cancer risk: a systematic review and meta-analysis. Environ Health Perspect. 2020;128:1–11.
Wang Q, Liu X, Ren S. Tofu intake is inversely associated with risk of breast cancer: a meta-analysis of observational studies. PLoS One. 2020;15:1–13.
Takkouche B, Regueira-Méndez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008;100:1439–47.
Wang Y, Zhao Y, Chong F, Song M, Sun Q, Li T, et al. A dose-response meta-analysis of green tea consumption and breast cancer risk. Int J Food Sci Nutr. 2020;71:656–67.
Conz L, Mota BS, Bahamondes L, Teixeira Dória M, Françoise Mauricette Derchain S, Rieira R, et al. Levonorgestrel-releasing intrauterine system and breast cancer risk: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2020;99:970–82.
Wang Y, Yan P, Fu T, Yuan J, Yang G, Liu Y, et al. The association between gestational diabetes mellitus and cancer in women: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2020;46:461–71.
Turati F, Dalmartello M, Bravi F, Serraino D, Augustin L, Giacosa A, et al. Adherence to the world cancer research fund/american institute for cancer research recommendations and the risk of breast cancer. Nutrients. 2020;12:607.
Li M, Han M, Chen Z, Tang Y, Ma J, Zhang Z, et al. Does marital status correlate with the female breast cancer risk? A systematic review and meta-analysis of observational studies. PLoS One. 2020;15:e0229899.
Adani G, Filippini T, Wise LA, Halldorsson TI, Blaha L, Vinceti M. Dietary intake of acrylamide and risk of breast, endometrial, and ovarian cancers: a systematic review and dose-response meta-analysis. Cancer Epidemiol Biomarkers Prev. 2020;29:1095–106.
Zeng J, Gu Y, Fu H, Liu C, Zou Y, Chang H. Association between one-carbon metabolism-related vitamins and risk of breast cancer: a systematic review and meta-analysis of prospective studies. Clin Breast Cancer. 2020;20:e469–80.
Farvid MS, Spence ND, Holmes MD, Barnett JB. Fiber consumption and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Cancer. 2020;126:3061–75.
Tong H, Wu Y, Yan Y, Dong Y, Guan X, Liu Y, et al. No association between abortion and risk of breast cancer among nulliparous women: evidence from a meta-analysis. Med. 2020;99:E20251.
Abbasalizad Farhangi M, Vajdi M. Dietary total antioxidant capacity (TAC) significantly reduces the risk of site-specific cancers: an updated systematic review and meta-analysis. Nutr Cancer. 2021;73:721–39.
Suzuki R, Orsini N, Saji S, Key TJ, Wolk A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status–a meta-analysis. Int J Cancer. 2009;124:698–712.
Zhang D, Dai C, Zhou L, Li Y, Liu K, Deng YJ, et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging (Albany NY). 2020;12:10772–94.
Liu J, Li X, Hou J, Sun J, Guo N, Wang Z. Dietary intake of N-3 and N-6 polyunsaturated fatty acids and risk of cancer: meta-analysis of data from 32 studies. Nutr Cancer. 2021;73:901–13.
Song HJ, Jeon N, Squires P. The association between acid-suppressive agent use and the risk of cancer: a systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76:1437–56.
Filippini T, Torres D, Lopes C, Carvalho C, Moreira P, Naska A, et al. Cadmium exposure and risk of breast cancer: a dose-response meta-analysis of cohort studies. Environ Int. 2020;142:105879.
Chong F, Wang Y, Song M, Sun Q, Xie W, Song C. Sedentary behavior and risk of breast cancer: a dose–response meta-analysis from prospective studies. Breast Cancer. 2021;28:48–59.
Okekunle AP, Gao J, Wu X, Feng R, Sun C. Higher dietary soy intake appears inversely related to breast cancer risk independent of estrogen receptor breast cancer phenotypes. Heliyon. 2020;6:e04228.
Zhou W, Chen X, Huang H, Liu S, Xie A, Lan L. Birth weight and incidence of breast cancer: dose-response meta-analysis of prospective studies. Clin Breast Cancer. 2020;20:e555–68.
Simin J, Tamimi RM, Engstrand L, Callens S, Brusselaers N. Antibiotic use and the risk of breast cancer: a systematic review and dose-response meta-analysis. Pharmacol Res. 2020;160:105072.
Zhang H, Guo L, Tao W, Zhang J, Zhu Y, Abdelrahim MEA, et al. Possible breast cancer risk related to background parenchymal enhancement at breast MRI: a meta-analysis study. Nutr Cancer. 2021;78:1371–7.
Wu Y, Wang M, Sun W, Li S, Wang W, Zhang D. Age at last birth and risk of developing breast cancer: a meta-analysis. Eur J Cancer Prev. 2020;29:424–32.
Vojtechova P, Martin RM. The association of atopic diseases with breast, prostate, and colorectal cancers: a meta-analysis. Cancer Causes Control. 2009;20:1091–105.
Wong ATY, Heath AK, Tong TYN, Reeves GK, Floud S, Beral V, et al. Sleep duration and breast cancer incidence: results from the million women study and meta-analysis of published prospective studies. Sleep. 2021;44:zsaa166.
Sealy N, Hankinson SE, Houghton SC. Olive oil and risk of breast cancer: a systematic review and dose–response meta-analysis of observational studies. Br J Nutr. 2021;125:1148–56.
