Abstract
Background
Colorectal cancer (CRC) is the 3rd most common malignancy with the liver being the most common site of metastases. The recurrence rate of colorectal liver metastases (CRLM) after liver resection (LR) is notably high, with an estimated 40% of patients experiencing recurrence within 6 months. In this context, we conducted a meta-analysis to synthesize and evaluate the reliability of evidence pertaining to prognostic factors associated with early recurrence (ER) in CRLM following LR.
Methods
Systematic searches were conducted from the inception of databases to July 14, 2023, to identify studies reporting prognostic factors associated with ER. The Quality in Prognostic Factor Studies (QUIPS) tool was employed to assess risk-of-bias for included studies. Meta-analysis was then performed on these prognostic factors, summarized by forest plots. The grading of evidence was based on sample size, heterogeneity, and Egger’s P value.
Results
The study included 24 investigations, comprising 12705 individuals, during an accrual period that extended from 2007 to 2023. In the evaluation of risk-of-bias, 22 studies were rated as low/moderate risk, while two studies were excluded because of high risk. Most of the studies used a postoperative interval of 6 months to define ER, with 30.2% (95% confidence interval [CI], 24.1–36.4%) of the patients experiencing ER following LR. 21 studies were pooled for meta-analysis. High-quality evidence showed that poor differentiation of CRC, larger and bilobar-distributed liver metastases, major hepatectomy, positive surgical margins, and postoperative complications were associated with an elevated risk of ER. Additionally, moderate-quality evidence suggested that elevated levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA199), lymph node metastases (LNM) of CRC, and a higher number of liver metastases were risk factors for ER.
Conclusion
This review has the potential to enhance the efficacy of surveillance strategies, refine prognostic assessments, and guide judicious treatment decisions for CRLM patients with high risk of ER. Additionally, it is essential to undertake well-designed prospective investigations to examine additional prognostic factors and develop salvage therapeutic approaches for ER of CRLM.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the 3rd most common malignancy and the 2nd most deadly cancer worldwide [1]. It is highly prevalent in developed countries but has started to show an increasing trend in China, partially attributed to shifts toward a high-fat, low-fiber diet [2]. CRC is prone to distant metastases, affecting over 50% of patients, with the liver being the primary site in approximately 70% of cases [3, 4]. Therapeutic options for colorectal liver metastases (CRLM) include hepatectomy, chemotherapy, radiotherapy, hepatic artery embolization, and thermal ablation, such as microwave coagulation therapy, radiofrequency ablation [5]. Currently, liver resection (LR) is acknowledged as the most effective treatment for CRLM patients, which can offer prolonged survival and, in selected cases, a chance of cure [6]. Increasing effectiveness of chemotherapy regimens, advances in surgical techniques, and improvements in perioperative patient management have expanded the boundaries of resectability [7, 8]. The current consensus proposes that a disease should be considered technically resectable as long as complete macroscopic resection is feasible while maintaining at least a 30% future liver remnant [9, 10]. Nevertheless, not all technically resectable patients experience a survival benefit from surgery, with 3-year recurrence rates reaching 60–70% [11,12,13]. The earlier the recurrence, the worse the prognosis, but the definition of early recurrence(ER) varies from 6 to 24 months [14,15,16].
Therefore, this meta-analysis aims to elucidate prognostic factors associated with ER in CRLM patients undergoing LR. Subsequently, our objective is to identify individuals with high risk of ER, who might benefit from closer surveillance and appropriate salvage therapy.
Materials and methods
Protocol and reporting
The protocol for this study was registered on PROSPERO (International Prospective Register of Systematic Reviews, www.crd.york.ac.uk/prospero) with the registration number CRD42023444091. This review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [17]. The PRISMA checklist is available in Supplementary Table S1.
Data sources and search strategy
All potentially eligible publications were retrieved from PubMed, Embase, Cochrane, and Web of Science from database inception until July 14, 2023. The search, employing the keywords “colorectal liver metastases”, “surgery”, “early”, and “recurrence”, was carried out by two investigators (YT, SFW). Supplementary File 1 included detailed information on the search strategy. Additionally, the bibliographies of included articles and relevant reviews were manually scrutinized to identify additional research and explore potentially relevant studies.
Eligibility criteria and study selection
Subjects were eligible for inclusion if the following criteria were met: (1) Prospective or retrospective studies including patients with CRLM who received liver resection; (2) Articles presenting ER rates categorized by a prognostic factor; (3) Articles reporting a relative ratio (RR) or an odds ratio (OR) (with a 95% confidence interval [CI]) or offering adequate data for RR/OR estimation; (4) No language restrictions.
