Abstract
Background
Central nervous system (CNS) tumors are the most common solid tumors in children and the leading cause of cancer-related death in the latter. Currently, the incidence rate exceeds that of leukemia and ranks first in the incidence of malignant tumors in children.
Methods
The epidemiological data on childhood CNS tumors were collected from the Chinese Cancer Registry Annual Report. The annual percent change (APC) of incidence and mortality-rate changes were estimated via Joinpoint regression. Due to a lack of pertinent data, we performed a system review on the clinical-pathological characteristics in Chinese publications.
Results
There was no significant increase in the incidence rate (APC: -0.1, 95% CI: -1.5 to 1.3), but there was a significant increase in the mortality rate (APC: 1.8, 95% CI: 0.3 to 3.4) for childhood CNS tumors. In the subgroup analysis, there were significant increases in both the incidence and mortality rates in rural areas (APC in the incidence: 6.2, 95% CI: 2.4 to 10.2; APC in mortality: 4.4, 95% CI: 0.4 to 8.4). The most common location and type of childhood CNS were, respectively, the cerebral hemisphere (25.5%, 95% CI: 21.7% to 29.4%) and astrocytomas (26.8%, 95% CI: 23.9% to 29.6%).
Conclusions
The epidemiological trends, and the relevant prediction, highlighted the need to pay continual attention to childhood CNS tumors, and the clinicopathology evinced its own distinctive characteristics. Timely detection and effective treatment must be further promoted regarding childhood CNS tumors with a view to decreasing the disease burden, especially in rural areas.
Similar content being viewed by others
Background
In comparison with adult cancers, childhood cancers are less common. Nonetheless, the poor prognosis for childhood cancers generates a serious impact on quality of life, especially in the case of childhood central nervous-system (CNS) tumors [1]. Currently, the incidence rate of childhood CNS tumors exceeds that of childhood leukemia, as reflected in the 2020 CBTRUS Statistical Report. Childhood CNS tumors have become the most common cause of cancer death in children, and the leading cause of childhood cancer mortality [2]. Conversely, while many studies have focused on the epidemiological trends and clinicopathological characteristics of adult cancers, few have focused on the status of childhood cancers in China. Although one study did report differences in the incidence rate of CNS between Chinese and American children, the results did not represent the overall situation in China, because the relevant cohort merely addressed Hong Kong [3].
The common locations and types of childhood CNS tumor differ from those in adults. The epidemiology of childhood CNS tumors was found to be affected by the location and pathological type of the lesion [4]. Moreover, the low specificity of CNS tumors in children can easily lead to misdiagnosis or missed diagnosis. Our previous research systematically analyzed the epidemiological changes of CNS tumors in all age groups in China [5]. We found a lack of relevant research on the epidemiological and clinicopathological patterns of childhood CNS tumors within the country. A comprehensive analysis of such tumors is urgently required, to provide medical evidence for future prevention and multimodal treatment approaches.
We have, therefore, conducted this study to describe the epidemiological trend from registry data, and for all cancer cases by sex, age, and area. Due to lack of data regarding the clinicopathological characteristics of childhood CNS tumors, we have performed a system review to analyze the locations and types of the tumors.
Methods
Data source and subjects
Thus far, the Chinese Cancer Registry Annual Report has released cancer data for the period 2005 to 2017. In our study, the incidence and mortality data for childhood CNS tumors (0–19 age group) were collected from the same Report, which by 2017 had addressed approximately 31.39% of the Chinese population. The changes in the number of registries, and the coverage of the population from 2005 to 2017, are detailed in Table S1, and the distribution of cancer registries in China is shown in Figure S1. With the continuous improvement of economic conditions and tumor-monitoring capacity in China, the number of monitoring units is increasing, the quality of monitoring data is improving, and the coverage of the population is also rising. At present, the Chinese Cancer Registry Annual Report comprehensively reflects the development of cancer incidence and death within the country. The resource includes all individuals diagnosed with, or dying from, childhood CNS tumors (ICD-10 codes C70 ~ C72, D32 ~ D33 and D42 ~ D43) between 1 January and 31 December each year.
