Abstract
We examined the expression of programmed death-ligand 1 (PD-L1) in carcinoma of unknown primary (CUP) and its potential implications. Tissue microarrays were constructed for 72 CUP cases (histologic subtypes: 22 adenocarcinoma, 15 poorly differentiated carcinoma, 19 squamous cell carcinoma, and 14 undifferentiated carcinoma; clinical subtype: favorable type 17 [23.6%], unfavorable type 55 [76.4%]), with immunohistochemical staining performed for PD-L1 (22C3, SP142, SP263, and 28 − 8), CK7, and CK20 to determine the association between staining results and clinicopathological parameters. In CUP, the PD-L1 positivity rate was 5.6–48.6% (tumor cells [TC] or tumor proportion score [TPS]: 5.6–36.1%, immune cell score [IC]: 8.3–48.6%, combined positive score [CPS]: 16.7%) using different cutoff values for 22C3 (TPS ≥ 1%, CPS ≥ 10), SP142 (TC ≥ 50%, IC ≥ 10%), SP263, and 28 − 8 (TC and IC ≥ 1%). PD-L1 SP142 TC and PD-L1 SP263 IC showed the lowest (5.6%) and highest (48.6%) positivity rates, respectively. The PD-L1 positivity rate did not significantly differ based on the histologic subtype, clinical subtype, or CK7/CK20 across clones. Considering TC κ ≥ 1%, TC κ ≥ 50%, IC κ ≥ 1%, and IC κ ≥ 10%, the PD-L1 positivity rate was TC = 4.2–36.1% and IC = 9.7–48.6%; the overall agreement between antibodies ranged from 69.4 to 93.1%, showing fair or better agreement (κ ≥ 0.21). In CUP, PD-L1 positivity varied depending on antibodies and scoring systems, with no difference observed according to histologic or clinical subtypes.
Simple Summary
Carcinoma of unknown primary (CUP) refers to a heterogeneous collection of cancers where metastatic growth is observed, but the origin of the primary tumor remains unidentified. The type of primary cancer is critical for establishing the treatment strategy in metastatic carcinoma, presenting a considerable challenge in CUP. Patients with programmed death-ligand 1 (PD-L1)–positive tumors are well-known to benefit from targeted therapy against PD-L1. However, the expression of PD-L1 in CUP remains poorly explored. The present study demonstrated that PD-L1 was expressed in CUP with varying positivity rates depending on the antibody and scoring system employed. There was no difference in PD-L1 expression based on histological or clinical subtypes. Based on PD-L1 expression, immune checkpoint inhibitors could afford an effective treatment strategy in CUP.
Similar content being viewed by others
Introduction
Carcinoma of unknown primary origin (CUP) is a metastatic carcinoma in which the primary tumor remains elusive even after evaluation of the clinical history, physical examination, radiological findings, laboratory tests and other diagnostic investigations [1]. CUP accounts for approximately 5–15% of malignant tumors [2,3,4], and advances in imaging and molecular testing have reduced this proportion to 1–2% in recent years [5]. Histologically, CUP comprises adenocarcinomas (50–60%) or poorly differentiated carcinomas (30–40%), with other histological types, including squamous cell carcinomas (5–8%) and undifferentiated carcinomas (2–5%) [4, 6]. Although the precise nature of CUP remains uncertain, two main hypotheses have been suggested: the first postulates that CUP represents a true metastatic tumor with a primary focus that is markedly small to be identified; the second suggests that CUP is a distinct entity with independent characteristics due to regression or dormancy of the primary lesion, known as the ‘true’ or ‘true” genuine’ or ‘genuine’ CUP hypothesis [6].
Treatment planning for metastatic carcinoma is generally determined by the type of primary cancer, making the absence of a known primary tumor in CUP a critical treatment challenge. The traditional diagnostic and treatment algorithm for CUP involves identifying favorable subgroups by undertaking a traditional diagnostic work-up and administering tissue origin-specific therapy while administering empirical chemotherapy or tissue origin-specific therapy based on the characteristics of each CUP in unfavorable subgroups [7]. Techniques such as immunohistochemistry (IHC) and molecular tools such as gene expression profiling, miRNA expression, and DNA methylation analysis have been employed to determine the most appropriate tissue-of-origin for a specific CUP [8]. Furthermore, precision medicine concepts based on advances in genomic tools are being applied to CUP to attempt targeted therapy by identifying possible treatment targets [9]. Therefore, identifying an appropriate treatment target for CUP is crucial to ensure proper treatment.