Studies were excluded according to the following criteria: (1) Articles on palliative surgery; (2) Articles without sufficient data for analysis; (3) Experimental animal studies; (4) Reviews, commentaries, conference proceedings, letters, case reports, editorials, and meta-analysis.
Article screening and study selection were independently performed by two reviewers (SFW, YQW). In instances of discordance, resolution was achieved through collaborative deliberation within the research team, culminating in a final consensus.
Data extraction
The following data were extracted from each included study, and missing data were noted: (1) first author, publication year, country, period of recruitment, study type, patient count, follow-up period, overall recurrence rate, ER definition, ER rate, 5-year OS in the ER group, and inclusion/exclusion criteria (Table 1); (2) Prognostic factors, including patient characteristics (continuous variables: age, carcinoembryonic antigen [CEA], carbohydrate antigen 19–9 [CA199], binary variables: gender), primary tumor characteristics (binary variables: tumor differentiation [poor vs moderate/good], lymph node metastases [LNM], tumor stage [T3-4 vs T1-2], tumor location [rectum vs colon]), liver metastases characteristics (binary variables: number [more vs less], diameter [> 5 cm vs ≤ 5 cm], synchronous metastases, bilobar distribution, extrahepatic metastases), and therapeutic factors (binary variables: laparoscopic resection,simultaneous resection, major hepatectomy,surgical margins [positive vs negative],preoperative chemotherapy, postoperative chemotherapy,blood transfusion,postoperative complications) and clinical risk score [CRS, binary, > 2 vs ≤ 2]; (3) RRs or ORs and corresponding 95% CIs for association between each prognostic factor and ER.
Continuous variables were summarized using median and interquartile range values, while categorical variables were expressed as counts and percentages. In cases where RR was unavailable, we either convert OR to RR or employed the events and patients counts in both exposed and non-exposed groups to calculate RR. By using standardized forms, two authors (YT, YQW) independently extracted the data from each eligible study. Disagreements were resolved by consensus or discussion with the third person (NYW).
Risk-of-bias assessment
To evaluate the risk-of-bias (RoB) at the study level, the Quality in Prognostic Factor Studies (QUIPS) tool was employed. This tool has six domains, with each domain assigned a RoB rating categorized as high, moderate, or low [38]. Studies were deemed to have low RoB if all domains were rated as low RoB or only one domain scored moderate RoB. Conversely, studies were classified as high RoB if at least one domain scored high RoB or if three or more domains scored moderate RoB. The remaining studies were attributed a moderate RoB rating.
Statistical analysis
The primary outcomes of the study focused on the RRs depicting the association between ER and prognostic factors. When available, preference was given to the most adjusted effect estimate, specifically opting for the Cox multivariable coefficient over the univariable estimate. Subsequently, all pooled outcomes were derived utilizing a random-effects model (Mantel–Haenszel method). The magnitude of the summary effects was graphically represented through forest plots.
Between-study heterogeneity was assessed utilizing the I2 statistical estimate, with an I2 value > 50% regarded as severe heterogeneity [39]. Consequently, subgroup analyses were executed to identify potential sources of heterogeneity. Assessment of reporting bias was undertaken through funnel plots and the Egger's test, specifically for prognostic factors identified in over 10 studies. A P value below 0.1 was deemed indicative of significant publication bias, prompting the execution of Trim and Fill analysis in such instances. Additionally, sensitivity analysis was performed by switching to fixed-effects models to test the robustness of the conclusions.
All statistical analyses were conducted using Review Manager software (Version 5.4) and Stata software (version 14.1). A significant two-way P value for comparison was defined as P < 0.05.
Evidence strength assessment
The grading of evidence strength for the identified associations in observational studies was based on the following criteria: Egger's test P value > 0.1, a cumulative population > 1000, and I2 < 50%. The association attained Class I (high-quality) evidence status when all three conditions were satisfied simultaneously. If two out of these three conditions were met, the association was categorized as Class II (moderate-quality) evidence. Furthermore, class III (moderate-quality) evidence was conferred upon an association when only one of the three conditions was fulfilled. Conversely, the absence of satisfaction for all of these three conditions designated an association as Class IV (low-quality) evidence [40].