We extracted the population sizes and incidence/mortality cases for four age groups (0 years, 1–4 years, 5–9 years, 10–14 years, and 15–19 years). Because the incidence/mortality rate in the 0-year age group was too low for our purposes, we combined the related data in the 0-year age group with those for the 1–4-year age group. Incidence rates, mortality, and the annual percent change (APC) in each age group were calculated to capture the trend from 2005 to 2017.
This study had been approved by the Ethics Committee of Liaoning Cancer Hospital. Because the data for our study were derived from the Chinese Cancer Registry Annual Report (2005 to 2017), and the related published article, our study does not involve specific research objects, sensitive human information and data, or commercial interests, and an application for ethical approval was thus waived.
Search strategy
The Cancer Registry Annual Report did not provide the required information on childhood CNS-tumor positions and subtypes. Consequently, we performed a system review to analyze the locations and types of these tumors. Multiple literature databases (PubMed, Web of Science, China Science and Technology Journal Database (CQVIP), China National Knowledge Infrastructure (CNKI), and Wanfang Data) were searched via an established search strategy. This latter included (1) the key words “central nervous system tumors,” “brain tumor(s),” “brain neoplasm(s),” “spinal cord tumor(s),” “spinal cord tumor(s),” and “spinal cord neoplasm” for searches on PubMed and Web of Science. Meanwhile (2), the above terms were set as subject headings (including title, abstract, and keyword) for searches within the Chinese databases. The searches included all relevant studies published prior to June 30, 2023. All the retrieved references were restricted to “child” and “Chinese population.” We conducted this study, and we reported the related results, based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, as issued in 2020 (Table S2). We also registered our review on the Open Science Framework (https://doi.org/10.17605/OSF.IO/EX76Z.).
Study selection
We created an electronic library with Endnote bibliographic software (version X6; Thomson Reuters, Inc., Philadelphia, PA), and all pertinent studies were retrieved from this electronic library. After duplicate studies were identified via the software, two authors (XM W, B Z) independently screened the titles/abstracts and full texts of the remaining studies. When disagreements occurred between the authors, a further author (CY W) was consulted, and agreement was reached by discussion.
The criteria for inclusion were as follows: (1) observational studies on primary tumor; (2) studies evincing a specific research time period and area; (3) a clear diagnostic criterion; (4) all cases being examined via imaging, and evincing a clear location, pathological type and grade; (5) when two or more studies came from the same population, the most recent article, or the article with the largest sample size, was selected. The criteria for exclusion were as follows: (1) studies failing to fulfil the above inclusion criteria; (2) the publication type was that of a review, conference abstract, or case report; (3) the data were not derived from the population, being derived (e.g.) from animal experiments. The International Classification of Childhood Cancer, third edition (ICCC-3), was used as a classification system to distinguish the pathological types of childhood CNS tumor.
Data extraction and quality assessment
We extracted basic information (First author, Year of publication, Study design, Time interval of case collection, Hospital of diagnosis and treatment, Source of cancer cases, Diagnostic criteria, and Number of subjects) from the included studies. To avoid the inclusion of duplicate cases, we also used three factors (Time interval of case collection, Hospital of diagnosis and treatment, Source of cancer cases) to identify the latter. The quality of the included studies was independently assessed by two authors (XM W, B Z) via a cross-sectional/prevalence study-quality assessment [6]. The detailed contents of the latter are shown in Tables S3 and S4.
Statistical analyses
The incidence and mortality of childhood CNS tumors were classified by sex, age group (0–4, 5–9, 10–14 and 15–19), and area (urban or rural). Joinpoint software (Version 4.7.0.0) was used to assess the magnitude, and direction of trends over time, for incidence and mortality. The APC was calculated by means of generalized linear models, assuming a Poisson distribution. The Monte Carlo permutation method was deployed to assess the tests of significance, and the overall asymptotic significance level was maintained via a Bonferroni correction. The overall summary estimate for each clinicopathological characteristic was calculated via a random-effects model (DerSimonian-Laird) in the meta-analysis. Heterogeneity was assessed via I-square statistics (I2) and Cochran Q-statistics. The meta-analysis was performed using R software (Version 4.1.1). P < 0.05 was defined as statistically significant.