Luan FJ, Wan Y, Mak KC, Ma CJ, Wang HQ. Cancer and mortality risks of patients with scoliosis from radiation exposure: a systematic review and meta-analysis. Eur Spine J. 2020;29:3123–34.
Wang B, Lu Z, Huang Y, Li R, Lin T. Does hypothyroidism increase the risk of breast cancer: evidence from a meta-analysis. BMC Cancer. 2020;20:733.
Zhang D, Xu P, Li Y, Wei B, Yang S, Zheng Y, et al. Association of vitamin C intake with breast cancer risk and mortality: a meta-analysis of observational studies. Aging (Albany NY). 2020;12:18415–35.
Shamshirian A, Heydari K, Shams Z, Aref AR, Shamshirian D, Tamtaji OR, et al. Breast cancer risk factors in Iran: a systematic review & meta-analysis. Horm Mol Biol Clin Investig. 2020;41:20200021.
Peng R, Liang X, Zhang G, Yao Y, Chen Z, Pan X, et al. Association use of bisphosphonates with risk of breast cancer: a meta-analysis. Biomed Res Int. 2020;2020:5606573.
Ren X, Xu P, Zhang D, Liu K, Song D, Zheng Y, et al. Association of folate intake and plasma folate level with the risk of breast cancer: a dose-response meta-analysis of observational studies. Aging (Albany NY). 2020;12:21355–75.
Kazemi A, Barati-Boldaji R, Soltani S, Mohammadipoor N, Esmaeilinezhad Z, Clark CCT, et al. Intake of various food groups and risk of breast cancer: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2021;12:809–49.
Xu S, Wang H, Liu Y, Zhang C, Xu Y, Tian F, et al. Hair chemicals may increase breast cancer risk: a meta-analysis of 210319 subjects from 14 studies. PLoS One. 2021;16:e0243792.
Xu X, Dailey AB, Peoples-Sheps M, Talbott EO, Li N, Roth J. Birth weight as a risk factor for breast cancer: a meta-analysis of 18 epidemiological studies. J Womens Health (Larchmt). 2009;18:1169–78.
Li R, Li X, Yan P, Bing Z, Cao L, Hui X, et al. Relationship between antidepressive agents and incidence risk of breast cancer: systematic review and meta-analysis. Futur Oncol. 2021;17:1105–24.
Llaha F, Gil-Lespinard M, Unal P, de Villasante I, Castañeda J, Zamora-Ros R. Consumption of sweet beverages and cancer risk. A systematic review and meta-analysis of observational studies. Nutrients. 2021;13:516.
Wong ATY, Fensom GK, Key TJ, Charlotte Onland-Moret N, Tong TYN, Travis RC. Urinary melatonin in relation to breast cancer risk: nested case–control analysis in the DOM study and meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2021;30:97–103.
Chen S, Wu F, Hai R, You Q, Xie L, Shu L, et al. Thyroid disease is associated with an increased risk of breast cancer: a systematic review and meta-analysis. Gland Surg. 2021;10:336.
Manouchehri E, Taghipour A, Ghavami V, Ebadi A, Homaei F, Latifnejad RR. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Womens Health. 2021;21:89.
Ba DM, Ssentongo P, Beelman RB, Muscat J, Gao X, Richie JP. Higher mushroom consumption is associated with lower risk of cancer: a systematic review and meta-analysis of observational studies. Adv Nutr. 2021;12:1691–704.
Ma S, Guo C, Sun C, Han T, Zhang H, Qu G, et al. Aspirin use and risk of breast cancer: a meta-analysis of observational studies from 1989 to 2019. Clin Breast Cancer. 2021;21:552–65.
Lovrics O, Butt J, Lee Y, Lovrics P, Boudreau V, Anvari M, et al. The effect of bariatric surgery on breast cancer incidence and characteristics: a meta-analysis and systematic review. Am J Surg. 2021;222:715–22.
Liu G, Cai W, Liu H, Jiang H, Bi Y, Wang H. The association of bisphenol A and phthalates with risk of breast cancer : a meta-analysis. Int J Environ Res Public Health. 2021;18:2375.
Xie Y, Wang M, Xu P, Deng Y, Zheng Y, Yang S, et al. Association between antihypertensive medication use and breast cancer: a systematic review and meta-analysis. Front Pharmacol. 2021;12:609901.
Cohen JM, Hutcheon JA, Julien SG, Tremblay ML, Fuhrer R. Insufficient milk supply and breast cancer risk: a systematic review. PLoS One. 2009;4:e8237.
Michels N, Specht IO, Heitmann BL, Chajès V, Huybrechts I. Dietary trans-fatty acid intake in relation to cancer risk: a systematic review and meta-analysis. Nutr Rev. 2021;79:758–76.
Lee J, Lee JY, Lee DW, Kim HR, Kang MY. Sedentary work and breast cancer risk: a systematic review and meta-analysis. J Occup Health. 2021;63:e12239.
Wei W, Wu BJ, Wu Y, Tong ZT, Zhong F, Hu CY. Association between long-term ambient air pollution exposure and the risk of breast cancer: a systematic review and meta-analysis. Environ Sci Pollut Res. 2021;28:63278–96.
Arafat HM, Omar J, Muhamad R, Al-Astani TAD, Shafii N, Al Laham NA, et al. Breast cancer risk from modifiable and non-modifiable risk factors among Palestinian women: a systematic review and meta-analysis. Asian Pacific J Cancer Prev. 2021;22:1987–95.