Result
Study selection
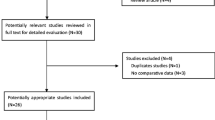
Our initial search strategy identified a total of 3157 pertinent studies, of which 1064 were removed due to duplication. Following the preliminary screening of titles and abstracts, 1883 abstracts were excluded because they did not meet the inclusion criteria. Furthermore, 12 reports were inaccessible, and 198 potentially relevant articles underwent a thorough review in full text. Ultimately, 174 articles were excluded for diverse reasons and 24 selected studies were included [12, 14,15,16, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], as illustrated in the PRISMA flow diagram (Fig. 1).
Study characteristics
This review included 24 studies, comprising 12,705 patients who underwent LR for CRLM, with a comprehensive summary presented in Table 1. Among these, twelve studies adopted a prospective cohort design [12, 18, 21, 22, 28,29,30,31,32, 34,35,36], with the remaining adopting a retrospective cohort approach [14,15,16, 19, 20, 23,24,25,26,27, 33, 37]. The publication years of the studies spanned from 2007 to 2023. In terms of geographical distribution, 15 studies originated from Asia [14,15,16, 19, 20, 23, 24, 26, 27, 29,30,31, 33, 35, 37], 7 from Europe [12, 18, 22, 25, 28, 32, 34], 1 from Australia [36], and the other one from the United States [21]. The recruitment period ranged from 1986 to 2020, with the median duration of follow-up ranging varying from 22 to 86.3 months.
Definition of early recurrence
The definition of ER exhibited variation among the studies. Twelve studies defined ER as six-month following surgery [15, 23, 24, 26, 28,29,30,31,32, 34,35,36], while five studies utilized 12 months as the cutoff for ER [16, 21, 25, 27, 37]. The ER rate ranged from 8.6% to 54.3%, and the pooled ER rate was 30.2% (95% CI, 24.1%–36.4%), indicating substantial heterogeneity across the studies (I2 = 98%, P < 0.001) (Fig. S1). Additionally, Viganò et al. applied a 3-month threshold to define very early recurrence (VER), with 11.6% of patients experiencing VER [12]. The overall CRLM recurrence rate was reported to be between 40.7% and 78.8%. For those patients who underwent early recurrence (ER), the 5-year overall survival (OS) spanned from 11.1% to 45.0% (Table 1).
Prognostic factors
A total of 22 potential prognostic factors were identified before the study, categorized into patient-related factors, primary tumor factors, liver metastasis factors, and treatment-related factors. The characteristics of these prognostic factors in the ER group were presented in Tables 2, 3, 4 and 5. As shown, the median age among patients with ER ranged from 55 to 66 years, and the proportion of males varied between 44.6% and 67.3%. Regarding primary tumor characteristics, poor tumor differentiation in the ER group ranged from 5.6% to 66.7%, and 42.3% to 55.7% of patients had LNM. As factors of liver metastases, bilobar distribution was noted in 26.7% to 74.2% of the ER group, and Jung et al. reported that up to 93.3% had synchronous metastases [24]. As reported, 11.5% to 63.3% of patients with ER had positive surgical margins, and approximately 30% to 80% of patients received chemotherapy.
Assessment on risk-of-bias
The results of the RoB assessment were presented in Table 6. Employing the QUIPS tool and the criteria described above, 17 studies received a classification of low overall RoB, whereas 5 studies were assigned a moderate RoB rating. Notably, two studies were excluded due to high RoB at this stage [33, 34].
Meta-analysis for prognostic factors
A total of 21 studies, involving 5791 patients, met the eligibility criteria for the meta-analysis. One study was omitted from consideration due to its utilization of VER (3 months) as the outcome, and two additional studies were excluded on account of high RoB.
All results graphically depicted using forest plots, illustrated in Figs. 2, 3, 4 and 5. Patient- related factors such as age and male gender exhibited no correlation with ER. Elevated concentrations of preoperative CEA (RR, 1.56; 95% CI, 1.19–2.04; I2 = 81%) and CA199 (RR, 1.48; 95% CI, 1.20–1.81; I2 = 36%) were identified as potential risk factors for ER (Fig. 2). Besides, primary tumor factors associated with an increased hazard of ER encompassed poor differentiation (RR, 1.13; 95% CI, 1.03–1.25; I2 = 0%) and LNM (RR, 1.31; 95% CI, 1.17–1.48; I2 = 47%) (Fig. 3). Concerning liver metastases, an elevated risk of ER was associated with factors such as a higher number of metastases (RR, 1.46; 95% CI, 1.26–1.68; I2 = 57%), larger metastases (RR, 1.18; 95% CI, 1.04–1.34; I2 = 29%), and bilobar distribution (RR, 1.37; 95% CI, 1.21–1.55; I2 = 40%) (Fig. 4). Regarding therapeutic factors, major hepatectomy (RR, 1.16; 95% CI, 1.07–1.25; I2 = 0%), positive surgical margins (RR, 1.33; 95% CI, 1.20–1.48; I2 = 34%), and postoperative complications (RR, 1.28; 95% CI, 1.13–1.44; I2 = 30%) have been recognized as risk factors associated with ER (Fig. 5a and b).