Results
Trends in the incidence and mortality rates of childhood CNS tumors
In China, from 2005 to 2017, a total of 8216 childhood CNS tumors were diagnosed among children aged 0 to 19. There was no significant increase in the incidence rate (APC: -0.1, 95% CI: -1.5 to 1.3). In terms of the area analysis, the incidence rate in rural areas increased significantly (APC: 6.2, 95% CI: 2.4 to 10.2), while no significant change was found in urban areas (APC: -1.6, 95% CI: -3.2 to 0.2). There was a significant decrease in the 15–19-year age group (APC: -2.2, 95% CI: -4.4 to -0.8). The incidence rate in males was higher than that in females, and the incidence in urban areas was also higher than that in rural areas (Fig. 1A, C, E and Table S5).
The trends of incidence and mortality rates of Childhood CNS tumors in China from 2005 to 2017. A The trend of the incidence rate of Childhood CNS tumors by sex in China from 2005 to 2017. B The trend of the mortality rate of Childhood CNS tumors by sex in China from 2005 to 2017. C The trend of the incidence rate of Childhood CNS tumors by area in China from 2005 to 2017. D The trend of the mortality rate of Childhood CNS tumors by area in China from 2005 to 2017. E The trend of the incidence rate of Childhood CNS tumors by age group in China from 2005 to 2017. F The trend of the mortality rate of Childhood CNS tumors by age group in China from 2005 to 2017
The number of childhood CNS tumor deaths was 4577. There was a significant increase in the mortality rate (APC:1.8, 95% CI: 0.3 to 3.4). There were also significant increases in CNS mortality from 2005 to 2017, in both the 5–9-year age group and the 10–14-year age group (APC in 5–9-year age group: 3.9, 95% CI: 2.2 to 5.7; APC in 10–14-year age group: 3.3, 95% CI: 0.5 to 6.1). As with the incidence rate, the mortality rate decreased with age. In terms of sex, the mortality rate in males increased significantly (APC: 1.9, 95% CI: 0.3 to 3.5), while no significant change was found in females (APC: 1.6, 95% CI: 0.1 to 3.3), and the mortality rate in males was also higher than that in females. As regards the area analysis, the mortality rate increased significantly in both urban and rural areas (APC in urban:1.6, 95% CI: 0.2 to 3.1; APC in rural: 4.4, 95% CI: 0.4 to 8.4). The mortality rate in urban areas was higher than that in rural areas. The extent of the increase in rural areas was, however, higher than that in urban areas, and the two gradually converged from 2005 to 2017 (Figs. 1B, D and F, and Table S5).
The clinicopathological characteristics of childhood CNS tumors
In terms of literature collection, 1654 potential studies were searched, across multiple databases. After a review of the titles and abstracts, 1594 studies were excluded. Of the remaining 60 studies, we found that 27 studies had duplicate cases, while studies with larger sample sizes and higher quality were included. Following a careful review of the full texts, 27 studies were excluded. In total, 16 studies satisfied the inclusion criteria for our meta-analysis [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. The study-selection process, and the results from the literature search, are shown in Fig. 2. Table S6 shows the characteristics of the included studies within the meta-analysis. All the included studies evinced a cross-sectional design.