Wang F, Zhang W, Cheng W, Huo N, Zhang S. Preeclampsia and cancer risk in women in later life: a systematic review and meta-analysis of cohort studies. Menopause. 2021;28:1070–8.
Zhao G, Ji Y, Ye Q, Ye X, Wo G, Chen X, et al. Effect of statins use on risk and prognosis of breast cancer: a meta-analysis. Anticancer Drugs. 2022;33:E507–18.
Van NTH, Hoang T, Myung SK. Night shift work and breast cancer risk: a meta-analysis of observational epidemiological studies. Carcinogenesis. 2021;42:1260–9.
Farvid MS, Sidahmed E, Spence ND, Mante Angua K, Rosner BA, Barnett JB. Consumption of red meat and processed meat and cancer incidence: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2021;36:937–51.
Hao Y, Jiang M, Miao Y, Li X, Hou C, Zhang X, et al. Effect of long-term weight gain on the risk of breast cancer across women’s whole adulthood as well as hormone-changed menopause stages: a systematic review and dose–response meta-analysis. Obes Res Clin Pract. 2021;15:439–48.
Urbano T, Vinceti M, Wise LA, Filippini T. Light at night and risk of breast cancer: a systematic review and dose–response meta-analysis. Int J Health Geogr. 2021;20:1–26.
Santos MCL, Horta BL, do Amaral JJF, Fernandes PFCBC, Galvão CM, Fernandes AFC. Association between stress and breast cancer in women: a meta-analysis. Cad Saude Publica. 2009;25 suppl 3:S453–63.
Drummond AE, Swain CTV, Brown KA, Dixon-Suen SC, Boing L, Van Roekel EH, et al. Linking physical activity to breast cancer via sex steroid hormones, part 2: the effect of sex steroid hormones on breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2022;31:28–37.
Poorolajal J, Heidarimoghis F, Karami M, Cheraghi Z, Gohari-Ensaf F, Shahbazi F, et al. Factors for the primary prevention of breast cancer: a meta-analysis of prospective cohort studies. J Res Health Sci. 2021;21:e00520.
Li C, Fan Z, Lin X, Cao M, Song F, Song F. Parity and risk of developing breast cancer according to tumor subtype: a systematic review and meta-analysis. Cancer Epidemiol. 2021;75:102050.
Wei L, Han N, Sun S, Ma X, Zhang Y. Sleep-disordered breathing and risk of the breast cancer: a meta-analysis of cohort studies. Int J Clin Pract. 2021;75:e14793.
Mokhtary A, Karakatsanis A, Valachis A. Mammographic density changes over time and breast cancer risk: a systematic review and meta-analysis. Cancers (Basel). 2021;13:4805.
Hayati Z, Jafarabadi MA, Pirouzpanah S. Dietary inflammatory index and breast cancer risk: an updated meta-analysis of observational studies. Eur J Clin Nutr. 2022;76:1073–87.
Monroy-iglesias MJ, Moss C, Beckmann K, Hammar N, Walldius G, Bosco C, et al. Serum total bilirubin and risk of cancer: a swedish cohort study and meta-analysis. Cancers (Basel). 2021;13:5540.
Schwarz C, Pedraza-Flechas AM, Pastor-Barriuso R, Lope V, de Larrea NF, Jiménez-Moleón JJ, et al. Long-term nightshift work and breast cancer risk: an updated systematic review and meta-analysis with special attention to menopausal status and to recent nightshift work. Cancers (Basel). 2021;13:5952.
Wang K, Ge M, Liu L, Lv H, Wang S, Jia F, et al. Birth weight and the risk of overall breast cancer, premenopausal and postmenopausal breast cancer in adulthood: a dose-response meta-analysis of observational studies. Menopause. 2022;29:114–24.
Markellos C, Ourailidou ME, Gavriatopoulou M, Halvatsiotis P, Sergentanis TN, Psaltopoulou T. Olive oil intake and cancer risk: a systematic review and meta-analysis. PLoS One. 2022;17:e0261649.
Brennan S, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review. Am J Clin Nutr. 2010;91:1294–302.
Long T, Liu K, Long J, Li J, Cheng L. Dietary glycemic index, glycemic load and cancer risk: a meta-analysis of prospective cohort studies. Eur J Nutr. 2022;61:2115–27.
Nappi C, Klain M, Cantoni V, Green R, Piscopo L, Volpe F, et al. Risk of primary breast cancer in patients with differentiated thyroid cancer undergoing radioactive iodine therapy: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2022;49:1630–9.
Bommareddy K, Hamade H, Lopez-Olivo MA, Wehner M, Tosh T, Barbieri JS. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275–82.
Hesari E, Ahmadinezhad M, Arshadi M, Azizi H, Khodamoradi F. The association between migraine and breast cancer risk: a systematic review and meta-analysis. PLoS One. 2022;17:1–13.
Cullinane C, Gillan H, Geraghty J, Evoy D, Rothwell J, Mccartan D, et al. Fertility treatment and breast-cancer incidence: meta-analysis. BJS Open. 2022;6:zrab149.
Wang Y, Song Z, Zhang S, Wang X, Li P. Risk-reducing salpingo-oophorectomy and breast cancer risk in BRCA1 or BRCA2 mutation carriers: a systematic review and meta-analysis. Eur J Surg Oncol. 2022;48:1209–16.