However, the stage and location of the primary tumor, synchronous metastases, extrahepatic metastases, laparoscopic surgery, preoperative or postoperative chemotherapy, and blood transfusion were not found to be statistically associated with ER. All the above results are presented in Table 7.
The CRS ranges from 0 to 5 points, with 1 point assigned for each of the following: LNM of the primary tumor, the interval < 1 year from primary tumor resection to the detection of liver metastasis, preoperative CEA > 200 ng/ml, more than one liver tumor, and largest tumor > 5 cm [41]. The combination of RRs in three studies showed that CRS > 2 had the potential to increase the risk of ER (RR, 1.44; 95% CI, 1.17–1.77; I2 = 0%; Egger’s P value = 0.232) (Fig. S2).
Reporting bias
Reporting bias was evaluated by funnel plot and Egger's test. Our results comparing the ER rates between groups with and without LNM of primary tumor revealed an asymmetric funnel, with a P value of 0.035 for the Egger's test (Fig. 6). By filling 4 studies using the Trim and Fill method, the recalculated pooled RR was 1.20, 95% CI (1.06, 1.37) (Fig. 7), which was not significantly changed from the initial estimate (RR, 1.31; 95% CI, 1.17–1.48). Therefore, the presence of publication bias has little significant effect on the overall finding.
Study quality
Using the rating rules mentioned above, no evidence was rated as Class IV. High-quality (Class I) evidence showed that poor differentiation of CRC, larger and bilobar-distributed liver metastases, major hepatectomy, positive surgical margins, and postoperative complications were factors linked to an elevated hazard of ER. Among other meaningful prognostic factors, elevated levels of CEA and CA199, LNM, and a higher number of liver metastases were rated as Class II (Table 7).
Subgroup analyses
Among the prognostic factors analyzed, an elevated number of metastases was reported of having high heterogeneity (I2 > 50%). Subgroup analyses were conducted, employing diverse thresholds for defining an increased number of metastases, categorized as multiple, > 3, and > 4 metastases. As shown in Fig. 8, all subgroup analyses showed significant differences in the ER rate between cases with more and fewer metastases. Notably, the subgroup of multiple metastases showed great heterogeneity (I2 = 79%), whereas the other two groups did not. Therefore, we found that the divergent definitions of “multiple” across different articles constituted the primary source of heterogeneity.
Sensitivity analyses
We performed sensitivity analysis by switching to fixed-effects models on all variables. The results were consistent across all variables except in the case of preoperative chemotherapy, wherein a fixed-effects model revealed an association with diminished risk of ER (RR, 1.11; 95% CI, 1.02–1.21; I2 = 58%) (Fig. S3). However, this result was deemed unreliable and excluded.
Discussion
This is the first-ever published meta-analysis summarizing prognostic factors associated with ER following LR for CRLM. Specifically, most of the studies used a postoperative interval of 6 months to define ER, which is earlier than the ER definition for other tumors in the liver. For example, hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) often use 1 year or 2 years as a cutoff value [42,43,44]. This review reveals that following LR for CRLM, the occurrence of ER is approximated at 30.2% (95% CI, 24.1%–36.4%). However, evidence shows that the 5-year OS rate after ER of CRLM ranges from 11.1% to 45%, while that of iCCA is only 8–11.6% [43,44,45]. This suggests that postoperative ER of CRLM is more common but the prognosis is relatively better, compared with other intrahepatic tumors. Furthermore, with the development of surgical techniques and minimally invasive local treatment strategies, CRLM patient is more likely to undergo re-resection and/or ablation after recurrence, and the 5-year OS after repeat hepatectomy is as high as 50% [46].
After pooling data from 21 studies involving 5791 patients, this meta-analysis identified ten prognostic factors that could play a crucial role in ER across four domains: patient-related factors, primary tumor characteristics, liver metastases attributes, and therapeutic factors.
As patient-related factors, elevated levels of preoperative CEA and CA199 were identified as potential risk factors for ER. However, the evidence was classified as level II due to high heterogeneity, which was attributed to varying cutoff values in different studies. Studies indicated that postoperative serum molecular markers had stronger predictive potential. Even within the normal limits, higher levels of postoperative CA199 were effective in predicting ER [15, 47, 48].
