Abstract
Background
Prognosis prediction for pancreatic cancer has always been difficult in clinical practice because of its high heterogeneity and mortality. The aim of the study was to assess the value of prognostic immune-inflammatory-nutritional (PIIN) score on overall survival (OS) in postoperative patients with pancreatic cancer and to develop a nomogram incorporating PIIN score.
Methods
This study retrospectively analyzed the clinic pathological data of 155 patients with pancreatic cancer who underwent radical surgery. PIIN score was calculated by measuring the fibrinogen (FIB), neutrophil to lymphocyte ratio (NLR), systemic immune-inflammation index (SII), albumin-bilirubin (ALBI) score, and prognostic nutritional index (PNI). Patients were divided into two groups by PIIN score levels over a threshold of 37.2. Univariate and multivariate analysis were performed using the Cox regression analysis model. The time-dependent receiver operating characteristic (ROC) curve was plotted to compare the prognostic values of the scoring systems. Finally, a nomogram based on PIIN score was constructed and validated.
Results
Multivariate regression analysis showed that PIIN score (hazard ratio (HR) = 2.171, 95% confidence interval (CI) = 1.207–3.906, P = 0.010), lymphovascular invasion (HR = 1.663, 95% CI = 1.081–2.557, P = 0.021), poor tumor grade (HR = 2.577, 95% CI = 1.668–3.982, P < 0.001), bad TNM stage (I vs. II: HR = 1.791, 95% CI = 1.103–2.906, P = 0.018; I vs. III: HR = 4.313, 95% CI = 2.365–7.865, P < 0.001) and without adjuvant chemotherapy (HR = 0.552, 95% CI = 0.368–0.829, P = 0.004) were independent risk factors for OS. The time-dependent ROC curves revealed that PIIN score was better than the other scoring systems in predicting survival prognosis. And last, the nomogram established from independent factors such as PIIN score had good predictive power for OS. The ROC curve results showed that the AUC values for 1, 3 and 5 years were 0.826, 0.798 and 0.846, respectively. The calibration plots showed the superior clinical applicability of the nomogram.
Conclusion
The nomogram model based on PIIN score can be utilized as one of the prognosis stratifications as well as postoperative follow-up for the development of individual treatment for pancreatic cancer.
Similar content being viewed by others
Introduction
Pancreatic cancer is a common malignant tumor of the digestive system with high morbidity and mortality [1, 2]. Radical surgical resection is the most effective treatment for pancreatic cancer. However, early diagnosis of pancreatic cancer is difficult because its early symptoms are often non-specific. In addition, it is highly invasive and has a poor prognosis, with an overall 5-year survival rate of less than 11% [3]. Current options to predict the overall survival (OS) in pancreatic cancer remain unsatisfying. Because of the heterogeneous nature of pancreatic cancer, the treatment strategy and outcomes are diverse, even for tumors with the same TNM stage. Therefore, it is important to find more accurate predictive markers for the treatment of pancreatic cancer.
Pathological factors are widely recognized prognostic measures that can significantly reflect tumor phenotypic differences and have significant predictive power [4, 5]. However, many studies have shown that the outcomes of patients with cancer are determined not only by tumor-related factors but also by patient-related factors. Inflammation-related scoring systems, such as neutrophil to lymphocyte ratio (NLR) and systemic inflammation score, have been shown to correlate with prognosis for pancreatic cancer [6, 7]. Moreover, some scoring systems composed of immunity and nutrition-related markers could also predict the prognosis of pancreatic cancer, such as the prognostic nutritional index (PNI) and controlling nutritional status score [8, 9]. However, a single blood marker can not reflect the landscape of a patient’s immune function, nutrition status, and inflammation.
The prognostic immune-inflammatory-nutritional (PIIN) score is a new scoring system which includes all of the markers that have been predominantly used now. Recently, a retrospective study of 571 patients found that PIIN score could predict the prognosis in patients with resected intrahepatic cholangiocarcinoma, which helped surgeons identify high-risk patients and develop individualized treatment plans [10]. However, the significance of PIIN score in the prognosis of patients with pancreatic cancer has not been explored. Thus, this study aimed to investigate whether PIIN score is associated with survival after surgery in patients with pancreatic cancer.