Li N, Guo X, Sun C, Lowe S, Su W, Song Q, et al. Dietary carbohydrate intake is associated with a lower risk of breast cancer: a meta-analysis of cohort studies. Nutr Res. 2022;100:70–92.
Liu F, Peng Y, Qiao Y, Huang Y, Song F, Zhang M, et al. Consumption of flavonoids and risk of hormone-related cancers: a systematic review and meta-analysis of observational studies. Nutr J. 2022;21:27.
Zare Sakhvidi MJ, Yang J, Mehrparvar AH, Dzhambov AM, Ebrahimi AA, Dadvand P, et al. Exposure to greenspace and cancer incidence, prevalence, and mortality: a systematic review and meta-analyses. Sci Total Environ. 2022;838(Pt 2):156180.
Parra-Soto S, Ahumada D, Petermann-Rocha F, Boonpoor J, Gallegos JL, Anderson J, et al. Association of meat, vegetarian, pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer: findings from the UK Biobank prospective cohort study and meta-analysis. BMC Med. 2022;20:79.
Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr. 2010;92:141–53.
Hong J, He Y, Fu R, Si Y, Xu B, Xu J, et al. The relationship between night shift work and breast cancer incidence: a systematic review and meta-analysis of observational studies. Open Med. 2022;12:712–31.
Liu H, Shi S, Gao J, Guo J, Li M, Wang L. Analysis of risk factors associated with breast cancer in women: a systematic review and meta-analysis. Transl Cancer Res. 2022;11:1344–53.
Najdi N, Esmailzadeh A, Shokrpour M, Nikfar S, Razavi SZ, Sepidarkish M, et al. A systematic review and meta-analysis on tubal ligation and breast cancer risk. Syst Rev. 2022;11:126.
Jiang H, Liu H, Liu G, Yu J, Liu N, Jin Y, et al. Associations between polyfluoroalkyl substances exposure and breast cancer: a meta-analysis. Toxics. 2022;10:318.
Zhang J, Yang J. Allium vegetables intake and risk of breast cancer: a meta-analysis. Iran J Public Health. 2022;51:746–57.
Kacimi SEO, Elgenidy A, Cheema HA, Ould Setti M, Khosla AA, Benmelouka AY, et al. Prior tonsillectomy and the risk of breast cancer in females: a systematic review and meta-analysis. Front Oncol. 2022;12:925596.
Chen C, Chen X, Wu D, Wang H, Wang C, Shen J, et al. Association of birth weight with cancer risk: a dose–response meta-analysis and Mendelian randomization study. J Cancer Res Clin Oncol. 2023;149:3925–35.
Nouri M, Mohsenpour MA, Katsiki N, Ghobadi S, Jafari A, Faghih S, et al. Effect of serum lipid profile on the risk of breast cancer: systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11:4503.
Leung JCN, Ng DWY, Chu RYK, Chan EWW, Huang L, Lum DH, et al. Association of antipsychotic use with breast cancer: a systematic review and meta-analysis of observational studies with over 2 million individuals. Epidemiol Psychiatr Sci. 2022;31:e61.
Baek K, Park JT, Kwak K. Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies. Epidemiol Health. 2022;44:e2022070.
EHaBCCG, Key TJ, Appleby PN, Reeves GK, Roddam AW, Helzlsouer KJ, et al. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–42.
Bodewes FTH, van Asselt AA, Dorrius MD, Greuter MJW, de Bock GH. Mammographic breast density and the risk of breast cancer: a systematic review and meta-analysis. Breast. 2022;66:62–8.
Reng Q, Zhu LL, Feng L, Li YJ, Zhu YX, Wang TT, et al. Dietary meat mutagens intake and cancer risk: a systematic review and meta-analysis. Front Nutr. 2022;9:962688.
Tran TVT, Kitahara CM, Leenhardt L, de Vathaire F, Boutron-Ruault MC, Journy N. The effect of thyroid dysfunction on breast cancer risk: an updated meta-analysis. Endocr Relat Cancer. 2023;30:1–42.
Thalib L, Doi SAR, Daher-Nashif S, Kane T, Furuya-Kanamori L. Does the sex of the firstborn child affect the breast cancer risk and survival: a systematic review and meta-analysis of over 1 million cases. Balk Med J. 2022;39:429–64.
Bamia C, Turati F, Guha N, van den Brandt P, Loomis D, Ferraroni M, et al. The role of coffee consumption in breast and ovarian cancer risk: updated meta-analyses. Epidemiol Biostat Public Heal. 2019;16:e13078.
Rezaianzadeh A, Ghorbani M, Rezaeian S, Kassani A. Red meat consumption and breast cancer risk in premenopausal women: a systematic review and meta-analysis. Middle East J Cancer. 2018;9:5–12.
Kalankesh LR, Rodríguez-Couto S, Zazouli MA, Moosazadeh M, Mousavinasab S. Do disinfection byproducts in drinking water have an effect on human cancer risk worldwide? A meta-analysis. Environ Qual Manag. 2019;29:105–19.
Raza W, Krachler B, Forsberg B, Sommar JN. Health benefits of leisure time and commuting physical activity: a meta-analysis of effects on morbidity. J Transp Heal. 2020;18:100873.
Han M, Wang Y, Jin Y, Zhao X, Cui H, Wang G, et al. Benign thyroid disease and the risk of breast cancer: an updated systematic review and meta-analysis. Front Endocrinol (Lausanne). 2022;13:984593.