For primary tumor factors, the analysis of aggregated RR values indicated a heightened risk of ER associated with poor differentiation and LNM, both indicative of a more advanced tumor stage. The impact of LNM on ER was categorized as class II evidence due to reporting bias, but the Trim and Fill analysis indicated that the presence of publication bias had no substantial influence on the overall findings. Besides, previous investigations have validated that individuals with LNM in primary tumors exhibit an adverse OS and progression-free survival (PFS) [49,50,51]. This reveals the significance of pathological characteristics of primary colorectal tumors in the prognosis assessment following LR. Particularly for metachronous liver metastases, defined as liver metastases discovered after primary tumor surgery, these primary tumor factors may assist surgeons in identifying patients who would derive greater benefits from LR [52, 53].
Characteristics of liver metastases, such as increased size, number, and bilobar distribution, have been recognized as potential risk factors associated with ER. Tumor size and number were frequently treated as dichotomous variables, with varying cutoff values, leading to heterogeneity across studies. However, these two variables can be used to calculate the tumor burden score (TBS) [TBS2 = (maximum tumor diameter)2 + (number of liver lesions)2], and the predictive efficacy of this index has been proved to exhibit higher specificity and sensitivity compared to relying solely on tumor size and number in patients with CRLM [54, 55]. Preoperative radiological imaging can provide first indications about the risk of ER, especially gadoxetate disodium-enhanced magnetic resonance imaging (EOB-MRI) can provide greater sensitivity [56]. Furthermore, the implementation of intraoperative hepatic ultrasonography (IOUS) has been reported to identify more occult hepatic lesions missed by preoperative imaging, thereby potentially mitigating the risk of ER [57, 58].
In terms of therapeutic factors, major hepatectomy, positive surgical margins, and postoperative complications have been identified to increase the risk of ER. But no statistically significant difference was observed in the impact on ER between laparoscopic and open hepatectomy, suggesting the viability of the laparoscopic approach. Major hepatectomy is traditionally defined as the resection of three or more liver segments [59]. Indeed, the presence of more and larger, and bilobar-distributed metastases mentioned above not only represents worse tumor behavior but also increases the probability of occult intrahepatic spread and affects the radicality of the treatment. Consequently, to ensure the complete removal of all lesions, more extensive liver resection may be performed, leaving little room for salvageability [60]. Besides, R1 resection has been identified as a risk factor, implying that overlooked lesions and residual microscopic tumor cells left after surgery contribute to ER. However, it remains uncertain whether recurrence at the surgical margin or the emergence of new metastases are the primary contributors to ER in patients with positive surgical margins. Severe postoperative complications could potentially extend the immunosuppression induced by major surgeries and delay the initiation of adjuvant chemotherapy [14].
The current study failed to demonstrate the benefit of preoperative or postoperative chemotherapy in preventing ER. The reason is that, on the one hand, patients with more advanced tumors and R1 resection have a greater tendency to receive adjuvant therapy, on the other hand, the effects of different regimens and cycles of adjuvant treatment are combined. But a study revealed that > 1 chemotherapy line and progression of disease during last-line chemotherapy, were identified as independent predictors of ER, suggesting that the response to chemotherapy was more important than the chemotherapy itself [22]. Therefore, the cooperation between surgeons and oncologists is essential, especially when aggressive indications are present.
In addition to individual prognostic factors, the CRS combines tumor markers, primary tumor factors, and liver metastasis factors and was widely adopted for the prognosis of CRLM patients. In this study, class I evidence demonstrated that a CRS > 2 increase the risk of ER. Furthermore, several studies have utilized multiple prognostic factors to develop nomograms to better predict ER of CRLM [15, 19, 20]. However, the generalizability of these nomograms requires further research, and there is an urgent need to develop a universal ER risk prediction score.
When liver recurrence occurs, salvage resection had the potential to extend long-term survival [22]. However, it was noteworthy that the secondary resection rate was notably diminished in individuals with ER compared to those experiencing late recurrence, due to worsened condition status and potential surgical complications [26]. Additionally, studies indicated that the survival benefit associated with salvage resection disappeared within the subgroup of patients exhibiting more than two risk factors for ER [29]. For these patients, salvage treatment may accelerate disease progression and postoperative complications, thereby mitigating survival benefits rather than effectively controlling local recurrence. Therefore, the indications for salvage resection in patients with ER should be strictly controlled.