Patients and methods
Patients
A total of 180 patients with resected pancreatic cancer between November 2011 and August 2022 at the Department of Hepatobiliary and Pancreatic Surgery, First Hospital of Jiaxing were retrospectively assessed. The inclusion criteria for this study were as follows: (1) pathologically diagnosed with pancreatic ductal adenocarcinoma, (2) patients received no prior anti-cancer treatment, (3) complete clinicopathological and follow-up data, and (4) radical resection performed with R0 margin. The exclusion criteria were as follows: (1) other concurrent malignancies, (2) active or chronic infectious or inflammatory status. (3) patients with distant metastasis, (4) tumor progression or death occurred within 1 month after surgery. In the end, 155 patients that met the criteria were included.
Surgical methods and adjuvant therapy
Based on the location and size of the tumor, each patient underwent pancreaticoduodenectomy, distal pancreatectomy or total pancreatectomy, and routine dissection of the abdominal lymph nodes. Gemcitabine and fluorouracil combined with adjuvant chemotherapy were routinely administered to patients with pancreatic cancer, without contraindications, after radical surgery.
Postoperative follow-up
In this study, overall survival (OS) was defined as the time between surgery and all-cause death or the last follow up. All patients were followed up every 3 months for 2 years after surgery, and the number of visits was reduced to every 6 months after 2 years. Survival data were extracted from outpatient or telephone records during follow-up. All patients were followed up until death or December 2022.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the First Hospital of Jiaxing (batch number: 2023-KY-670). We retrospectively used information about the participants’ previous clinical visits without direct contact with them and protected their privacy. The Ethics Committee of the First Hospital of Jiaxing approved the requirement for the waiver of informed consent for this study.
PIIN score and other prognostic scoring systems
Data on the following clinical characteristics and clinicopathological information were obtained from electronic medical records of the hospital information system. Including: age, sex, tumor location, lymphovascular and nerve invasion, tumor size, grade, T stage, N stage, TNM stage, adjuvant chemotherapy, chemotherapy completion rate, and postoperative complications. Besides, lymphocyte count, neutrophil count, platelet count, fibrinogen (FIB), albumin, carbohydrate antigen 19 − 9 (CA 19 − 9) and bilirubin levels were determined within 7 days before surgery.
NLR = neutrophil count/lymphocyte count [11]. Systemic immune-inflammation index (SII) = platelet count × neutrophil count/lymphocyte count [12]. albumin-bilirubin (ALBI) score = -0.085× albumin (g/L) + 0.66 × log10 bilirubin (µmol/L) [13]. PNI = 10 × albumin (g/L) + 5 × lymphocyte count [14].
The PIIN score was calculated according to Jiang et al.’s method, with five parameters including NLR, SII, FIB, ALBI, and PNI [10]. PIIN score = NLR × 0.876 + SII × 0.0174 + FIB × 14.355 + ALBI × 2.209 − PNI × 0.386. In this study, we further adjusted the cut-off value using X-tile software. The ideal cut-off point was found to be 37.2 for PIIN score.
Statistical analysis
In this study, chi-square test or Fisher’s exact probability method was used to assess the relationship between PIIN score and the clinicopathological features of the patients. Univariate and multivariate analyses were performed using Cox proportional hazards regression to assess the prognostic factors. The Kaplan-Meier method was used to draw the survival curve, and a parallel log-rank test was performed. A time-dependent receiver operating characteristic (ROC) curve and area under curve (AUC) were used to compare the prognostic abilities of PIIN score and other prognostic scoring systems. A nomogram was then built by using the variables in the multivariate analysis to predict OS at 1, 3, and 5 years after surgery. ROC curve analyses were performed to compare the predicting efficiency of the prediction model. Calibration curves were plotted to evaluate the consistency between predicted and observed survival. The cut-off values of age and CA 19 − 9 level were generated by the X-tile software (3.6.1; Yale University, New Haven, USA). Statistical significance was set at a two-sided P-value equal to 0.05.