Khatami A, Pormohammad A, Farzi R, Saadati H, Mehrabi M, Kiani SJ, et al. Bovine Leukemia virus (BLV) and risk of breast cancer: a systematic review and meta-analysis of case-control studies. Infect Agent Cancer. 2020;15:48.
Jansen-Van Der Weide MC, Greuter MJW, Jansen L, Oosterwijk JC, Pijnappel RM, De Bock GH. Exposure to low-dose radiation and the risk of breast cancer among women with a familial or genetic predisposition: a meta-analysis. Eur Radiol. 2010;20:2547–56.
Vrieling A, Buck K, Kaaks R, Chang-Claude J. Adult weight gain in relation to breast cancer risk by estrogen and progesterone receptor status: a meta-analysis. Breast Cancer Res Treat. 2010;123:641–9.
Zreik TG, Mazloom A, Chen Y, Vannucci M, Pinnix CC, Fulton S, et al. Fertility drugs and the risk of breast cancer: a meta-analysis and review. Breast Cancer Res Treat. 2010;124:13–26.
Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: a meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127:23–31.
Chan ALF, Leung HWC, Wang SF. Multivitamin supplement use and risk of breast cancer: a meta-analysis. Ann Pharmacother. 2011;45:476–84.
Fulan H, Changxing J, Yi Baina W, Wencui Z, Chunqing L, Fan W, et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control. 2011;22:1383–96.
Hu F, Wang Yi B, Zhang W, Liang J, Lin C, Li D, et al. Carotenoids and breast cancer risk: a meta-analysis and meta-regression. Breast Cancer Res Treat. 2012;131:239–53.
Walker K, Bratton DJ, Frost C. Premenopausal endogenous oestrogen levels and breast cancer risk: a meta-analysis. Br J Cancer. 2011;105:1451–7.
Kim HS, Woo OH, Park KH, Woo SU, Yang DS, Kim AR, et al. The relationship between twin births and maternal risk of breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;131:671–7.
Chen L, Zhou WB, Zhao Y, Liu XA, Ding Q, Zha XM, et al. Bloody nipple discharge is a predictor of breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2012;132:9–14.
Aune D, Chan DSM, Greenwood DC, Vieira AR, Navarro Rosenblatt DA, Vieira R, et al. Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23:1394–402.
Hardefeldt PJ, Eslick GD, Edirimanne S. Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;133:1169–77.
Nagata C, Hu YH, Shimizu H. Effects of menstrual and reproductive factors on the risk of breast cancer: meta-analysis of the case-control studies in Japan. Japanese J Cancer Res. 1995;86:910–5.
Aune D, Chan DSM, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;134:479–93.
Aune D, Chan DSM, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, et al. Dietary compared with blood concentrations of carotenoids and breast cancer risk: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2012;96:356–73.
Du X, Zhang R, Xue Y, Li D, Cai J, Zhou S, et al. Insulin glargine and risk of cancer: a meta-analysis. Int J Biol Markers. 2012;27:e241–6.
Liu X, Lv K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: a meta-analysis. Breast. 2013;22:309–13.
Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19:793–803.
Ni XJ, Xia TS, Zhao YC, Ma JJ, Zhao J, Liu XA, et al. Postmenopausal hormone therapy is associated with in situ breast cancer risk. Asian Pacific J Cancer Prev. 2012;13:3917–25.
Boyle P, Koechlin A, Pizot C, Boniol M, Robertson C, Mullie P, et al. Blood glucose concentrations and breast cancer risk in women without diabetes: a meta-analysis. Eur J Nutr. 2013;52:1533–40.
Colmers IN, Bowker SL, Tjosvold LA, Johnson JA. Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab. 2012;38:485–506.
Bonifazi M, Tramacere I, Pomponio G, Gabrielli B, Avvedimento EV, La Vecchia C, et al. Systemic sclerosis (scleroderma) and cancer risk: systematic review and meta-analysis of observational studies. Rheumatology. 2013;52:143–54.
Antoni S, Sasco AJ, Dos Santos SI, McCormack V. Is mammographic density differentially associated with breast cancer according to receptor status? A meta-analysis. Breast Cancer Res Treat. 2013;137:337–47.
Bernier MO, Bossard N, Ayzac L, Thalabard JC. Breastfeeding and risk of breast cancer: a meta-analysis of published studies. Hum Reprod Update. 2000;6:374–86.
Kim JS, Kang EJ, Woo OH, Park KH, Woo SU, Yang DS, et al. The relationship between preeclampsia, pregnancy-induced hypertension and maternal risk of breast cancer: a meta-analysis. Acta Oncol. 2013;52:1643–8.
Tang X, Yang L, He Z, Liu J. Insulin glargine and cancer risk in patients with diabetes: a meta-analysis. PLoS One. 2012;7:e51814.
Kamdar BB, Tergas AI, Mateen FJ, Bhayani NH, Oh J. Night-shift work and risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;138:291–301.
Gaudet MM, Patel AV, Sun J, Teras LR, Gapstur SM. Tubal sterilization and breast cancer incidence: results from the cancer prevention study II nutrition cohort and meta-analysis. Am J Epidemiol. 2013;177:492–9.
Song JK, Bae JM. Citrus fruit intake and breast cancer risk: a quantitative systematic review. J Breast Cancer. 2013;16:72–6.
Anothaisintawee T, Wiratkapun C, Lerdsitthichai P, Kasamesup V, Wongwaisayawan S, Srinakarin J, et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia-Pacific J Public Heal. 2013;25:368–87.
