Abstract
Background
A consensus has not been reached on the value of prostate-specific antigen density (PSAD) as a predictor of biochemical recurrence of prostate cancer. This meta-analysis aimed to evaluate the association between PSAD and biochemical recurrence of prostate cancer after primary treatment.
Methods
Two authors systematically searched PubMed, Web of Science, and Embase databases (up to August September 10, 2023) to identify studies that assessed the value of pretreatment PSAD in predicting biochemical recurrence after primary treatment (radical prostatectomy or radiotherapy) of prostate cancer. A random effect model was used to pool adjusted hazard ratios (HR) with 95% confidence intervals (CI) for biochemical recurrence.
Results
Nine studies with 4963 patients were eligible for the meta-analysis. The reported prevalence of biochemical recurrence ranged from 4 to 55.1%. For patients with higher PSAD compared to those with low PSAD, the pooled HR of biochemical recurrence was 1.59 (95% CI 1.21–2.10). Subgroup analysis showed that the pooled HR of biochemical recurrence was 1.80 (95% CI 1.34–2.42) for patients who received radical prostatectomy, and 0.98 (95% CI 0.66–1.45) for patients who received radiotherapy.
Conclusions
Elevated pretreatment PSAD may be an independent predictor for biochemical recurrence of prostate cancer after radical prostatectomy. Determining PSAD could potentially improve the prediction of biochemical recurrence in patients with prostate cancer.
Similar content being viewed by others
Background
Prostate cancer is the second most common type of cancer and the fifth leading cause of mortality among men [1]. Worldwide, there were approximately 1,414,259 newly diagnosed cases and 375,304 deaths from prostate cancer reported in 2020 [2]. Biochemical recurrence (BCR) after primary curative treatment may may indicate a more advanced or aggressive form of the disease. Almost one-third of men with prostate cancer experience BCR after primary treatment [3]. BCR is considered a marker for local recurrence, distant metastasis, and prostate-specific survival [4]. Determining BCR after primary treatment can help identify treatment failure and determine the need for salvage therapy. Therefore, improving the risk stratification of BCR is critical for better management of prostate cancer patients.
Prostate-specific antigen (PSA), a kallikrein-related serine protease, is widely used for prostate cancer screening. However, the blood PSA level can be affected by the size of the prostate gland. Prostate-specific antigen density (PSAD) is typically calculated by determining the ratio between the blood PSA level (ng/mL) and the estimated prostate volume (cm3) before treatment. Initially, PSAD was used to differentiate between benign prostatic disease and prostate cancer [5]. Subsequently, has been studied as a potential indicator for adverse pathological features [6, 7] or BCR [8, 9] after primary prostate cancer treatment. However, there is still conflicting evidence regarding whether pretreatment PSAD can independently predict BCR in patients with prostate cancer [8,9,10,11,12,13,14].
No previous meta-analysis has been conducted to investigate the association of PSAD with BCR of prostate cancer to date. Consequently, we conducted the present meta-analysis to further elucidate the significance of pretreatment PSAD as a prognostic factor for BCR in patients with prostate cancer.
Materials and methods
Study guideline and ethics approval
This study was prepared according to the checklist of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15]. Ethical approval was not necessary as the study did not involve individual patient data.
Literature search
Two authors conducted a thorough search on PubMed, Web of Science, and Embase databases until August September 10, 2023. The search utilized the following keywords (Supplemental Text S1): (“prostate neoplasms” OR “prostate cancer” OR “prostate tumor” OR “prostate carcinoma”) AND (“prostate-specific antigen density” OR “PSAD”) AND (“biochemical recurrence” OR “biochemical failure” OR “relapse”). Additionally, the authors manually reviewed references from included studies and relevant reviews for potential inclusion.
Study selection
Two authors independently evaluated the eligibility of the retrieved studies using the following criteria. The inclusion criteria included:1) patients with a diagnosis of prostate cancer who received radical prostatectomy or radiotherapy, 2) pretreatment PSAD level as a predictor,3) BCR defined as at least two consecutive PSA level elevation after primary curative treatment as the outcome of interest, 4) reported multivariable adjusted risk estimates of BCR for the categorical analysis of PSAD, and 5) prospective or retrospective observational study as the design. The cutoff value of PSAD elevation was defined by the individual study. The criteria for exclusion were as follow: (1) studies lacking risk estimate data, (2) reporting unadjusted risk estimates, and (3) reporting risk estimate by continuously coding PSAD.