SPSS (version 22.0; IBM Corporation, Armonk, NY, USA) and R software (R Project for Statistical Computing, Vienna, Austria) were used for data processing. The R-packet “timeROC” was used for time-dependent ROC curve analysis, and the nomogram was drawn using the R-packet “rms”.
Results
Relationships between PIIN score and clinicopathological characteristics
A total of 155 patients with pancreatic cancer were enrolled in this study according to the inclusion and exclusion criteria. Among them, 85 (54.8%) were males and 70 (45.2%) were females. Of the 155 patients, the median age was 66 years (IQR: 60.5–71 years); the median CA 19 − 9 level was 159.7 U/mL (IQR: 48.0-421.6 U/mL). As for tumor site, 107 (69.0%) tumors were primarily located in the head of the pancreas, while 48 (31.0%) tumors primarily occurred in the body or tail of the pancreas. According to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system classification, 55 cases in stage I, 74 cases in stage II, and 26 cases in stage III. 89 cases (57.4%) received postoperative adjuvant chemotherapy. Postoperative complications occured in 43.9% of cases. The most common complications were related to infection (14.8%), followed by pancreatic leak (11.0%) and bleeding (5.8%).
Table 1 summarizes the relationships between PIIN score and clinicopathological characteristics. PIIN score was closely related to the tumor location (P = 0.003) and postoperative complications (P < 0.001). However, it had no significant correlation with age, sex, lymphovascular and nerve invasion, grade, tumor size, T stage, N stage, TNM stage, CA 19 − 9, adjuvant chemotherapy, type of adjuvant chemotherapy, chemotherapy completion rate, and type of postoperative complications (all P > 0.05).
Univariate and multivariate cox regression analysis
The results of the univariate analyses based on OS are shown in Table 2. Univariate analysis demonstrated that age, lymphovascular invasion, grade, N stage, TNM stage, adjuvant chemotherapy and PIIN score were significantly correlated with OS (all P < 0.05).
A Cox multivariate model was established to identify independent risk factors affecting OS. And PIIN score was identified as an independent factor to predict the OS (Table 3; hazard ratio (HR) = 2.171, 95% confidence interval (CI) = 1.207–3.906, P = 0.010). In addition, OS was markedly impaired among cases with lymphovascular invasion (HR = 1.663, 95% CI = 1.081–2.557, P = 0.021), poor tumor grade (HR = 2.577, 95% CI = 1.668–3.982, P < 0.001), bad TNM stage (I vs. II: HR = 1.791, 95% CI = 1.103–2.906, P = 0.018; I vs. III: HR = 4.313, 95% CI = 2.365–7.865, P < 0.001) and without adjuvant chemotherapy (HR = 0.552, 95% CI = 0.368–0.829, P = 0.004).
Prognostic value of the PIIN score and other prognostic scoring systems
The time-dependent ROC curve was generated for the PIIN score and other prognostic scoring systems, and the AUC values were calculated at different time points. Time-dependent ROC curve analysis revealed that PIIN score was significantly superior to SII, ALBI, NLR, and PNI in predicting 1-, 3-, and 5-year OS (Fig. 1).
Nomogram development and validation
Based on the results of univariate and multivariate COX regression analysis, independent prognostic factors were integrated into the construction of nomogram model to predict OS at 1-, 3-, and 5-year (Fig. 2).
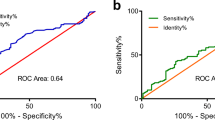
The C-index value for patients with pancreatic cancer were 0.711 (95% CI: 0.676–0.746). The C-index was between 0.7 and 0.9 meant that the prediction accuracy of the model was high. The ROC curve results showed that the AUC values for 1, 3, and 5 years are 0.826, 0.798, and 0.846, respectively (Fig. 3A). The calibration curves for the probability of survival at 1, 3, and 5 years demonstrated good agreement between nomogram predictions and actual observations (Fig. 3B-D).