Miao SY, Zhou W, Chen L, Wang S, Liu XA. Influence of ABO blood group and Rhesus factor on breast cancer risk: a meta-analysis of 9665 breast cancer patients and 244768 controls. Asia Pac J Clin Oncol. 2014;10:101–8.
Ijaz S, Verbeek J, Seidler A, Lindbohm ML, Ojajärvi A, Orsini N, et al. Night-shift work and breast cancer - a systematic review and meta-analysis. Scand J Work Environ Heal. 2013;39:431–47.
Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706.
Sergentanis TN, Diamantaras AA, Perlepe C, Kanavidis P, Skalkidou A, Petridou ET. IVF and breast cancer: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:106–23.
Erren T. A meta-analysis of epidemiologic studies of electric and magnetic fields and breast cancer in women and men. Bioelectromagnetics. 2001;Suppl 5:S105–19.
Wu W, Kang S, Zhang D. Association of vitamin B6, vitamin B12 and methionine with risk of breast cancer: a dose-response meta-analysis. Br J Cancer. 2013;109:1926–44.
Ingber SZ, Buser MC, Pohl HR, Abadin HG, Edward Murray H, Scinicariello F. DDT/DDE and breast cancer: a meta-analysis. Regul Toxicol Pharmacol. 2013;67:421–33.
Liu T, Xu QE, Zhang CH, Zhang P. Occupational exposure to methylene chloride and risk of cancer: a meta-analysis. Cancer Causes Control. 2013;24:2037–49.
Qin Y, Zhou Y, Zhang X, Wei X, He J. Sleep duration and breast cancer risk: a meta-analysis of observational studies. Int J Cancer. 2014;134:1166–73.
Moorman PG, Havrilesky LJ, Gierisch JM, Coeytaux RR, Lowery WJ, Urrutia RP, et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J Clin Oncol. 2013;31:4188–98.
Huang Y, Zhang X, Li W, Song F, Dai H, Wang J, et al. A meta-analysis of the association between induced abortion and breast cancer risk among Chinese females. Cancer Causes Control. 2014;25:227–36.
Catalá-López F, Suárez-Pinilla M, Suárez-Pinilla P, Valderas JM, Gómez-Beneyto M, Martinez S, et al. Inverse and direct cancer comorbidity in people with central nervous system disorders: a meta-analysis of cancer incidence in 577,013 participants of 50 observational studies. Psychother Psychosom. 2014;83:89–105.
Gao Y, Huang YB, Liu XO, Chen C, Dai HJ, Song FJ, et al. Tea consumption, alcohol drinking and physical activity associations with breast cancer risk among Chinese females: a systematic review and meta-analysis. Asian Pacific J Cancer Prev. 2013;14:7543–50.
Si R, Qu K, Jiang Z, Yang X, Gao P. Egg consumption and breast cancer risk: a meta-analysis. Breast Cancer. 2014;21:251–61.
Yang B, Ren XL, Fu YQ, Gao JL, Li D. Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies. BMC Cancer. 2014;14:105.
Boyd NF, Stone J, Vogt KN, Connelly BS, Martin LJ, Minkin S. Dietary fat and breast cancer risk revisited: a meta-analysis of the published literature. Br J Cancer. 2003;89:1672–85.
Chen M, Rao Y, Zheng Y, Wei S, Li Y, Guo T, et al. Association between soy isoflavone intake and breast cancer risk for pre- and post-menopausal women: a meta-analysis of epidemiological studies. PLoS One. 2014;9:e89288.
Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, et al. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197.
Li J, Zou L, Chen W, Zhu B, Shen N, Ke J, et al. Dietary mushroom intake may reduce the risk of breast cancer: evidence from a meta-analysis of observational studies. PLoS One. 2014;9:e93437.
Tio M, Andrici J, Eslick GD. Folate intake and the risk of breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;145:513–24.
Hernandez AV, Guarnizo M, Miranda Y, Pasupuleti V, Deshpande A, Paico S, et al. Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PLoS One. 2014;9:e99317.
Wang F, Hou J, Shen Q, Yue Y, Xie F, Wang X, et al. Mouse mammary tumor virus-like virus infection and the risk of human breast cancer: a meta-analysis. Am J Transl Res. 2014;6:248–66.
Park JH, Cha ES, Ko Y, Hwang MS, Hong JH, Lee WJ. Exposure to dichlorodiphenyltrichloroethane and the risk of breast cancer: a systematic review and meta-analysis. Osong Public Heal Res Perspect. 2014;5:77–84.
Lv M, Zhu X, Zhong S, Chen W, Hu Q, Ma T, et al. Radial scars and subsequent breast cancer risk: a meta-analysis. PLoS One. 2014;9:e102503.
Nie XC, Dong DS, Bai Y, Xia P. Meta-analysis of black tea consumption and breast cancer risk: update 2013. Nutr Cancer. 2014;66:1009–14.
He C, Anand ST, Ebell MH, Vena JE, Robb SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health. 2015;88:533–47.
González-Pérez A, García Rodríguez LA, López-Ridaura R. Effects of non-steroidal anti-inflammatory drugs on cancer sites other than the colon and rectum: a metal-analysis. BMC Cancer. 2003;3:1–12.
Hu F, Wu Z, Li G, Teng C, Liu Y, Wang F, et al. The plasma level of retinol, vitamins A, C and α-tocopherol could reduce breast cancer risk? A meta-analysis and meta-regression. J Cancer Res Clin Oncol. 2015;141:601–14.