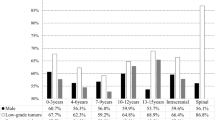
A total of 12,932 children with CNS tumors were addressed by the 16 studies. Among the total population of included children, 56.4% were males (95% CI: 51.7% to 61.0%), and the male-to-female ratio was approximately 1.28:1. Regarding the age-group analysis, the proportion of low-grade tumors increased by age, but the ratio of high-grade and low-grade tumors decreased by age (Table 1). In terms of the analysis of childhood CNS-tumor location, intracranial tumors accounted for 92.6% (95% CI: 91.3% to 93.9%), while spinal-cord tumors accounted for only 7.4% (95% CI: 6.2% to 8.7%), with a ratio of 13.2:1. The ratio of supratentorial tumors to infratentorial tumors was 1.71:1. Among supratentorial tumors, the cerebral hemisphere (25.5%, 95% CI: 21.7% to 29.4%) and sellar region (18.4%, 95% CI: 14.4% to 24.4%) were the main tumor locations, accounting for 67.7% of supratentorial tumors. Among infratentorial tumors, meanwhile, the cerebellum (20.8%, 95% CI: 16.7% to 24.8%) and the vermis of cerebellum (12.4%, 95% CI: 6.8% to 18.1%) were the main tumor locations, accounting for 75.0% of infratentorial tumors (Fig. 3 and Table S7). In the pathological types of childhood CNS tumor, the top five pathological types were astrocytomas (26.8%, 95% CI: 23.9% to 29.6%), tumors of the sellar region (craniopharyngiomas) (14.0%, 95% CI: 12.0% to 16.0%), medulloblastomas (12.8%, 95% CI: 11.4% to 14.2%), intracranial and intraspinal germ-cell tumors (7.0%, 95% CI: 5.7% to 8.3%) and pituitary adenomas and carcinomas (6.7%, 95% CI: 2.8% to 10.6%) (Fig. 4 and Table S8).
Discussion
The incidence and mortality trends for childhood CNS tumors in China have rarely been reported. In our study, conversely, we systematically analyzed the relevant data on childhood CNS tumors. There was a significant increase in the mortality rate, but no significant changes in the incidence rate from 2005 to 2017. Similar incidence and mortality trends were observed between males and females. Nonetheless, in China, there were significant increases in both the incidence and mortality rate in rural areas, while the incidence and mortality rates in rural areas were close to those in urban areas by 2017. This change may be caused by the shrinking gap between urban and rural socioeconomic conditions and health-care levels.
With the discovery of the increased mortality rate, we noticed a difference in the proportion of lesion sites and pathological types for childhood CNS tumors. In terms of the clinicopathological characteristics of childhood CNS tumors in China, due to a lack of relevant data for the country, we collected pertinent articles and used the meta-analysis to pool the associated data. Recent studies have found that most childhood CNS tumors are, in fact, low-grade tumors [23]. In China, the proportion of low-grade tumors increased by age, and it was higher than the proportion of high-grade tumors. Conversely, the ratio of high-grade and low-grade childhood CNS tumors decreased by age, and these figures were different from those in the USA [2].
In our study, astrocytoma was the most common type of childhood CNS tumor, followed by craniopharyngioma and medulloblastoma. Reportedly, high birth weight has been one of the risk factors for astrocytoma and embryonic tumors [24]. In the more economically developed areas of China, the incidence of high birth weight has exceeded 10% [25], and this can partly explain the high incidence of CNS among children in urban areas. Gliomas also have a high incidence rate in adults, but meningiomas and pituitary tumors were the most common non-malignant tumors in adults [26]. The incidence of childhood astrocytoma was partially attributed to cancer predisposition syndromes [27]. Pilocytic astrocytoma comprised the most common form of CNS tumor in patients with neurofibromatosis type 1 (NF1), accounting for approximately 49% of cases [28]. It was noteworthy, nevertheless, that NF1-associated pilocytic astrocytoma evinced a better prognosis in children than sporadic pilocytic astrocytoma [29]. Conversely, the clinical monitoring and treatment strategies for NF1-derived CNS tumors are still in the process of optimization [30]. Timely management of NF1 patients, meanwhile, prevented the occurrence of childhood CNS tumors. In addition, parents with higher educational levels were associated with a higher risk of CNS tumors among their children [31]. Large-scale childhood CNS tumor data from China will be needed to explore the issue in the future.