There are several limitations in this review. Initially, all the included studies are non-randomized control trials, introducing the possibility of confounding bias. Moreover, the combination of various ER definitions, ranging from 6 to 24 months, and amalgamation of diverse cutoff values for prognostic factors contributed to the substantial heterogeneity. In addition, only RRs were extracted and combined, not the hazard ratios (HRs), which were less persuasive due to the absence of time-related data. Despite these limitations, pooling evidence from available observational studies enabled us to synthesize relevant and generalizable prognostic factors.
Conclusion
This review offers a consolidated summary of the prognostic factors associated with ER subsequent to LR for CRLM. These findings have the potential to enhance the efficacy of surveillance strategies, refine prognostic assessments, and guide judicious treatment decisions for CRLM patients with high risk of ER. Additionally, it is essential to undertake well-designed prospective investigations to examine additional prognostic factors and develop salvage therapeutic approaches for ER of CRLM.
Availability of data and materials
Yes, I have research data to declare. Data is provided within the manuscript or supplementary information files.
Abbreviations
- CA199:
-
carbohydrate antigen 19–9
- CEA:
-
carcinoembryonic antigen
- CI:
-
confidence interval
- CRC:
-
colorectal cancer
- CRLM:
-
colorectal liver metastases
- CRS:
-
clinical risk score
- ER:
-
early recurrence
- HCC:
-
hepatocellular carcinoma
- HR:
-
hazard ratio
- iCCA:
-
intrahepatic cholangiocarcinoma
- IOUS:
-
intraoperative hepatic ultrasonography
- LNM:
-
lymph node metastases
- LR:
-
liver resection
- OR:
-
odds ratio
- OS:
-
overall survival
- PFS:
-
progression-free survival
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- QUIPS:
-
Quality in Prognostic Factor Studies
- RoB:
-
risk-of-bias
- RR:
-
relative ratio
- VER:
-
very early recurrence
References
Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Translational Oncology. 2021;14(10):101174.
Wang Y, Yan Q, Fan C, et al. Overview and countermeasures of cancer burden in China. Sci China Life Sci. 2023;66(11):2515–26.
Patel RK, Rahman S, Schwantes IR, et al. Updated Management of Colorectal Cancer Liver Metastases: Scientific Advances Driving Modern Therapeutic Innovations. Cell Mol Gastroenterol Hepatol. 2023;16(6):881–94.
Kim EK, Song MJ, Jung Y, Lee WS, Jang HH. Proteomic Analysis of Primary Colon Cancer and Synchronous Solitary Liver Metastasis. Cancer Genomics Proteomics. 2019;16(6):583–92.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. The Lancet. 2019;394(10207):1467–80.
Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3(1):50–6.
Allard MA, Adam R, Giuliante F, et al. Long-term outcomes of patients with 10 or more colorectal liver metastases. Br J Cancer. 2017;117(5):604–11.
Adam R, De Gramont A, Figueras J, et al. The Oncosurgery Approach to Managing Liver Metastases from Colorectal Cancer: A Multidisciplinary International Consensus. Oncologist. 2012;17(10):1225–39.
Cutsem EV, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Annals of Oncology. 2016;27(8):1386–422.
Kambakamba P, Hoti E, Cremen S, Braun F, Becker T, Linecker M. The evolution of surgery for colorectal liver metastases: A persistent challenge to improve survival. Surgery. 2021;170(6):1732–40.
Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301.
Viganò L, Gentile D, Galvanin J, et al. Very Early Recurrence After Liver Resection for Colorectal Metastases: Incidence, Risk Factors, and Prognostic Impact. J Gastrointest Surg. 2022;26(3):570–82.
Ros J, Salva F, Dopazo C, et al. Liver transplantation in metastatic colorectal cancer: are we ready for it? British Journal of Cancer. 2023;128(10):1797.
Kaibori M, Iwamoto Y, Ishizaki M, et al. Predictors and outcome of early recurrence after resection of hepatic metastases from colorectal cancer. Langenbeck’s Archives of Surgery. 2012;397(3):373–81.
Dai S, Ye Y, Kong X, Li J, Ding K. A predictive model for early recurrence of colorectal-cancer liver metastases based on clinical parameters. Gastroenterology Report. 2021;9(3):241–51.
Sakai N, Furukawa K, Takayashiki T, Kuboki S, Takano S, Ohtsuka M. Recurrence patterns and their effects on clinical outcomes after R1 resection of colorectal liver metastases: a propensity score-matched analysis. Langenbecks Arch Surg. 2021;406(8):2739–47.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses the PRISMA statement. BMJ. 2009;339:2535.
Bhogal RH, Hodson J, Bramhall SR, et al. Predictors of early recurrence after resection of colorectal liver metastases. World J Surg Oncol. 2015;13:135.