Programmed death 1 (PD-1) is an immune checkpoint molecule found on different immune cells, playing a crucial role in immune responses [10]. Conversely, programmed death-ligand 1 (PD-L1) acts as a ligand for PD-1. Tumor cells express PD-L1, which facilitates their evasion of antitumor immune responses by interacting with PD-1 and forming a suppressive pathway [11, 12]. PD-L1 is expressed in 20–70% of tumors, including lung cancer [11, 13,14,15,16], urinary bladder cancer [17], malignant melanoma [18], ovarian cancer [19], breast cancer [20, 21], and gastric cancer [22, 23]. In patients with PD-L1-positive tumors, targeted therapy against PD-L1 can be used to induce an antitumor immune response. Notably, PD-L1 inhibitors have been approved as effective treatments for non-small cell lung cancer, urothelial carcinoma, gastric carcinoma, esophageal carcinoma, cervical cancer, and triple-negative breast cancer (TNBC) [24]. In addition, various drugs such as pembrolizumab, atezolizumab, durvalumab, nivolumab, and ipilimumab have been developed as PD-L1 inhibitors [25]. Therefore, it is important to determine whether PD-L1 is expressed in tumor cells prior to targeted therapy. The most common and simple method for detecting PD-L1 expression is IHC using a monoclonal PD-L1 antibody on formalin-fixed paraffin-embedded (FFPE) specimens. Monoclonal PD-L1 antibodies, such as clone 28 − 8 [26], 22C3 [27], SP142 [14, 17], and SP263 [28] are commercially available, and appropriate antibodies and scoring systems have been established as companion diagnostics for different types of cancer. Although several studies have investigated PD-L1 expression in various tumors using various antibodies, PD-L1 expression in CUP has been poorly explored. Therefore, the purpose of the present study was to examine PD-L1 expression in CUP according to different antibodies and scoring systems and to explore its implications.
Materials and methods
Patient selection and clinicopathologic evaluation
In this study, we utilized FFPE tissue samples obtained from patients with Carcinoma of Unknown Primary (CUP) at Severance Hospital. The study adhered to the principles of the Declaration of Helsinki and obtained approval from the Institutional Review Board of Yonsei University Severance Hospital (IRB number: 4-2022-1380). Due to the retrospective nature of the study, patient consent was exempted by the Institutional Review Board of Yonsei University Severance Hospital.
The selected patients were diagnosed with metastatic carcinoma by a pathologist between January 1999 and December 2012. In this study, needle biopsies yielding insufficient tissue for TMA construction were excluded, while excisional biopsies suitable for TMA construction were included. Cases that received chemotherapy or targeted therapy before tissue diagnosis were excluded. All available hematoxylin and eosin (H&E)-stained slides were carefully reviewed. Clinicopathological parameters, including patient age, sex, histological type, organ involvement, and patient outcomes, were assessed for each tumor. Based on histological criteria, CUPs were categorized into four distinct groups [29]: adenocarcinomas (ADCs) displayed glandular differentiation, while squamous cell carcinomas (SCCs) exhibited evidence of squamous differentiation. Poorly differentiated carcinomas (PDCs) did not exhibit any specific lineage differentiation, and undifferentiated carcinomas (UDCs) consisted of syncytial tumor cell nests or individual tumor cells closely intertwined with dense lymphoplasmacytic infiltration, resembling the pattern seen in nasopharyngeal undifferentiated carcinomas. Additionally, CUPs were classified into favorable and unfavorable subgroups according to international guidelines [7, 30]. In accordance with international guidelines, the following nine scenarios are defined as the favorable subgroup. In this study, these same nine scenarios were also defined as the favorable subgroup; (1) poorly differentiated neuroendocrine CUP, (2) well-differentiated neuroendocrine tumor of unknown primary, (3) peritoneal adenocarcinomatosis of a serous papillary in females, (4) isolated axillary nodal metastases in females, (5) SCC involving non-supraclavicular cervical lymph nodes, (6) CUP with a colorectal IHC or molecular profile, (7) single metastatic deposit from unknown primary, (8) males with blastic bone metastases or IHC/serum prostate-specific antigen expression, and (9) SCC involving isolated inguinal adenopathy. CUP cases outside the defined favorable subgroup were categorized as the unfavorable subgroup.
Tissue microarray
Following the assessment of H&E-stained slides, suitable FFPE tumor tissue samples were retrospectively gathered, focusing on the most representative tumor region, which was carefully demarcated. A punch machine was utilized to extract the chosen area, and a 3 mm tissue core was inserted into a 6 × 5 recipient block. For each sample, tissue microarrays were created, with two tissue cores included in each array.