Regarding tumor location, as with the results of childhood CNS tumors in previous studies [32,33,34], our study found that supratentorial tumors were more common than infratentorial tumors. Meanwhile, the cerebral hemisphere and sellar region were common locations of supratentorial tumors, and the cerebellum and vermis of cerebellum were common locations for infratentorial tumors. Nonetheless, some common subtypes of childhood CNS tumor in China are inconsistent with those in other countries and regions. Generally, it has been considered that the cerebral hemisphere is the most common site of intracranial tumors in adults (this being relatively rare in children), but in recent years, some studies have reported that the proportion of supratentorial tumors is roughly the same as the proportion of infratentorial tumors, even though the proportion of supratentorial tumors is slightly higher [4, 35]. Our results also showed that the cerebral hemisphere was the most common site of intracranial tumors in children, indicating a gradual diversification trend in intracranial tumors among the latter. Due to differences in pathological type and location, childhood CNS tumors in China evinced their own characteristics. In future, we should consider the key locations and types of childhood CNS tumor when we address medical investment for diagnosis and treatment.
The symptoms of CNS tumors in children lack specificity, and they are difficult to identify and diagnose in a timely manner [36]. Supratentorial tumors, which are mainly composed of cerebral-hemisphere tumors and sellar-region tumors, can damage the normal functions of the cerebral-hemisphere, thalamus and sellar region. The main early symptoms of supratentorial tumors are limb weakness, visual-field defects, epileptic seizures, slow reactions, etc. [37]. Most of the infratentorial tumors were found to be cerebellar tumors, and this may be related to the high incidence of neuroepithelial tumors. The main symptoms of infratentorial tumors are vomiting and dizziness [37]. The early symptoms are not serious, and because of the poor expressive abilities of very young children, infratentorial tumors are often ignored or misdiagnosed. This delays treatment.
In the last 20 years, in the USA, there has been a significant decline in the mortality rate and no change in incidence rates, while survival rates have increased by 16% [38]. In fact, the disease burden of childhood CNS tumors has decreased significantly in the USA. This reflects the former, substantial gap in the diagnosis and treatment of childhood CNS tumors between China and more developed countries. Due to the unique characteristics of age, symptoms, and prognosis, childhood CNS tumor patients require specialized professional pediatric care during the treatment and care process [39, 40]. Nonetheless, in low- and middle-income countries, the numerical proportion of childhood CNS tumor patients to professional pediatric neurosurgeons has not yet reached optimal levels. Thus, there has been a lack of experienced health-care professionals [41].
For these reasons, sufficient and professional pediatric neuro-oncology centers, as well as multidisciplinary services staffed by trained surgeons in line with recognized treatment protocols, could not be provided in China or other, less-developed states [42, 43]. The lack of suitable diagnostic conditions often caused delayed diagnoses for children [44], thus affecting the incidence rate [39]. In addition, many childhood CNS tumors required advanced treatment methods. On the one hand, due to high operating costs, commercial medical companies were not willing to conduct advanced clinical trials in low- and middle-income countries [45]. Indeed, with advances in molecular-diagnostic and targeted-therapy technologies, the gap in professional care provision for children became increasingly wide. On the other hand, in order to access advanced treatment technology, patients and their families were often transferred to economically developed, but geographically distant, countries for medical treatment. While such families would benefit from advanced technology, they also faced high treatment costs, a problem exacerbated by insurance policies that did not provide appropriate funding. These issues undoubtedly led to the abandonment of treatment, thus increasing the mortality rate [46]. Therefore, while one may raise awareness of cancer prevention and treatment among Chinese families, the capacities of domestic professional pediatric care, and the question of medical costs, cannot be ignored.
Some limitations of our study should be noted. First, the data collection in cancer registries in China was carried out in accordance with unified steps and procedures. The establishment of cancer registration points has been restricted by regional medical and economic disparities, so coverage of the entire Chinese population could not be guaranteed. The fact that only around 1/3 of Chinese population data was covered in this way caused a certain degree of selection bias in our results. As the most authoritative cancer-registration information source in China, however, the Cancer Registry Annual Report provides the largest available reservoir of population information, so our research results are still representative.