Woo HD, Park S, Oh K, Kim HJ, Shin HR, Moon HK, et al. Diet and cancer risk in the Korean population: a meta-analysis. Asian Pacific J Cancer Prev. 2014;15:8509–19.
Pelucchi C, Bosetti C, Galeone C, La Vecchia C. Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer. 2015;136:2912–22.
de Pedro M, Baeza S, Escudero MT, Dierssen-Sotos T, Gómez-Acebo I, Pollán M, et al. Effect of COX-2 inhibitors and other non-steroidal inflammatory drugs on breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2015;149:525–36.
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. JNCI J Natl Cancer Inst. 2015;107:88.
Gennari A, Costa M, Puntoni M, Paleari L, De Censi A, Sormani MP, et al. Breast cancer incidence after hormonal treatments for infertility: systematic review and meta-analysis of population-based studies. Breast Cancer Res Treat. 2015;150:405–13.
Guo J, Huang Y, Yang L, Xie Z, Song S, Yin J, et al. Association between abortion and breast cancer: an updated systematic review and meta-analysis based on prospective studies. Cancer Causes Control. 2015;26:811–9.
Wu YC, Zheng D, Sun JJ, Zou ZK, Ma ZL. Meta-analysis of studies on breast cancer risk and diet in Chinese women. Int J Clin Exp Med. 2015;8:73–85.
Chen LX, Ning GZ, Zhou ZR, Li YL, Zhang D, Wu QL, et al. The carcinogenicity of alendronate in patients with osteoporosis: evidence from cohort studies. PLoS One. 2015;10:e0123080.
Akinyemiju TF, Genkinger JM, Farhat M, Wilson A, Gary-Webb TL, Tehranifar P. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer. 2015;15:191.
Shi R, Yu H, McLarty J, Glass J. IGF-I and breast cancer: a meta-analysis. Int J Cancer. 2004;111:418–23.
Sun HL, Dong XX, Cong YJ, Gan Y, Deng J, Cao SY, et al. Depression and the risk of breast cancer: a meta-analysis of cohort studies. Asian Pacific J Cancer Prev. 2015;16:3233–9.
Jones L, Bates G, McCoy E, Bellis MA. Relationship between alcohol-attributable disease and socioeconomic status, and the role of alcohol consumption in this relationship: a systematic review and meta-analysis. BMC Public Health. 2015;15:400.
Guo L, Liu S, Zhang S, Chen Q, Zhang M, Quan P, et al. C-reactive protein and risk of breast cancer: a systematic review and meta-analysis. Sci Rep. 2015;5:10508.
Zhou Y, Zhao H, Peng C. Association of sedentary behavior with the risk of breast cancer in women: update meta-analysis of observational studies. Ann Epidemiol. 2015;25:687–97.
Simmonds M, Burch J, Llewellyn A, Griffiths C, Yang H, Owen C, et al. The use of measures of obesity in childhood for predicting obesity and the development of obesity-related diseases in adulthood: a systematic review and meta-analysis. Health Technol Assess (Rockv). 2015;19:1–336.
Touvier M, Fassier P, His M, Norat T, Chan DSM, Blacher J, et al. Cholesterol and breast cancer risk: a systematic review and meta-analysis of prospective studies. Br J Nutr. 2015;114:347–57.
Keum N, Lee DH, Marchand N, Oh H, Liu H, Aune D, et al. Egg intake and cancers of the breast, ovary and prostate: a dose-response meta-analysis of prospective observational studies. Br J Nutr. 2015;114:1099–107.
Zhang B, Shu XO, Delahanty RJ, Zeng C, Michailidou K, Bolla MK, et al. Height and breast cancer risk: evidence from prospective studies and mendelian randomization. J Natl Cancer Inst. 2015;107:djv219.
Zhong S, Chen L, Zhang X, Yu D, Tang J, Zhao J. Aspirin use and risk of breast cancer: systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2015;24:1645–55.
Zhou Y, Wang T, Zhai S, Li W, Meng Q. Linoleic acid and breast cancer risk: a meta-analysis. Public Health Nutr. 2016;19:1457–63.
Hoxha I, Sadiku F, Hoxha L, Nasim M, Christine Buteau MA, Grezda K, et al. Breast cancer and lifestyle factors: umbrella review. Hematol Oncol Clin North Am. 2023;38:137–70.
Jabbari M, Pourmoradian S, Eini-Zinab H, Mosharkesh E, Hosseini Balam F, Yaghmaei Y, et al. Levels of evidence for the association between different food groups/items consumption and the risk of various cancer sites: an umbrella review. Int J Food Sci Nutr. 2022;73:861–74.
Cao F, Li YZ, Zhang DY, Wang XY, Chen WX, Liu FH, et al. Human papillomavirus infection and the risk of cancer at specific sites other than anogenital tract and oropharyngeal region: an umbrella review. eBioMedicine. 2024;104:105155.
Kühn T, Kalotai N, Amini AM, Haardt J, Lehmann A, Schmidt A, et al. Protein intake and cancer: an umbrella review of systematic reviews for the evidence-based guideline of the German Nutrition Society. Eur J Nutr. 2024;13:1–16. https://doi.org/10.1007/S00394-024-03380-4/TABLES/2.
Aramburu A, Dolores-Maldonado G, Curi-Quinto K, Cueva K, Alvarado-Gamarra G, Alcalá-Marcos K, et al. Effect of reducing saturated fat intake on cardiovascular disease in adults: an umbrella review. Front Public Heal. 2024;12:1396576.