IHC
Immunohistochemistry (IHC) was conducted on FFPE tissue sections, and the antibodies employed are specified in Supplementary Table 1. Briefly, 3-µm thick tissue sections were prepared from paraffin blocks and then deparaffinized and rehydrated using xylene and alcohol solution. The IHC procedure was carried out using a Ventana Discovery XT automated stainer (Ventana Medical System, Tucson, AZ, USA). Antigen retrieval was achieved using CC1 buffer (Cell Conditioning 1; citrate buffer, pH 6.0; Ventana Medical System). Immunohistochemical staining was performed, incorporating appropriate positive and negative controls. For the negative control group, the primary antibody incubation step was omitted. Each antibody’s recommended positive control, as specified by the manufacturer, was utilized.
Interpretation of immunohistochemical results
Immunohistochemical staining of PD-L1 was performed according to the antibody used. PD-L1 22C3 expression was evaluated using tumor cells (TC) (tumor proportion score [TPS]), immune cell score (IC), and combined positive score (CPS). TPS was calculated by dividing the number of PD-L1 staining tumor cells by the number of viable tumor cells and multiplying by 100%. The CPS was calculated by dividing the number of PD-L1 staining cells (including tumor cells, lymphocytes, and histiocytes) by the number of viable tumor cells and multiplying by 100%. PD-L1 28 − 8, SP142, and SP263 were evaluated for TC and IC. TC was defined as the percentage of tumor cells showing any intensity of membranous staining for PD-L1, while IC was defined as the percentage of the tumor area occupied by PD-L1 staining immune cells (including lymphocytes, histiocytes, dendritic cells, and granulocytes). In this study, PD-L1 interpretation was conducted by two pathologists (HM Kim and JS Koo) who participated in the study, using a multi-view microscope. They determined TC, IC, and CPS of PD-L1 for each case while reviewing the TMA slides. For cases near the cut-off value, the two pathologists reached a final decision through consensus. The pathologist (JS Koo) who interpreted the PD-L1 IHC in this study is a board-certified pathologist with over 20 years of experience in the field. Their expertise lies particularly in breast cancer, where they have been routinely interpreting PD-L1 (SP142 and 22C3) for several years in daily practice. Additionally, they have published research papers on PD-L1 [31,32,33].
Two different methods were used to analyze the TPS, IC, and CPS. First, the cutoff values established for each PD-L1 clone in other tumor types were used. For PD-L1 22C3, TPS of ≥ 1 [34] and CPS of ≥ 10 were considered positive [35]. For PD-L1 SP142, TC of ≥ 50 and IC of ≥ 10 were considered positive [36]. For PD-L1 28 − 8 and SP263, TC and IC of ≥ 1 were considered positive [37]. Second, to compare the results for each antibody, the criteria for positivity were set as TC(TPS) ≥ 1%, TC(TPS) ≥ 50%, IC ≥ 1%, and IC ≥ 10%. For CK7 and CK20, the cutoff value was set at 10%; cases with < 10% staining were considered negative, whereas those with ≥ 10% staining were considered positive [38].
Statistical analysis
Data analysis was performed using SPSS for Windows (version 24.0; IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using Student’s t-test, while categorical variables were assessed using Fisher’s exact tests. The threshold for statistical significance was set at p < 0.05. To evaluate the agreement between any two PD-L1 antibody clones for each scoring method, Cohen’s kappa coefficient was utilized. The interpretation of the kappa coefficient values was as follows: <0 indicated no agreement, 0.0–0.20 represented slight agreement, 0.21–0.40 indicated fair agreement, 0.41–0.60 signified moderate agreement, 0.61–0.80 suggested substantial agreement, and 0.81–1.00 denoted almost perfect agreement [39]. Kaplan-Meier survival curves and log-rank statistics were employed to assess the survival time. Additionally, multivariate regression analysis was conducted using a Cox proportional hazards model.
Results
Basal characteristics of patients with CUP according to the histologic and clinical subtypes
Supplementary Tables 2 and 3 show the basal characteristics according to histological and clinical subtypes in the 72 CUP cases. Overall, 22 (30.6%) patients had ADC, 15 (20.8%) had PDC, 19 (26.4%) had SCC, and 16 (22.2%) had UDC. The clinical subtype was favorable in 17 (23.6%) and unfavorable in 55 (76.4%) cases. The involved organs were as follows: lymph nodes 49 (68.1%), bone 8 (11.1%), brain 7 (9.7%), and other 8 (11.1%). Moreover, there was a difference in clinical subtype according to the histologic subtype, with ADC and UDC showing a higher proportion of the unfavorable type, while SCC showed a higher proportion of the favorable type (p = 0.003). Additionally, postoperative treatment differed according to the histologic subtype, with chemotherapy most commonly employed in ADC, chemoradiotherapy in PDC, and surgery only in UDC (p = 0.007). Among the CUP cases, 37 (51.4%) were CK7 (+)/CK20 (-), 3 (4.2%) were CK7 (+)/CK20 (+), 3 (4.2%) were CK7 (-)/CK20 (+), and 29 (40.3%) were CK7 (-)/CK20 (-), with no significant difference in histologic subtype (p = 0.522).