Data extraction and quality assessment
Using a pre-designed data extraction form, two independent authors abstracted the following information: surname of the first author, year of publication, country of origin, design of study, type of prostate cancer, baseline age of the patients, number of patients, treatment approach, cutoff category of PSAD, definition of BCR, percentage of patients with BCR, follow-up duration, adjusted risk estimate, and adjusted confounders. The methodological quality of included studies was assess by two independent authors using the Newcastle-Ottawa Scale (NOS) [16]. The studies with the total score 7 or higher were considered high-quality. Disagreements in the data extraction and quality evaluation process were resolved by consensus.
Statistical analysis
All analyzes were conducted with Stata version12.0 (Stata Corporation, College Station, TX). The prognostic value of PSAD for BCR was summarized by combining adjusted hazard ratios (HR) with 95% confidence intervals (CI) for high vs. low PSAD category. The degree of heterogeneity across studies was evaluated using the I2 statistics and Cochran Q test. Statistical heterogeneity was determined by a p-value < 0.1 for the Cochran Q test and/or an I2 statistic greater than 50%. If statistical heterogeneity was present, a random effects model was used for the meta-analysis. To assess the reliability of the pooled summary, a sensitivity analysis was performed by excluding individual studies from the overall analysis each turn. Subgroup analyses were conducted based on the risk of patients according to the D’Amico criteria [17], country of origin, treatment approach, number of patients, cutoff value of PSAD, follow-up duration, and whether adjusted pretreatment PSA level. Funnel plots, Begg’s rank correlation test [18], and Egger’s linear regression test [19] were used to evaluate publication bias.
Results
Search results and characteristics of included studies
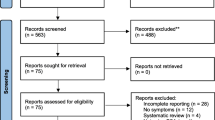
Figure 1 presents the meticulous process of study selection. Initially, a total of 727 publications were identified through our literature search. After eliminating duplicates, 316 articles remained for evaluation of their titles and abstracts. Of these, 32 full-text articles were retrieved for eligibility assessment. Following the application of predetermined inclusion and exclusion criteria, 23 articles were excluded. Finally, 9 studies [8, 9, 11,12,13,14, 20,21,22] were included in this meta-analysis.
Table 1 presents the baseline characteristics of the included studies. These studies were published between 1997 and 2022 and were all retrospective in nature. The eligible studies were conducted in various regions including Europe [11, 12], USA [8], Canada [9], Japan [13, 14, 21], and China [20, 22]. The sample sizes of individual studies ranged between 95 and 1334, resulting in a total of 4963 prostate cancer patients. The follow-up duration varied between 21 and 60.3 months. The reported prevalence of BCR ranged from 4 to 55.1%. The median/mean age of the patients ranged from 63 to 69.5 years old from studies reporting such data. Based on the NOS criteria, 2 studies [14, 21] were graded as moderate quality, while the remaining studies were graded as high-quality (Table S1).
Association of PSAD with BCR
As shown in Fig. 2, a meta-analysis using a random effect model showed that the pooled adjusted HR for BCR was 1.59 (95% CI 1.21–2.10) in the high PSAD category compared to the low category. There was significant heterogeneity (I2 = 57.6%; p = 0.016) across studies. Leave-one-out sensitivity analysis revealed that none of the individual studies had a significant impact on the overall pooling result. In the subgroup analysis (Table 2), the pooled HR for BCR was 1.80 (95% CI 1.34–2.42) among patients receiving radical prostatectomy [11,12,13,14, 20,21,22], while 0.98 (95% CI 0.66–1.45) in those receiving radiotherapy [8–9].
Publication bias
There was no evidence of publication bias according to the Begg’s test (p = 0.175), Egger’s test (p = 0.368), and funnel plots (Fig. 3).
Discussion
To the best of our knowledge, this is the first meta-analysis to assess the impact of pretreatment PSAD on BCR of prostate cancer after primary treatment. Our main finding suggests that elevated pretreatment PSAD may be an independent predictor for BCR of prostate cancer after radical prostatectomy. Compared to patients with low PSAD, those with high PSAD had an 80% higher risk of BCR after radical prostatectomy. However, there was no clear association between pretreatment PSAD and BCR of prostate cancer in the radiotherapy subgroup.
Apart from the categorical analysis of the PSAD, when considered as a continuous variable, was an independent risk factor for BCR in patients with intermediate-risk prostate cancer who underwent radical prostatectomy [23]. This association was also observed in patients with high-risk and very high-risk prostate cancer, even after adjusting for other factors [24]. These findings provide additional evidence for the predictive value of pretreatment PSAD in predicting BCR in prostate cancer patients.