Chen Q, Zhang Y, Li X, Huang Z, Zhao H, Cai J. Nomogram Incorporating Preoperative Testing Markers for the prediction of Early Recurrence for Colorectal Liver Metastases with Neoadjuvant Chemotherapy followed by Hepatectomy. J Cancer. 2022;13(6):1758–67.
Deng Y, Chen Q, Li C, et al. Nomogram predicting early recurrence defined by the minimum P value approach for colorectal liver metastasis patients receiving colorectal cancer resection with simultaneous liver metastasis resection: development and validation. J Gastrointest Oncol. 2023;14(3):1279–92.
Finkelstein SE, Fernandez FG, Dehdashti F, et al. Unique site- and time-specific patterns of recurrence following resection of colorectal carcinoma hepatic metastases in patients staged by FDG-PET. J HepatoBiliary-Pancreat Sci. 2008;15(5):483–7.
Imai K, Allard MA, Benitez CC, et al. Early Recurrence After Hepatectomy for Colorectal Liver Metastases: What Optimal Definition and What Predictive Factors? Oncologist. 2016;21(7):887–94.
Inoue Y, Fujii K, Kagota S, et al. The Management of Recurrence within Six Months after Hepatic Resection for Colorectal Liver Metastasis. Dig Surg. 2020;37(4):282–91.
Jung SW, Kim DS, Yu YD, Han JH, Suh SO. Risk factors for cancer recurrence or death within 6 months after liver resection in patients with colorectal cancer liver metastasis. Annals Surg Treat Res. 2016;90(5):257–64.
Lalmahomed ZS, Mostert B, Onstenk W, et al. Prognostic value of circulating tumour cells for early recurrence after resection of colorectal liver metastases. British J Cancer. 2015;112(3):556–61.
Lin J, Peng J, Zhao Y, et al. Early recurrence in patients undergoing curative resection of colorectal liver oligometastases: identification of its clinical characteristics, risk factors, and prognosis. J Cancer Res Clin Oncol. 2018;144(2):359–69.
Liu W, Yan X, Wang K, et al. Risk factor analysis of early recurrence after resection of colorectal liver metastasis. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18(11):1098–101.
Malik HZ, Gomez D, Wong V, et al. Predictors of early disease recurrence following hepatic resection for colorectal cancer metastasis. Eur J Surg Oncol. 2007;33(8):1003–9.
Mao R, Zhao JJ, Bi XY, et al. A postoperative scoring system for post-hepatectomy early recurrence of colorectal liver metastases. Oncotarget. 2017;8(60):102531–9.
Narita M, Oussoultzoglou E, Chenard MP, et al. Predicting early intrahepatic recurrence after curative resection of colorectal liver metastases with molecular Markers. World J Surg. 2015;39(5):1167–76.
Sun Y, Yan X, Wang K, et al. Predictors and outcomes of recurrence after resection of colorectal liver metastases. Zhonghua Yi Xue Za Zhi. 2014;94(16):1232–6.
Tabchouri N, Gayet B, Okumura S, et al. Recurrence patterns after laparoscopic resection of colorectal liver metastases. Surg Endosc. 2018;32(12):4788–97.
Tanaka K, Murakami T, Yabushita Y, et al. Maximal debulking liver resection as a beneficial treatment strategy for advanced and aggressive colorectal liver metastases. Anticancer Res. 2014;34(10):5547–54.
Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases risk factors, prognosis, and treatment a livermetsurvey-based study of 6,025 Patients. Ann Surg Oncol. 2014;21(4):1276–86.
Watanabe G, Mise Y, Ito H, et al. Repeat Hepatectomy for Early Recurrence of Colorectal Liver Metastases-Prognostic Impacts Assessed from the Recurrence Pattern. World J Surg. 2020;44(1):268–76.
Wong GYM, Mol B, Bhimani N, et al. Recurrence patterns predict survival after resection of colorectal liver metastases. ANZ J Surg. 2022;92(9):2149–56.
Yamashita Y ichi, Adachi E, Toh Y, et al. Risk factors for early recurrence after curative hepatectomy for colorectal liver metastases. Surg Today. 2011;41(4):526–32.
Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Mei Z, Wang Q, Zhang Y, et al. Risk Factors for Recurrence after anal fistula surgery: A meta-analysis. Int J Surg. 2019;69:153–64.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical Score for Predicting Recurrence After Hepatic Resection for Metastatic Colorectal Cancer. Ann Surg. 1999;230(3):309.