PD-L1 expression in CUP
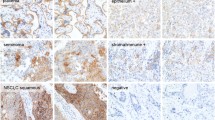
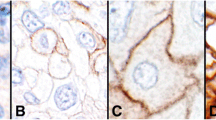
In CUP, tumor and immune cells exhibited PD-L1 expression at varying proportions and intensities (Fig. 1). PD-L1 expression was examined in CUP using cutoff values as follows: TPS ≥ 1%, CPS ≥ 10 for SP142; TC ≥ 50%, IC ≥ 10% for 22C3; TC and IC ≥ 1% for 28 − 8 and SP263. PD-L1 positivity rates ranged between 5.6 and 48.6%, with the lowest rate of 5.6% observed in PD-L1 SP142 TC and the highest rate of 48.6% in PD-L1 SP263 IC. PD-L1 positivity rates did not show significant differences according to histologic subtype (Table 1), clinical subtype (Table 2), or CK7/CK20 pattern (Table 3) across clones.
PD-L1 expression in tumor cells and immune cells in CUP histologic subtypes. In CUP, PD-L1 expression can be observed in both tumor and immune cells with varying proportions and intensities for the four histologic subtypes of ADC, PDC, SCC, and UDC using the four PD-L1 antibodies: 22C3, SP142, SP263, and 28 − 8. ADC, adenocarcinoma; CUP, carcinoma of unknown primary; PDC, poorly differentiated carcinoma; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma; UDC, undifferentiated carcinoma
Difference and concordance of PD-L1 expression in CUP according to PD-L1 antibody clones and Scoring systems
We then analyzed differences in PD-L1 expression among the four clones and scoring systems in CUP. For the TC system, PD-L1 positivity ranged between 18.1 and 36.1% for a cutoff value of 1% and between 4.2 and 20.8% for a cutoff value of 50%. Among the examined clones, 22C3 and SP263 showed the lowest and highest positivity rates, respectively. For the IC system, PD-L1 positivity ranged between 26.4 and 48.6% for a cutoff value of 1% and between 9.7 and 38.9% for a cutoff value of 10%. Among the clones, 28 − 8 and SP263 exhibited the lowest and highest positivity rates, respectively (Table 4).
Next, we examined the concordance of PD-L1 expression among clones according to the scoring system (Table 5). For TC ≥ 1%, all clones showed moderate or high agreement, with the highest agreement between 22C3 and SP142 (OA = 93.1%, ≥ =0.772) and the lowest agreement between 22C3 and SP263 (OA = 83.3%, ≥ =0.599). For TC κ 50%, all clones showed fair or higher agreement, with the highest agreement between 28 − 8 and SP263 (OA = 90.3%, κ = 0.664) and the lowest agreement between 22C3 and SP263 (OA = 83.3%, κ = 0.284). For IC ≥ 1%, all clones showed moderate or higher agreement, with the highest agreement between SP263 and SP142 (OA = 90.3%, κ = 0.805) and the lowest agreement between 28 − 8 and SP263 (OA = 77.8%, κ = 0.550). For IC κ 10%, all clones showed fair or high agreement, with the highest agreement between 22C3 and SP142 (OA = 91.7%, κ = 0.578) and the lowest agreement between SP142 and SP263 (OA = 69.4%, κ = 0.261).
Impact of clinicopathologic factors and PD-L1 status on prognosis of CUP
We subsequently performed univariate analysis to determine the impact of clinicopathological factors and PD-L1 expression on prognosis. We observed that the histological subtype was associated with shorter overall survival (OS) (UDC > SCC > ADC > PDC, p = 0.030), whereas PD-L1 expression was not significantly associated with shorter OS (Table 6). In subgroup analysis, PD-L1 SP263 TC positivity (p = 0.030) and PD-L1 SP263 IC negativity (p = 0.007) were significantly associated with shorter OS for CUP with ADC histologic subtypes. For CK7 positive CUP, PD-L1 SP263 IC negativity (p = 0.041) and PD-L1 28 − 8 IC negativity (p = 0.029) were significantly associated with a shorter OS. For CK7 and CK20 positive CUP and unfavorable clinical type CUP, PD-L1 28 − 8 IC negativity (p = 0.037 and p = 0.040, respectively) was significantly associated with shorter OS (Fig. 2).