One potential issue with the interpretation of the findings is the treatment pattern. Our subgroup analysis revealed that elevated PSAD was only a significant predictor of BCR in patients who underwent radical prostatectomy, but not in those who received radiotherapy. This suggests that PSAD may have a stronger predictive value after surgery compared to after radiotherapy. Additionally, the predictive value of PSAD seemed to be more pronounced in high-risk prostate cancer patients according to the D’Amico criteria. However, it should be noted that these findings were based on a small number of studies. Therefore, further research is needed to validate these results.
The prevalence of patients developing BCR was up to 55.1% in the included studies. Our meta-analysis provides some evidence to support elevated pretreatment PSAD was associated with an increased risk of BCR in prostate cancer patients. Incorporating PSAD into active surveillance protocols may improve the accuracy of predicting BCR in these patients. Additionally, elevated PSAD levels have been linked to high Gleason scores [6], advanced pathological stages [25], and extracapsular extension [12]. Therefore, assessing PSAD in prostate cancer patients has the potential to aid in clinical decision making.
Our meta-analysis has certain limitations that should be acknowledged. Firstly, all of the studies included in our analysis were retrospective designs, which carry inherent selection bias and may have unmeasured confounders. Secondly, the use of different cutoff values for PSAD in the included studies makes it difficult to apply our findings in a clinical setting. Thirdly, there was no uniform definition of BCR in the included studies. We only selected studies that reported at least two consecutive rises in PSA levels after curative treatment, and therefore, some studies that reported a single rise in PSA levels were not included in our analysis. However, these studies still provided valuable data on the association between PSAD and BCR in prostate cancer. Fourthly, there was significant heterogeneity in both the overall and subgroup analyses, which may be attributed to variations in patient and tumor characteristics, PSAD cutoff values, methods for measuring prostate volume, and treatment approaches. Fifthly, this meta-analysis was not prospectively registered in PROSPERO and other international databases. Finally, the number of patients included in our analysis was relatively small, particularly in some subgroups.
In conclusion, our analysis suggests that an elevated pretreatment PSAD may be an independent predictor for BCR of prostate cancer after radical prostatectomy. Therefore, incorporating the assessment of pretreatment PSAD may improve risk stratification for BCR in prostate cancer patients. However, it is important to note that our conclusion is limited by the fact that we only analyzed retrospective studies. Future high-quality prospective studies are required to validate the our findings.
Data availability
All data generated or analyzed during this study are included in this published article and its additional files.
Abbreviations
- PSAD:
-
Prostate-specific antigen density
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- BCR:
-
Biochemical recurrence
- NOS:
-
Newcastle-Ottawa Scale
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Gandaglia G, Leni R, Bray F, Fleshner N, Freedland SJ, Kibel A, Stattin P, Van Poppel H, La Vecchia C. Epidemiology and Prevention of prostate Cancer. Eur Urol Oncol. 2021;4:877–92.
Simon NI, Parker C, Hope TA, Paller CJ. Best approaches and updates for prostate Cancer biochemical recurrence. Am Soc Clin Oncol Educ Book. 2023;42:1–8.
Van den Broeck T, van den Bergh RCN, Arfi N, Gross T, Moris L, Briers E, Cumberbatch M, De Santis M, Tilki D, Fanti S, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate Cancer: a systematic review. Eur Urol. 2019;75:967–87.
Benson MC, Whang IS, Pantuck A, Ring K, Kaplan SA, Olsson CA, Cooner WH. Prostate specific antigen density: a means of distinguishing benign prostatic hypertrophy and prostate cancer. J Urol. 1992;147:815–6.
Magheli A, Hinz S, Hege C, Stephan C, Jung K, Miller K, Lein M. Prostate specific antigen density to predict prostate cancer upgrading in a contemporary radical prostatectomy series: a single center experience. J Urol. 2010;183:126–31.
Oh JJ, Hong SK, Lee JK, Lee BK, Lee S, Kwon OS, Byun SS, Lee SE. Prostate-specific antigen vs prostate-specific antigen density as a predictor of upgrading in men diagnosed with Gleason 6 prostate cancer by contemporary multicore prostate biopsy. BJU Int. 2012;110:E494–499.
Ingenito AC, Ennis RD, Hsu IC, Begg MD, Benson MC, Schiff PB. Re-examining the role of prostate-specific antigen density in predicting outcome for clinically localized prostate cancer. Urology. 1997;50:73–8.
Aref I, Eapen L, Agboola O, Cross P. Is prostate specific antigen density an important prognostic indicator for patients with prostate cancer treated with external beam therapy? Br J Radiol. 1998;71:868–71.