Evaluation of the nomogram model. A ROC curve of a predictive model that predicts 1-, 3-, and 5-year survival. B-D Calibration curves of prediction models for predicting 1-, 3-, and 5-year survival. The horizontal axis represents the nomogram-predicted survival, and the vertical axis symbolizes the actual survival. The curve in color closest to the 45° gray line gets the best prediction performance
Risk stratification based on the nomogram
Based on different cut-off values of the total points determined by the X-tile software, we subdivided patients into low-, middle -, and high-risk groups, and applied Kaplan-Meier survival analysis to assess their survival. Patients were divided into low risk group (< 140), medium risk group (140–220) and high risk group (>220). The results of survival curve showed a significant difference in prognosis among the three risk groups (Fig. 4; P < 0.001). Therefore, the nomogram-based risk stratification system can significantly enhance the discrimination of survival of pancreatic cancer patients.
Discussion
A single indicator is insufficient for prognosis risk stratification, highlighting the urgent need to integrate these markers. The PIIN score, which is a new scoring system that includes serum fibrinogen, NLR, SII, ALBI score, and PNI, comprehensively reflects the patient’s inflammatory, immune and nutritional status.
Previous studies have shown that postoperative complication was significantly associated with prognosis [15, 16]. A retrospective study by Aoyama et al. revealed that postoperative complication was associated with poor prognosis in patients with pancreatic cancer [17]. In our cohort, the most common complication was related to infection followed by pancreatic leak and bleeding. A previous study showed that poor nutritional status with low albumin level (< 3.5 g/dL) or low BMI (< 18.5 kg/m2) before surgery for pancreatic head cancer was a predictor of postoperative complications [18]. Similarly, PIIN score was markedly related to postoperative complications (P < 0.001). Therefore, PIIN score has great clinical significance for predicting postoperative complications and aiding the assessment of treatment tolerance.
Differences of the tumor location may also affect clinical and surgical outcomes. surgical margin positivity was more likely for tumors located in the uncinate process than for other tumors in a retrospective study [19]. Moreover, different surgical approach according to tumor location could have an impact on patient’s gastrointestinal function and delay in recovery to a different degree. In our study, a significantly higher PIIN score was observed in patients with pancreatic head cancer (P = 0.003). However, tumor location was not associated with the prognosis of pancreatic cancer. Larger clinical samples and prospective studies are needed to examine the relationship between prognosis and the tumor location.
Based on the prognostic analysis of 155 patients with pancreatic cancer who underwent radical surgery, we observed that PIIN score was an independent prognostic factor for OS. We compared the predictions of different prognostic scores by time-dependent ROC curve analysis. The results showed that PIIN score had the highest AUC value compared with NLR, SII, ALBI score, and PNI, and the prognosis prediction effect was the best. Thus, clinicians can better monitor patients considering the better accuracy of PIIN score in predicting prognosis at an individual level. Moreover, close follow-up and individualized adjuvant therapy after surgery are of great clinical significance for improving patient prognosis.
Systemic inflammatory status and local immune response in cancer patients are significantly associated with tumor progression and prognosis [20]. Tissue damage caused by chronic inflammation produces local anti-inflammatory cytokines that promote tumor cell proliferation [21]. As a major player in the innate immune system, neutrophils respond to various inflammatory signals, including cancer, and directly promote tumor progression, metastasis, and angiogenesis [22]. The secretion of cytokines such as IL-6 and IL-8 induces neutrophil recruitment to the tumor site and has pro-inflammatory and angiogenic effects [23,24,25]. Lymphocytes can be used to assess immune status, and they induce an antitumor immune response in the systemic circulation and tumor microenvironment to kill tumor cells [26]. Lymphocytopenia has been reported to be associated with prognosis of patients with pancreatic cancer [27]. Additionally, monocytes promote immune escape by limiting the infiltration of activated CD8 T cells into tumor microenvironment [28].