Impact of clinicopathologic factors and PD-L1 status on the prognosis of CUP. In the case of ADC, PD-L1 SP263 TC positivity (p = 0.030) and PD-L1 SP263 IC negativity (p = 0.007) show a significant association with shorter overall survival. In CK7 positive CUP, PD-L1 SP263 IC negativity (p = 0.041) and PD-L1 28 − 8 IC negativity (p = 0.029) are significantly associated with shorter overall survival, while in CK7 and CK20 positive CUP and unfavorable clinical type CUP, PD-L1 28 − 8 IC negativity (p = 0.037 and p = 0.040, respectively) shows a significant association with shorter overall survival. ADC, adenocarcinoma; CUP, carcinoma of unknown primary; IC, immune cell score; PDC, poorly differentiated carcinoma; PD-L1, programmed death-ligand 1; SCC, squamous cell carcinoma; UDC, undifferentiated carcinoma
Discussion
In the present study, we determined the expression of PD-L1 in various CUP clones, detecting various positive rates depending on the antibodies used, the applied scoring system, and cutoff values. Although PD-L1 expression in CUP remains poorly established, a positivity rate of 22% in tumor cells has been reported [40] using the antibody SP142, with the positivity criteria defined as moderate (2+) membranous positivity in at least 5% of tumor cells, indicating that the previous criteria were TC ≥ 5%. In addition, PD-L1 was found to be expressed in tumor cells in 14% of the CUP cases [41]; the antibody used was 22C3, and the positivity criteria were defined as at least 50% of tumor cells being positive, indicating that the previous criteria were TPS ≥ 50%. In the current study, the positivity rates were 18.1% (for TC ≥ 1%) and 5.6% (for TC ≥ 50%) using SP142, and 19.4% (for TC ≥ 1%) and 4.2% (for TC ≥ 50%) with 22C3. The PD-L1 positivity rate varied depending on the PD-L1 antibody clone, scoring system, and cutoff values, as well as based on the interpretation by the pathologist and sample tissue type. Therefore, a direct comparison can be challenging. Although some studies have examined the expression of PD-L1 using only one PD-L1 antibody, no study has explored PD-L1 expression using multiple PD-L1 antibodies with various scoring systems or cutoff values. As previously mentioned, various factors can impact the results of PD-L1 IHC in tumors, including the PD-L1 antibody clone, scoring system, cutoff value, interpretation pathologist, sample tissue type (biopsy or resection), and primary or metastasis. Accordingly, several studies have investigated the expression and consistency of PD-L1 according to these factors in various types of cancers. PD-L1 expression has been extensively explored in cancers such as non-small cell lung carcinoma (NSCLC), TNBC, melanoma, renal cell carcinoma, bladder cancer, and gastric cancer. The positivity rates of PD-L1 in each cancer type were as follows: NSCLC (TC = 23–86%, IC = 23–68%) [42], breast TNBC (IC = 23–74%, CPS = 17–81%) [43], renal cell carcinoma (TPS = 25–60%) [44], bladder cancer (TC = 12–72%) [45], and gastric cancer (TC = 15–69%) [23].
In tumors, the main function of PD-L1 is to predict the response to immune checkpoint inhibitors (ICI), and various clinical trials are underway to optimize its function as a predictive factor, depending on the type of tumor. Accordingly, a companion diagnosis has been established in clinical practice for each cancer type, determining the optimal PD-L1 antibody clone, IHC platform, scoring system, cutoff value, and specific ICI. Representative tumors include NSCLC, TNBC, urothelial carcinoma, uterine cervical cancer, and gastric/esophageal cancer. Therefore, additional preclinical and clinical studies are required to determine the optimal PD-L1 conditions for CUP. Although the possibility of an ICI therapy response according to PD-L1 expression status in CUP warrants clinical trials and extensive research, a potential response to ICI therapy according to the PD-L1 expression status can be sufficiently suggested.
Currently, the treatment approach in CUP involves site-specific therapy if the tissue-of-origin is determined using an IHC panel and/or molecular tissue-of-origin assay [7,8,9, 30]. Given that the efficacy of ICI therapy based on PD-L1 has been confirmed in NSCLC, TNBC, urothelial carcinoma, uterine cervical cancer, and gastric/esophageal cancer, if the tissue origin is determined for CUP using an IHC panel and/or molecular tissue-of-origin assay, ICI therapy could be initiated on assessing PD-L1 expression. However, it is necessary to consider that the currently defined PD-L1 clones, IHC platforms, scoring systems, and cutoff values for each cancer type tend to differ; therefore, additional research is needed to determine whether different PD-L1 evaluation systems should be used according to the tissue origin in CUP.