Corn BW, Hanks GE, Lee WR, Bonin SR, Hudes G, Schultheiss T. Prostate specific antigen density is not an independent predictor of response for prostate cancer treated by conformal radiotherapy. J Urol. 1995;153:1855–9.
Busch J, Hamborg K, Meyer HA, Buckendahl J, Magheli A, Lein M, Jung K, Miller K, Stephan C. Value of prostate specific antigen density and percent free prostate specific antigen for prostate cancer prognosis. J Urol. 2012;188:2165–70.
Gandaglia G, Ploussard G, Isbarn H, Suardi N, De Visschere PJ, Futterer JJ, Ghadjar P, Massard C, Ost P, Sooriakumaran P, et al. What is the optimal definition of misclassification in patients with very low-risk prostate cancer eligible for active surveillance? Results from a multi-institutional series. Urol Oncol. 2015;33:164e161–169.
Hashimoto T, Yoshioka K, Nagao G, Nakagami Y, Ohno Y, Horiguchi Y, Namiki K, Nakashima J, Tachibana M. Prediction of biochemical recurrence after robot-assisted radical prostatectomy: analysis of 784 Japanese patients. Int J Urol. 2015;22:188–93.
Yashi M, Nukui A, Tokura Y, Takei K, Suzuki I, Sakamoto K, Yuki H, Kambara T, Betsunoh H, Abe H, et al. Performance characteristics of prostate-specific antigen density and biopsy core details to predict oncological outcome in patients with intermediate to high-risk prostate cancer underwent robot-assisted radical prostatectomy. BMC Urol. 2017;17:47.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Assessed September 28,: The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2023).
D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, Tomaszewski JE, Renshaw AA, Kaplan I, Beard CJ, Wein A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Peng C, Zhang J, Hou J. Performance characteristics of prostate-specific antigen density and biopsy primary Gleason score to predict biochemical failure in patients with intermediate prostate cancer who underwent radical prostatectomy. Cancer Manag Res. 2019;1:1133–9.
Shida Y, Hakariya T, Mitsunari K, Matsuo T, Ohba K, Miyata Y, Sakai H. Preoperative predictors of Lymph Node Invasion and biochemical recurrence in high-risk prostate Cancer. Cancer Diagn Progn. 2022;2:49–54.
Yan YH, Hu GM, Xie C, Geng ZX, Xue BX. Risk factors for biochemical recurrence of prostate cancer:a single-center 11-year retrospective analysis. J Contemp Urol Reprod Oncol. 2022;14:150–5.
Narita S, Mitsuzuka K, Tsuchiya N, Koie T, Kawamura S, Ohyama C, Tochigi T, Yamaguchi T, Arai Y, Habuchi T. Reassessment of the risk factors for biochemical recurrence in D’Amico intermediate-risk prostate cancer treated using radical prostatectomy. Int J Urol. 2015;22:1029–35.
Yang CK, Yang CR, Ou YC, Cheng CL, Ho HC, Chiu KY, Wang SS, Li JR, Chen CS, Hung CF, et al. Prostate–specific antigen density and preoperative MRI findings as predictors of biochemical recurrence in high–risk and very high–risk prostate cancer. Oncol Lett. 2023;26:284.
Koie T, Mitsuzuka K, Yoneyama T, Narita S, Kawamura S, Kaiho Y, Tsuchiya N, Tochigi T, Habuchi T, Arai Y, et al. Prostate-specific antigen density predicts extracapsular extension and increased risk of biochemical recurrence in patients with high-risk prostate cancer who underwent radical prostatectomy. Int J Clin Oncol. 2015;20:176–81.
Acknowledgements
None.
Funding
This work is supported by (1) Provincial Natural Science Foundation Project of Jiangsu of China (BK20191221), (2) Social Development Plan of Jiangsu Province-Standardization of Key Disease Diagnosis and Treatment Project (BE2016715), and (3) Commission of Health and Family Planning Foundation Jiangsu Provincial of China (H2017089).
Author information
Authors and Affiliations
Contributions
Y Fan and C Zou contributed to study conception/design and interpretation of data; FL Cui, Y Qiu, W Xu contributed to literature search, study selection, data extraction, quality assessment, and statistical analysis; Y Qiu wrote the manuscript; FL Cui editted the manuscript; All the authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cui, F., Qiu, Y., Xu, W. et al. Pretreatment prostate-specific antigen density as a predictor of biochemical recurrence in patients with prostate cancer: a meta-analysis. BMC Cancer 24, 305 (2024). https://doi.org/10.1186/s12885-024-12029-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12029-8