Pancreatic cancer is characterized by exocrine insufficiency and nutritional imbalance, which can lead to malnutrition and sarcopenia [29]. Cancer-related malnutrition makes patients more vulnerable to surgical injury and serves as a negative prognostic factor. Serum albumin is an important marker of host nutritional status and is closely correlated with the degree of malnutrition. In pancreatic cancer, PNI and Controlling Nutritional Status as the nutritional status indices not only reflect the overall nutritional status of the patient but also closely related to the prognosis [30, 31]. Therefore, carrying out a nutritional screening is a fundamental intervention in the diagnosis of pancreatic cancer and should be implemented at regular intervals during therapy.
Integrating multiple prognostic factors allows a more accurate evaluation of patients’ prognosis. In our study, the nomogram model based on the PIIN score can predict the prognosis of patients with pancreatic cancer after surgery. The nomogram excels in predictive performance, with ROC curves showing AUC values of 0.826, 0.798, and 0.846 for 1, 3, and 5 years, respectively. The calibration plots of the PIIN score-based nomogram model indicated that it has favorable discriminative ability in patients with pancreatic cancer. The developed nomogram not only comprehensively integrates numerous known clinicopathological features into a prognostic model but also expands the clinical applications of the PIIN score.
This study had some limitations. First, the present study, which lacked external validation, was a single-center cohort study, and selection bias might have affected the results. Second, the cut-off value of PIIN score needs to be further validated. In the future, prospective studies with larger sample sizes, and external validation of our findings in other populations, are essential.
Conclusion
PIIN score is an independent prognostic factor for OS in patients with pancreatic cancer. A new nomogram prediction model based on PIIN score is established and validated, which can independently predict disease progression and survival of patients with pancreatic cancer that are undergoing surgical management
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- OS:
-
overall survival
- NLR:
-
neutrophil to lymphocyte ratio
- PNI:
-
prognostic nutritional index
- PIIN:
-
prognostic immune-inflammatory-nutritional
- FIB:
-
fibrinogen
- CA 19 − 9:
-
carbohydrate antigen 19 − 9
- SII:
-
systemic immune-inflammation index
- ALBI:
-
albumin-bilirubin
- ROC:
-
receiver operating characteristic
- AUC:
-
area under curve
- AJCC:
-
American Joint Committee on Cancer
- HR:
-
hazard ratio
- CI:
-
confidence interval
References
The global. Regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–47.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493–502.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Muralidhar V, Nipp RD, Mamon HJ, Punglia RS, Hong TS, Ferrone C, et al. Association between very small Tumor size and decreased overall survival in node-positive pancreatic Cancer. Ann Surg Oncol. 2018;25:4027–34.
Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–79.
Iwai N, Okuda T, Sakagami J, Harada T, Ohara T, Taniguchi M, et al. Neutrophil to lymphocyte ratio predicts prognosis in unresectable pancreatic cancer. Sci Rep. 2020;10:18758.
Markus M, Abendroth A, Noureddine R, Paul A, Breitenbuecher S, Virchow I, et al. Combined systemic inflammation score (SIS) correlates with prognosis in patients with advanced pancreatic cancer receiving palliative chemotherapy. J Cancer Res Clin Oncol. 2021;147:579–91.
Li S, Tian G, Chen Z, Zhuang Y, Li G. Prognostic role of the Prognostic Nutritional Index in Pancreatic Cancer: a Meta-analysis. Nutr Cancer. 2019;71:207–13.
Dang C, Wang M, Zhu F, Qin T, Qin R. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with pancreatic cancer undergoing radical surgery. Asian J Surg. 2022;45:1237–45.
Zhu J, Wang D, Liu C, Huang R, Gao F, Feng X, et al. Development and validation of a new prognostic immune-inflammatory-nutritional score for predicting outcomes after curative resection for intrahepatic cholangiocarcinoma: a multicenter study. Front Immunol. 2023;14:1165510.