Based on the subgroup analysis of CUP, PD-L1 SP263 TC positivity, PD-L1 SP263 IC negativity, and PD-L1 28 − 8 IC negativity were associated with a poor prognosis. Other tumors, including urothelial carcinoma, NSCLC, head and neck cancer, and liver cholangiocarcinoma, have shown similar results, where PD-L1 expression in tumor cells was associated with poor prognosis, whereas PD-L1 expression in immune cells was associated with better prognosis [46,47,48,49,50].
In this study, only PD-L1 staining was conducted. However, previous studies in other cancer types have performed double staining such as CD68/PD-L1 to distinguish staining differences between PD-L1 and tumor-associated macrophages (TAMs) and other immune cells, and have presented differences in tumor subtypes and prognosis accordingly [51, 52]. Therefore, dual staining like CD68/PD-L1 can provide important insights into the role of immune cells in the tumor microenvironment and the mechanisms of tumor immune evasion. This could aid in developing treatment strategies and identifying the origin of tumors. Therefore, additional research on dual staining, such as CD68/PD-L1, is deemed necessary to accurately characterize the tumor properties and develop personalized treatment strategies, especially in cases like CUP where the tumor origin is unknown.
One limitation of this study is that PD-L1 staining was conducted on a limited amount of tissue using TMA, which may not adequately reflect tumor heterogeneity. Previous studies investigating the differences in PD-L1 expression between biopsy and surgical tissue in various cancer types have shown a concordance rate of 70% or higher in most cases [53,54,55,56]. Additionally, in clinical practice, obtaining small biopsies rather than excising the entire lesion surgically is more common in cases of CUP, suggesting that the results from TMA studies may be more similar to the actual clinical environment. Moreover, in cases where small biopsies are not feasible due to various clinical circumstances, cytological samples may be considered for assessing PD-L1 status in CUP patients. Previous studies have reported moderate or higher concordance rates between cytology and histology samples regarding PD-L1 expression [57,58,59], indicating the need for additional research on PD-L1 expression in cytological samples from CUP patients.
Conclusions
In conclusion, PD-L1 expression was observed in CUP, with varying positivity rates depending on the antibody and scoring system employed. There was no difference in PD-L1 expression based on histological or clinical subtypes. Therefore, ICI treatment based on PD-L1 expression in CUP can be an effective treatment strategy.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Pavlidis N, Fizazi K. Cancer of unknown primary (CUP). Crit Rev Oncol Hematol. 2005;54:243–50.
Haskell CM, Cochran AJ, Barsky SH, Steckel RJ. Metastasis of unknown origin. Curr Probl Cancer. 1988;12:5–58.
Krementz ET, Cerise EJ, Foster DS, Morgan LR Jr. Metastases of undetermined source. Curr Probl Cancer. 1979;4:4–37.
Lembersky BC, Thomas LC. Metastases of unknown primary site. Med Clin North Am. 1996;80:153–71.
Rassy E, Pavlidis N. The currently declining incidence of cancer of unknown primary. Cancer Epidemiol. 2019;61:139–41.
van de Wouw AJ, Jansen RL, Speel EJ, Hillen HF. The unknown biology of the unknown primary tumour: a literature review. Ann Oncol. 2003;14:191–6.
Olivier T, Fernandez E, Labidi-Galy I, Dietrich PY, Rodriguez-Bravo V, Baciarello G, Fizazi K, Patrikidou A. Redefining cancer of unknown primary: is precision medicine really shifting the paradigm? Cancer Treat Rev. 2021;97:102204.
Bochtler T, Löffler H, Krämer A. Diagnosis and management of metastatic neoplasms with unknown primary. Semin Diagn Pathol. 2018;35:199–206.
Kato S, Alsafar A, Walavalkar V, Hainsworth J, Kurzrock R. Cancer of unknown primary in the Molecular Era. Trends Cancer. 2021;7:465–77.
Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–95.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–66.
D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E, Chella A, Fontanini G, Filice ME, Tornillo L, Incensati RM, Sani S, Crino L, Terracciano L, Cappuzzo F. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28.
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–100.
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62.
Thierauf J, Veit JA, Affolter A, Bergmann C, Grunow J, Laban S, Lennerz JK, Grunmuller L, Mauch C, Plinkert PK, Hess J, Hoffmann TK. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res. 2015;25:503–9.
Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 + T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–5.