Hu J, Wang J, Li Y, Xue K, Kan J. Use of dietary fibers in reducing the risk of several cancer types: an umbrella review. Nutrients. 2023;15:2545.
Lee JS, Lee YA, Shin CH, Suh DI, Lee YJ, Yon DK. Long-term health outcomes of early menarche in women: an umbrella review. QJM An Int J Med. 2022;115:837–47.
Grosso G, Godos J, Galvano F, Giovannucci EL. Coffee, caffeine, and health outcomes: an umbrella review. Annu Rev Nutr. 2017;37:131–56.
Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, et al. Coffee drinking and cancer risk: an umbrella review of meta-analyses of observational studies. BMC Cancer. 2020;20:101.
Friedenreich C. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001;10:15–32.
Okasha M, McCarron P, Gunnell D, Smith G. Exposures in childhood, adolescence and early adulthood and breast cancer risk: a systematic review of the literature. Breast Cancer Res Treat. 2003;78:223–76.
Gunnell D, Okasha M, Smith G, Oliver S, Sandhu J, Holly J. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–42.
Lai FY, Nath M, Hamby SE, Thompson JR, Nelson CP, Samani NJ. Adult height and risk of 50 diseases: a combined epidemiological and genetic analysis. BMC Med. 2018;16:1–18.
Rosenfeld R. Insulin-like growth factors and the basis of growth. N Engl J Med. 2003;349:2184–6.
Sherman B, Wallace R, Bean J, Schlabaugh L. Relationship of body weight to menarcheal and menopausal age: implications for breast cancer risk. J Clin Endocrinol Metab. 1981;52:488–93.
Key T, Pike M. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24:29–43.
Siiteri P, Murai J, Hammond G, Nisker J, Raymoure W, Kuhn R. The serum transport of steroid hormones. Recent Prog Horm Res. 1982;38:457–510.
Russo J, Russo I. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–37.
Avvakumov G, Cherkasov A, Muller Y, Hammond G. Structural analyses of sex hormone-binding globulin reveal novel ligands and function. Mol Cell Endocrinol. 2010;316:13–23.
Rosner W, Hryb D, Kahn S, Nakhla A, Romas N. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol. 2010;316:79–85.
Farhat G, Parimi N, Chlebowski R, Manson J, Anderson G, Huang A, et al. Sex hormone levels and risk of breast cancer with estrogen plus progestin. J Natl Cancer Inst. 2013;105:1496–503.
Sieri S, Muti P, Claudia A, Berrino F, Pala V, Grioni S, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J Cancer. 2012;130:921–9.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol Consumption and Ethyl Carbamate. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer. 2010;96:3–1383.
World Health Organization. Alcohol is one of the biggest risk factors for breast cancer. https://www.who.int/europe/news/item/20-10-2021-alcohol-is-one-of-the-biggest-risk-factors-for-breast-cancer. Accessed 23 May 2024.
Starek-Świechowicz B, Budziszewska B, Starek A. Alcohol and breast cancer. Pharmacol Rep. 2023;75:69.
Jiang X, Dimou NL, Al-Dabhani K, Lewis SJ, Martin RM, Haycock PC, et al. Circulating vitamin D concentrations and risk of breast and prostate cancer: a Mendelian randomization study. Int J Epidemiol. 2019;48:1416.
Ong JS, Gharahkhani P, An J, Law MH, Whiteman DC, Neale RE, et al. Vitamin D and overall cancer risk and cancer mortality: a Mendelian randomization study. Hum Mol Genet. 2018;27:4315–22.
Dimitrakopoulou VI, Tsilidis KK, Haycock PC, Dimou NL, Al-Dabhani K, Martin RM, et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ. 2017;359:j4761.
Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, et al. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808.
Lange C, Richer J, Shen T, Horwitz K. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:31308–16.
Carvajal A, Espinoza N, Kato S, Pinto M, Sadarangani A, Monso C, et al. Progesterone pre-treatment potentiates EGF pathway signaling in the breast cancer cell line ZR-75. Breast Cancer Res Treat. 2005;94:171–83.
Zhao Y, Zhu S, Li X, Wang F, Hu F, Li D, et al. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–50.
Dontu G, El-Ashry D, Wicha M. Breast cancer, stem/progenytor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–7.
Acknowledgements
Not applicable.
Funding
Open access funding provided by the Cyprus Libraries Consortium (CLC). None declared.
Author information
Authors and Affiliations
Contributions
Conceptualisation, AY and GKN; Literature search, GM; Study selection, data extraction, and analysis KP, GM, AP, AG, AH, ME, KL, EK, CK, MC, and MT; Data curation, KP, GM, AP, and NC; Formal analysis, KP and GM; Investigation, AY, KP, and GM; Methodology, AY, KP, GM, DP, KKT, SB, and GKN; Project administration, AY, KP, GM, and GKN; Resources, AY and GKN; Interpretation, AY, KP, GM, DP, KKT, SB, and GKN; Writing – original draft, AY, KP, and GM; Critical review, DP, KKT, SB, and GKN; Writing – revision of original draft, all authors. All authors reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yiallourou, A., Pantavou, K., Markozannes, G. et al. Non-genetic factors and breast cancer: an umbrella review of meta-analyses. BMC Cancer 24, 903 (2024). https://doi.org/10.1186/s12885-024-12641-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12641-8