Maeda T, Hiura A, Uehara J, Toyoshima R, Nakagawa T, Yoshino K. Neutrophil-to-lymphocyte ratio is associated with survival and sentinel lymph node positivity in invasive cutaneous squamous cell carcinoma: a retrospective study. J Am Acad Dermatol. 2022;86:615–20.
Guo W, Song Y, Sun Y, Du H, Cai Y, You Q, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front Endocrinol. 2022;13:1071465.
Ho SY, Hsu CY, Liu PH, Lee RC, Ko CC, Huang YH, et al. Albumin-Bilirubin (ALBI) Grade-based Nomogram for patients with Hepatocellular Carcinoma undergoing Transarterial Chemoembolization. Dig Dis Sci. 2021;66:1730–8.
Wang Z, Zhao L, He S. Prognostic nutritional index and the risk of mortality in patients with hypertrophic cardiomyopathy. Int J Cardiol. 2021;331:152–7.
Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247:71–6.
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575–83.
Aoyama T, Murakawa M, Katayama Y, Yamaoku K, Kanazawa A, Higuchi A, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res. 2015;35:2401–9.
Lee B, Han HS, Yoon YS, Cho JY, Lee JS. Impact of preoperative malnutrition, based on albumin level and body mass index, on operative outcomes in patients with pancreatic head cancer. J Hepato-Biliary-Pancreat Sci. 2021;28:1069–75.
Lai CC, Wang SY, Liao CH, Hsu JT, Chiang KC, Yeh TS, et al. Surgical Margin Status of Patients with Pancreatic Ductal Adenocarcinoma Undergoing Surgery with Radical Intent: Risk Factors for the Survival Impact of Positive Margins. In vivo (Athens, Greece). 2018;32:1591–7.
Dupré A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44:566–70.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–67.
Kerfoot SM, Raharjo E, Ho M, Kaur J, Serirom S, McCafferty DM, et al. Exclusive neutrophil recruitment with oncostatin M in a human system. Am J Pathol. 2001;159:1531–9.
Chen MB, Hajal C, Benjamin DC, Yu C, Azizgolshani H, Hynes RO, et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc Natl Acad Sci USA. 2018;115:7022–7.
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–904.
Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res. 2013;19:28–33.
Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8.
Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. 2012;72:876–86.
De Luca R, Gianotti L, Pedrazzoli P, Brunetti O, Rizzo A, Sandini M, et al. Immunonutrition and prehabilitation in pancreatic cancer surgery: a new concept in the era of ERAS® and neoadjuvant treatment. Eur J Surg Oncol. 2023;49:542–9.
Kim KH, Hwang HK, Kang IC, Lee WJ, Kang CM. Oncologic impact of preoperative prognostic nutritional index change in resected pancreatic cancer following neoadjuvant chemotherapy. Pancreatology. 2020;20:247–53.
Ma X, Zou W, Sun Y. Prognostic value of pretreatment Controlling Nutritional Status score for patients with pancreatic Cancer: a Meta-analysis. Front Oncol. 2021;11:770894.
Acknowledgements
Not applicable.
Funding
This study was supported by the Jiaxing Science and Technology Project (2021AD30140).
Author information
Authors and Affiliations
Contributions
J Y: Conceptualization, Methodology, Investigation, Data curation, Writing-original draft. HK Z and HB L: Formal analysis, Validation, Visualization. FQ Z: Formal analysis, Software. K T: Project administration, Writing-review & editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of the First Hospital of Jiaxing (batch number: 2023-KY-670). We retrospectively used information about the participants’ previous clinical visits without direct contact with them and protected their privacy. The Ethics Committee of the First Hospital of Jiaxing approved the requirement for the waiver of informed consent for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, J., Zhou, H., Li, H. et al. Nomogram incorporating prognostic immune-inflammatory-nutritional score for survival prediction in pancreatic cancer: a retrospective study. BMC Cancer 24, 193 (2024). https://doi.org/10.1186/s12885-024-11948-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-11948-w