Bastaki S, Irandoust M, Ahmadi A, Hojjat-Farsangi M, Ambrose P, Hallaj S, Edalati M, Ghalamfarsa G, Azizi G, Yousefi M, Chalajour H, Jadidi-Niaragh F. PD-L1/PD-1 axis as a potent therapeutic target in breast cancer. Life Sci. 2020;247:117437.
Li CJ, Lin LT, Hou MF, Chu PY. PD–L1/PD–1 blockade in breast cancer: the immunotherapy era (review). Oncol Rep. 2021;45:5–12.
Chen K, Wang X, Yang L, Chen Z. The Anti-PD-1/PD-L1 immunotherapy for gastric esophageal Cancer: a systematic review and Meta-analysis and literature review. Cancer Control. 2021;28:1073274821997430.
Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, Zhong X, Li X, Qian H, Wang X. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS ONE. 2017;12:e0182692.
Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, Hirsch FR. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345–62.
Bagchi S, Yuan R, Engleman EG. Immune Checkpoint inhibitors for the treatment of Cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49.
Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, Zhao X, Martinez AJ, Wang W, Gibney G, Kroeger J, Eysmans C, Sarnaik AA, Chen YA. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–8.
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71.
Chauhan A, Siegel L, Freese R, Racila E, Stewart J 3rd, Amin K. Performance of Ventana SP263 PD-L1 assay in endobronchial ultrasound guided-fine-needle aspiration derived non-small-cell lung carcinoma samples. Diagn Cytopathol. 2021;49:355–62.
Pavlidis N, Pentheroudakis G. Cancer of unknown primary site. Lancet. 2012;379:1428–35.
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G. Cancers of unknown primary site: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v133–138.
Choi SH, Chang JS, Koo JS, Park JW, Sohn JH, Keum KC, Suh CO, Kim YB. Differential Prognostic Impact of strong PD-L1 expression and 18F-FDG uptake in Triple-negative breast Cancer. Am J Clin Oncol. 2018;41:1049–57.
Kim HM, Lee J, Koo JS. Clinicopathological and prognostic significance of programmed death ligand-1 expression in breast cancer: a meta-analysis. BMC Cancer. 2017;17:690.
Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14:173.
Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell Lung Cancer. N Engl J Med. 2016;375:1823–33.
Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–92.
Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A. Atezolizumab versus Docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–46.
García A, Recondo G, Greco M, de la Vega M, Perazzo F, Recondo G, Avagnina A, Denninghoff V. Correlation between PD-L1 expression (clones 28 – 8 and SP263) and histopathology in lung adenocarcinoma. Heliyon. 2020;6:e04117.
Tot T. The value of cytokeratins 20 and 7 in discriminating metastatic adenocarcinomas from pleural mesotheliomas. Cancer. 2001;92:2727–32.
Huang X, Ding Q, Guo H, Gong Y, Zhao J, Zhao M, Sui D, Wu Y, Chen H, Liu H, Zhang J, Resetkova E, Moulder SL, Wang WL, Huo L. Comparison of three FDA-approved diagnostic immunohistochemistry assays of PD-L1 in triple-negative breast carcinoma. Hum Pathol. 2021;108:42–50.
Gatalica Z, Xiu J, Swensen J, Vranic S. Comprehensive analysis of cancers of unknown primary for the biomarkers of response to immune checkpoint blockade therapy. Eur J Cancer. 2018;94:179–86.
Ross JS, Sokol ES, Moch H, Mileshkin L, Baciarello G, Losa F, Beringer A, Thomas M, Elvin JA, Ngo N, Jin DX, Krämer A. Comprehensive genomic profiling of Carcinoma of unknown primary origin: retrospective molecular classification considering the CUPISCO Study Design. Oncologist. 2021;26:e394–402.
Yu H, Boyle TA, Zhou C, Rimm DL, Hirsch FR. PD-L1 expression in Lung Cancer. J Thorac Oncol. 2016;11:964–75.
Núñez Abad M, Calabuig-Fariñas S, Lobo de Mena M, Torres-Martínez S, García González C, García García J. Iranzo González-Cruz V, Camps Herrero C: programmed death-ligand 1 (PD-L1) as immunotherapy biomarker in breast Cancer. Cancers (Basel) 2022, 14.
Carretero-González A, Lora D, Martín Sobrino I, Sáez Sanz I, Bourlon MT, Anido Herranz U, Martínez Chanzá N, Castellano D, de Velasco G. The value of PD-L1 expression as predictive biomarker in metastatic renal cell Carcinoma patients: a Meta-analysis of Randomized clinical trials. Cancers (Basel) 2020, 12.
Zhou TC, Sankin AI, Porcelli SA, Perlin DS, Schoenberg MP, Zang X. A review of the PD-1/PD-L1 checkpoint in bladder cancer: from mediator of immune escape to target for treatment. Urol Oncol. 2017;35:14–20.