Second, in the Chinese Cancer Registry Annual Report, we could not extract information on treatment type, time to diagnosis and treatment, etc. Thus, we only analyzed the effects of age, gender, and rural/urban disparity. This was a limiting factor for our results. Third, due to the lack of childhood CNS tumor-type information in Chinese cancer reports, we performed a system review to analyze the data of relevant articles, in order to reflect childhood CNS-tumor positions and subtypes. The results from our meta-analysis merely provided an estimate. Still, this reflected the general situation regarding childhood CNS tumor typologies, and the meta-analysis method was a suitable solution for our study. Therefore, after a strict inclusion and exclusion process, we used the cross-sectional/prevalence study quality assessment to assess the quality of the included studies. We found that the scores of all the included studies were above 9, signifying high quality.
Fourth, in the inclusion criteria for our systematic review, we restricted the location and pathological type of lesions to those confirmed by imaging. This may have caused some cases, which had not undergone biopsy or resection, to be overlooked. Fifth, we failed to distinguish between benign and malignant tumors in our systematic review. Future studies, with large sample sizes from multiple centers in China, are thus needed to provide further support.
Conclusion
There was a significant increase in the incidence and mortality rate of childhood CNS tumors in rural areas, which may be the main reason for the increased burden of childhood CNS tumors. The clinicopathology of childhood CNS tumors in China evinced its own, particular characteristics. The latter must be addressed, in order to improve the treatment of childhood CNS tumors in terms of key positions and types. It is thus essential to formulate preventive and control measures suitable for childhood CNS tumors in China.
Availability of data and materials
All our research datasets were extracted from the China Cancer Registry Annual Report from 2005 to 2017, which were all publication of books. The annual report we reference can be get in the Global Health Data Exchange (GHDx) online repository, https://ghdx.healthdata.org/series/china-national-central-cancer-registry. Readers can access the data used in our study from the Supplementary materials.
Abbreviations
- CNS:
-
Central nervous system
- CQVIP:
-
China Science and Technology Journal Database
- CNKI:
-
China National Knowledge Infrastructure
- APC:
-
Annual percent change
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- BCG:
-
Bacille Calmette-Guérin
- ICCC-3:
-
International Classification of Childhood Cancer, Third edition
- NF1:
-
Neurofibromatosis type 1
References
Stavinoha PL, Trinh-Wong T, Rodriguez LN, Stewart CM, Frost K. Educational pain points for pediatric brain tumor survivors: review of risks and remedies. Children (Basel). 2021;8:1125.
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol. 2022;24(Suppl 5):v1-95.
Liu APY, Liu Q, Shing MMK, Ku DTL, Fu E, Luk C-W, et al. Incidence and outcomes of CNS tumors in Chinese children: comparative analysis with the surveillance, epidemiology, and end results program. JCO Glob Oncol. 2020;6:JGO.19.00378.
Katchy KC, Alexander S, Al-Nashmi NM, Al-Ramadan A. Epidemiology of primary brain tumors in childhood and adolescence in Kuwait. Springerplus. 2013;2:58.
Zhu B, Wu X, Piao H, Xu S, Yao B. A comparison of epidemiological characteristics of central nervous system tumours in China and globally from 1990 to 2019. Neuroepidemiology. 2021;55:460–72.
Chang SM. The Agency for Healthcare Research and Quality (AHRQ) effective health care (EHC) program methods guide for comparative effectiveness reviews: keeping up-to-date in a rapidly evolving field. J Clin Epidemiol. 2011;64:1166–7.
Chen H, Wu J, Chen J, Zhou L, Zhang F. Central nervous system tumors in children-analysis of 766 cases. Chin Clin Neurosci. 2001;9:262–5.
Chen L, Ming Y, Liu L, Li D, Xia X, Gu Y, et al. Epidemiologic survey of intracranial tumors in childhood in southern area of Sichuan. J Luzhou Med Coll. 2007;30:476–9.
Gui T, Zhang Q, Zhang T, Zhou Z, Liu W, Li H, et al. Central nervous system tumors in childhood: a analysis of 330 cases. J Clin Exp Pathol. 2008;24:403–6.
Shen W. Clinical analysis of primary pediatric central nervous system tumors. Master. Fudan University; 2008.