Nevola R, Ruocco R, Criscuolo L, et al. Predictors of early and late hepatocellular carcinoma recurrence. World J Gastroenterol. 2023;29(8):1243–60.
Yang H, Wang J, Li Z, et al. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Front Oncol. 2019;9:854.
Wang C, Pang S, Si-Ma H, et al. Specific risk factors contributing to early and late recurrences of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol. 2019;17:2.
Tsilimigras DI, Sahara K, Wu L, et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg. 2020;155(9):823–31.
Hof J, Wertenbroek MWJLAE, Peeters PMJG, Widder J, Sieders E, de Jong KP. Outcomes after resection and or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg. 2016;103(8):1055–62.
Lin JK, Lin CC, Yang SH, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26(9):1135–41.
Araujo RLC, Gönen M, Allen P, et al. Positive postoperative CEA is a strong predictor of recurrence for patients after resection for colorectal liver metastases. Ann Surg Oncol. 2015;22(9):3087–93.
Rees M, Tekkis PP, Welsh FKS, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–35.
Tan MCB, Castaldo ET, Gao F, et al. A prognostic system applicable to patients with resectable liver metastasis from colorectal Carcinoma staged by positron emission tomography with [18F]Fluoro-2-Deoxy-D-Glucose: Role of Primary Tumor Variables. J Am College Surgeons. 2008;206(5):857–68.
Yamaguchi T, Mori T, Takahashi K, Matsumoto H, Miyamoto H, Kato T. A new classification system for liver metastases from colorectal cancer in Japanese multicenter analysis. Hepatogastroenterology. 2008;55(81):173–8.
Tan EK, Ooi L. Colorectal cancer liver metastases - understanding the differences in the management of synchronous and metachronous disease. Ann Acad Med, Singap. 2010;39:719–15.
Engstrand J, Strömberg C, Nilsson H, Freedman J, Jonas E. Synchronous and metachronous liver metastases in patients with colorectal cancer—towards a clinically relevant definition. World J Surg Oncol. 2019;17(1):228.
Peng J, Li W, Fan W, et al. Prognostic value of a novel biomarker combining DNA ploidy and tumor burden score for initially resectable liver metastases from patients with colorectal cancer. Cancer Cell Int. 2021;21:554.
Peng J, Liu Y, Li W, et al. Application of Tumor Burden Score for predicting conversion outcome in patients with initially unresectable colorectal liver metastases after first-line systemic therapy. Therap Adv Gastroenterol. 2021;14:17562848211066206.
Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol. 2011;46(9):548–55.
Sietses C, Meijerink MR, Meijer S, van den Tol MP. The impact of intraoperative ultrasonography on the surgical treatment of patients with colorectal liver metastases. Surg Endosc. 2010;24(8):1917–22.
Viganò L, Ferrero A, Amisano M, Russolillo N, Capussotti L. Comparison of laparoscopic and open intraoperative ultrasonography for staging liver tumours. Br J Surg. 2013;100(4):535–42.
Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204(5):854–62 discussion 862-864.
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg. 2016;263(1):146–52.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (Grant No.82002578), etc. The authors extend their sincere appreciation to National Natural Science Foundation of China for their financial support, which played a crucial role in the execution of this project.
The conception and design of the study were led by BL and GL. Their insights and expertise significantly shaped the research framework and objectives. YQW, NYW, and SFW made great contributions to the collection and analysis of data, as well as the manuscript writing. Their dedication and expertise greatly enriched the quality and depth of this study.
In the final stages, all authors critically reviewed and provided final approval for the manuscript. Their collaborative efforts and constructive feedback enhanced the quality of the final publication.
In conclusion, the authors would like to express their gratitude to those who contributed to the successful completion of this research.
Funding
This work was supported by National Natural Science Foundation of China (Grant No.82002578); Sichuan Science and Technology Program (Grant No.2022YSF0060, Grant No.2022YSF0114, Grant No.2022NSFSC0680, Grant No. 2023YFS0094); 1·3·5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (20HXFH021); 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21049).
Author information
Authors and Affiliations
Contributions
(i) Conception and design: YT, BL, GL; (ii) Administrative support: BL, GL; (ii) Collection and assembly of data: YT, SFW; (iii) Data analysis and interpretation: YT, YQW, NYW; (iv) Manuscript writing: YT, SFW; (v) Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, Y., Wang, Y., Wen, N. et al. Prognostic factors associated with early recurrence following liver resection for colorectal liver metastases: a systematic review and meta-analysis. BMC Cancer 24, 426 (2024). https://doi.org/10.1186/s12885-024-12162-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12162-4