Chan AWH, Tong JHM, Kwan JSH, Chow C, Chung LY, Chau SL, Lung RWM, Ng CSH, Wan IYP, Mok TSK, To KF. Assessment of programmed cell death ligand-1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non-small cell lung carcinoma. Mod Pathol. 2018;31:1381–90.
Kim HR, Ha SJ, Hong MH, Heo SJ, Koh YW, Choi EC, Kim EK, Pyo KH, Jung I, Seo D, Choi J, Cho BC, Yoon SO. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956.
Mocan LP, Craciun R, Grapa C, Melincovici CS, Rusu I, Al Hajjar N, Sparchez Z, Leucuta D, Ilies M, Sparchez M, Mocan T, Mihu CM. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for patients with intrahepatic cholangiocarcinoma. Cancer Immunol Immunother 2022.
Wen Y, Chen Y, Duan X, Zhu W, Cai C, Deng T, Zeng G. The clinicopathological and prognostic value of PD-L1 in urothelial carcinoma: a meta-analysis. Clin Exp Med. 2019;19:407–16.
Zhong Q, Shou J, Ying J, Ling Y, Yu Y, Shen Z, Zhang Y, Li N, Shi Y, Zhou A. High PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor in urothelial carcinoma. Future Oncol. 2021;17:2893–905.
Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, McDermott MW, Theodosopoulos PV, Aghi MK, Berger MS, Butowski NA, Barani I, Phillips JJ, Perry A, Okada H. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol. 2016;130:543–52.
Melotti S, Ambrosi F, Franceschini T, Giunchi F, Filippo GD, Franchini E, Massari F, Mollica V, Tateo V, Bianchi FM, Colecchia M, Acosta AM, Lobo J, Fiorentino M, Ricci C. TAMs PD-L1(+) in the reprogramming of germ cell tumors of the testis. Pathol Res Pract. 2023;247:154540.
de Jong JJ, Stoop H, Nieboer D, Boormans JL, van Leenders G. Concordance of PD-L1 expression in matched urothelial bladder cancer specimens. Histopathology. 2018;73:983–9.
Gradecki SE, Grange JS, Stelow EB. Concordance of PD-L1 expression between core biopsy and resection specimens of Non-small Cell Lung Cancer. Am J Surg Pathol. 2018;42:1090–4.
Kim SW, Jeong G, Ryu MH, Park YS. Comparison of PD-L1 immunohistochemical assays in advanced gastric adenocarcinomas using endoscopic biopsy and paired resected specimens. Pathology. 2021;53:586–94.
Zhao L, Chen P, Fu K, Li J, Dai Y, Wang Y, Zhuang Y, Sun L, Chen H, Lin Q. Concordance of PD-L1 status between image-guided percutaneous biopsies and Matched Surgical Specimen in Non-small Cell Lung Cancer. Front Oncol. 2020;10:551367.
Ambrosi F, Giunchi F, Capizzi E, Cancellieri A, Trisolini R, Ardizzoni A, Fiorentino M, Ricci C. Interobserver agreement of PD-L1 (SP263) assessment in advanced NSCLC on cytological smears and histological samples. Pathol Res Pract. 2022;233:153893.
Gagné A, Orain M, Ionescu D, Tsao MS, Joubert D, Joubert P. Comprehensive assessment of PD-L1 immunohistochemistry on paired tissue and cytology specimens from non-small cell lung cancer. Lung Cancer. 2020;146:276–84.
Tejerina E, Garca Tobar L, Echeveste JI, de Andrea CE, Vigliar E, Lozano MD. PD-L1 in Cytological samples: a review and a practical Approach. Front Med (Lausanne). 2021;8:668612.
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.S.K.; methodology, H.M.K and J.S.K.; validation, H.M.K and J.S.K.; formal analysis, H.M.K and J.S.K.; investigation, J.S.K.; resources, J.S.K.; data curation, H.M.K and J.S.K.; writing—original draft preparation, H.M.K and J.S.K.; writing—review and editing, J.S.K.; su-pervision, J.S.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study adhered to the principles of the Declaration of Helsinki and obtained approval from the Institutional Review Board of Yonsei University Severance Hospital (IRB number: 4-2022-1380). Due to the retrospective nature of the study, patient consent was exempted by the Institutional Review Board of Yonsei University Severance Hospital.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, H.M., Koo, J.S. Programmed death-ligand 1 expression in carcinoma of unknown primary. BMC Cancer 24, 689 (2024). https://doi.org/10.1186/s12885-024-12437-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12437-w