Zhou D, Zhang Y, Liu H, Luo S, Luo L, Dai K. Epidemiology of nervous system tumors in children: a survey of 1,485 cases in Beijing Tiantan hospital from 2001 to 2005. Pediatr Neurosurg. 2008;44:97–103.
Cui S, Qin J, Liu M, Jin S, Han T, Yan S, et al. A statistisc analysis of 5540 cases of central nervous system tumors with WHO classification. Chin J Contemp Neurol Neurosurg. 2009;9:65–9.
Tao ZHU, Yun-hu YU, Da-jian Z, Jian-ning Z. Clinical analysis of central nervous system tumors in children, a report of 468 cases. Chin J Neurosurg. 2012;28:8–12.
Liu W. A retrospective and prognostic analysis of pediatrics central nervous system tumors: evaluation of EGFR family gene amplification and overexpression in embryonal tumors and glioblastomas. Doctor. Shandong University; 2014
Cui M, Dong X. Clinical analysis of central nervous system tumors in children: a report of 163 cases. J Harbin Med Univ. 2015;49:533–6.
Tang X, Kong Y, Guo Q. Intracranial tumor in children:a clinicopathological analysis of 221 cases. J Clin Exp Pathol. 2015;31:298–301.
Wang J, Zhao Y, Yang Q, Sai K, Ke C, et al. Clinical analysis of pediatric central nervous system tumors: a retrospective study of 219 cases. Guangdong Med J. 2015;36:2621–4.
Ye Y, He X, Yang Y, Su X, Kong C, Bai W, et al. Clinical characteristics and epidemiology of intracranial tumor in childhood: a single center survey in northwest of China. Chin J Neurosurg Dis Res. 2018;17:198–202.
Li J, Chu H, Miao N, Jiao J, Zhang W. Pediatric tumors of nervous system tumors: a clinicopathologic study of 398 cases. J Diag Pathol. 2019;26:333–7.
Xu X, Li J, Lin J, Chen C, Yang R, Li F. Clinicopathological characteristics of central nervous system tumors in 483 children. Acad J Guangzhou Med College. 2022;50:51–4.
An X, Tian Y, Zheng J, Liu Z, Liu G, Wang J, et al. A disease type review of pediatric central nervous system tumors diagnosed and treated in 44 hospitals in Beijing. Chin J Neurosurg. 2022;38:934–40.
Wu X, Dangmurenjiafu G, Fan G, Zeng J, Zhao X, Sheng C, et al. Epidemiology of pediatric central nervous system tumors in Uyghur: experience from a single center. Childs Nerv Syst. 2023;39:909–14.
Schultz KAP, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25:3649–56.
Georgakis MK, Kalogirou EI, Liaskas A, Karalexi MA, Papathoma P, Ladopoulou K, et al. Anthropometrics at birth and risk of a primary central nervous system tumour: a systematic review and meta-analysis. Eur J Cancer. 2017;75:117–31.
Guo H, Gu B, Mao X, Jin Y, Wang X, et al. Analysis on risk factors of high birth weight in Changzhou. Matern Child Health Care China. 2011;26:1056–9.
Ostrom QT, Francis SS, Barnholtz-Sloan JS. Epidemiology of brain and other CNS tumors. Curr Neurol Neurosci Rep. 2021;21:68.
Adel Fahmideh M, Scheurer ME. Pediatric brain tumors: descriptive epidemiology, risk factors, and future directions. Cancer Epidemiol Biomark Prev. 2021;30:813–21.
Rodriguez FJ, Perry A, Gutmann DH, O’Neill BP, Leonard J, Bryant S, et al. Gliomas in Neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol. 2008;67:240–9.
Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–20.
Nix JS, Blakeley J, Rodriguez FJ. An update on the central nervous system manifestations of neurofibromatosis type 1. Acta Neuropathol. 2020;139:625–41.
Erdmann F, Hvidtfeldt UA, Sørensen M, Raaschou-Nielsen O. Socioeconomic differences in the risk of childhood central nervous system tumors in Denmark: a nationwide register-based case-control study. Cancer Causes Control. 2020;31:915–29.
Kaatsch P, Rickert CH, Kühl J, Schüz J, Michaelis J. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92:3155–64.
Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–11.
Cho K-T, Wang K-C, Kim S-K, Shin S-H, Chi JG, Cho B-K. Pediatric brain tumors: statistics of SNUH, Korea (1959–2000). Childs Nerv Syst. 2002;18:30–7.
Rosemberg S, Fujiwara D. Epidemiology of pediatric tumors of the nervous system according to the WHO 2000 classification: a report of 1,195 cases from a single institution. Childs Nerv Syst. 2005;21:940–4.
Patel V, McNinch NL, Rush S. Diagnostic delay and morbidity of central nervous system tumors in children and young adults: a pediatric hospital experience. J Neurooncol. 2019;143:297–304.
Wilne S, Collier J, Kennedy C, Koller K, Grundy R, Walker D. Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol. 2007;8:685–95.
Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103.
Fischer C, Petriccione M, Donzelli M, Pottenger E. Improving Care in Pediatric Neuro-oncology Patients: an overview of the unique needs of children with brain tumors. J Child Neurol. 2016;31:488–505.
Keating R, Curry S, Hussey J. Cardiorespiratory fitness and health-related quality of life in survivors of childhood central nervous system tumours. Support Care Cancer. 2023;31:395.
Choi J-U. The promotion of pediatric neurosurgery throughout the world. Childs Nerv Syst. 2007;23:929–36.
Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi. 2019;40:52–7.
Pak-Yin Liu A, Moreira DC, Sun C, Krull L, Gao Y, Yang B, et al. Challenges and opportunities for managing pediatric central nervous system tumors in China. Pediatr Investig. 2020;4:211–7.
Elhassan MMA, Mohamedani AA, Osman HHM, Yousif NO, Elhaj NM, Qaddoumi I. Patterns, treatments, and outcomes of pediatric central nervous system tumors in Sudan: a single institution experience. Childs Nerv Syst. 2019;35:437–44.
Bailey S, Davidson A, Parkes J, Tabori U, Figaji A, Epari S, et al. How can genomic innovations in pediatric brain tumors transform outcomes in low- and middle-income countries? JCO Glob Oncol. 2022;8:e2200156.
Roach JT, Baticulon RE, Campos DA, Andrews JM, Qaddoumi I, Boop FA, et al. The role of neurosurgery in advancing pediatric CNS tumor care worldwide. Brain Spine. 2023;3:101748.
Acknowledgements
The present study was carried out with the support of the Shenyang Science and Technology Talent Program (RC210496).
Funding
This study was supported by the Shenyang Science and Technology Talent Program (RC210496).
Author information
Authors and Affiliations
Contributions
XMW and B Z designed the whole research, HY W, CY W and B Y conducted the data collection, HY W, B Z and XMW analyzed the data. HY W, B Y, B Z and XMW wrote the manuscript. T T and B Y discussed the relevant results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Because the data of our study were from the Chinese Cancer Registry Annual Report from 2005 to 2017 and the related published article. Our study does not involve specific research objects, human-sensitive information, data, or commercial interests. Therefore, no ethical approval is required.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
The information of cancer registries in China between 2005 and 2017. Table S2. The PRISMA-2020 Checklist. Table S3. The cross-sectional/prevalence Study quality assessment. Table S4. The cross-sectional/prevalence Study quality assessment on our included studies. Table S5. The results by join point regression on the incidence and mortality rate of Childhood CNS tumors by age, sex, area, and period between 2005 and 2017. Table S6. The characteristics of the included studies in the meta-analysis. Table S7. The proportion of CNS tumor location by meta-analysis. Table S8. The proportion of CNS tumor type by meta-analysis. Figure S1. Distribution of cancer registries in China.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yao, B., Wang, H., Wu, X. et al. A system review of central nervous system tumors on children in China: epidemiology and clinical characteristics. BMC Cancer 24, 138 (2024). https://doi.org/10.1186/s12885-024-11883-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-11883-w