Abstract
Background
Routine clinical staging for hepatocellular carcinoma (HCC) incorporates liver function, general health, and tumor morphology. Further refinement of prognostic assessments and treatment decisions may benefit from the inclusion of tumor biological marker alpha-fetoprotein (AFP) and systemic inflammation indicator C-reactive protein (CRP).
Methods
Data from a multicenter cohort of 2770 HCC patients undergoing hepatectomy were analyzed. We developed the PACE risk score (Prognostic implications of AFP and CRP Elevation) after initially assessing preoperative AFP and CRP’s prognostic value. Subgroup analyzes were performed in BCLC cohorts A and B using multivariable Cox analysis to evaluate the prognostic stratification ability of the PACE risk score and its complementary utility for BCLC staging.
Results
Preoperative AFP ≥ 400ng/mL and CRP ≥ 10 mg/L emerged as independent predictors of poorer prognosis in HCC patients who underwent hepatectomy, leading to the creation of the PACE risk score. PACE risk score stratified patients into low, intermediate, and high-risk groups with cumulative 5-year overall (OS) and recurrence-free survival (RFS) rates of 59.6%/44.9%, 43.9%/38.4%, and 20.6%/18.0% respectively (all P < 0.001). Increased PACE risk scores correlated significantly with early recurrence and extrahepatic metastases frequency (all P < 0.001). The multivariable analysis identified intermediate and high-risk PACE scores as independently correlating with poor postoperative OS and RFS. Furthermore, the PACE risk score proficiently stratified the prognosis of BCLC stages A and B patients, with multivariable analyses demonstrating it as an independent prognostic determinant for both stages.
Conclusion
The PACE risk score serves as an effective tool for postoperative risk stratification, potentially supplementing the BCLC staging system.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) continues to be one of the most pervasive malignancies worldwide, with both disease burden and mortality rates escalating [1, 2]. Liver resection emerges as a pivotal, potentially curative approach for HCC treatment, proving particularly effective for those diagnosed at very early or early stages [3, 4]. Nevertheless, due to the complex biological characteristics of the tumor and individual patient differences, the prognosis can vary substantially even among those who have undergone liver resection [5,6,7].
The prognosis of HCC depends significantly on the staging of the disease and suitable treatment options [8, 9]. Clinical staging systems exemplified by the Barcelona Clinic Liver Cancer (BCLC) algorithm are utilized for prognosis evaluation and therapeutic recommendations [9]. Despite their pivotal role in HCC treatment decisions, these staging systems have limitations. They often solely account for tumor morphological characteristics and patient baseline conditions, thereby offering limited performance in refining patient prognosis and guiding treatment decisions. It’s not unusual to see patients within the same stage, who have received the same guideline-recommended treatment, exhibit markedly different clinical outcomes [7, 10].
In recent times, an increasing body of evidence has shown that the prognosis of HCC is associated not only with the tumor’s morphological features but also with its biological behavior and the host’s systemic inflammatory response [11,12,13,14,15]. Hematological parameters, as effective indicators of the host’s systemic inflammatory response and tumor biology, have gained wide acceptance. Among these, C-reactive protein (CRP) is a marker of systemic inflammation, and it has been proven to have a strong correlation with the prognosis of numerous tumors [16,17,18]. Similarly, alpha-fetoprotein (AFP), an HCC-specific biomarker, is considered to reflect the tumor’s biological behavior and clinical prognosis [19, 20]. In consideration of these factors, it is plausible that including hematological parameters like CRP and AFP, indicators of systemic inflammation and HCC biology respectively, in clinical staging evaluations may offer substantial aid in enhancing patient prognostic assessment and treatment allocation.
To this end, we conducted a large-scale retrospective study involving a multicenter cohort of 2770 patients, with the aim of exploring the value of CRP and AFP in the prognostic evaluation of HCC after resection, and constructed a PACE risk score (Prognostic implications of AFP and CRP Elevation), with the goal of providing clinicians with additional information on tumor biological aggressiveness and systemic inflammatory response. Our results show that the PACE score, when used in combination with the tumor morphology-based BCLC staging system, has the potential to provide clinicians with more accurate and personalized prognostic assessment and treatment recommendations before surgery, thereby enhancing assessment of suitability for liver resection, postoperative management, and improving the outcome of liver resection.
Methods
Study population
A retrospective review was conducted on consecutive patients with HCC who underwent hepatectomy as a curative-intent therapy, between April 2009 and April 2019 at six hospitals in China: Mengchao Hepatobiliary Hospital of Fujian Medical University, Eastern Hepatobiliary Surgery Hospital of Naval Medical University, Zhongshan Hospital affiliated to Xiamen University, the First Affiliated Hospital of Shandong First Medical University, the First affiliated Hospital of Ningbo University, and the Third Hospital of Zhangzhou. HCC diagnoses were confirmed histopathologically from resected specimens. The study’s inclusion criteria were: (1) patients who underwent R0 resection, which is characterized by complete tumor removal with pathologically confirmed absence of tumor cells at the surgical margin; (2) absence of macrovascular invasion and extrahepatic metastasis; (3) availability of preoperative serum AFP and CRP data (collected within one week prior to hepatectomy). Patients were excluded based on the following criteria: (1) recurrent or mixed HCC; (2) clinical evidence of preoperative infection; (3) prior anticancer treatments; (4) palliative resection (R1/R2); (5) emergency hepatectomy due to rupture HCC; (6) insufficient clinical information or missing follow-up data. This retrospective study was approved by the institutional review boards of each medical center and conducted according to the ethical guidelines of the 1975 Declaration of Helsinki.
Clinicopathologic and operational variables
Patients’ clinicopathologic variables included age, gender, hypertension, diabetes, liver cirrhosis, etiology of liver disease, liver function status, platelet count, total bilirubin, albumin, AFP, CRP, the number of tumors, tumor diameter, satellite nodules, microvascular invasion, degree of tumor cell differentiation, tumor capsule, and BCLC staging. All laboratory indices were based on the most recent tests within one week before hepatectomy. BCLC stage 0 was defined as a single lesion not larger than 2 cm, stage A as a single lesion larger than 2 cm, or multiple lesions numbering 2–3 with the largest tumor not larger than 3 cm, and stage B as more than three tumors or 2–3 tumors with any of them larger than 3 cm in diameter [8]. Operational variables included the extent of hepatectomy, type of hepatectomy, intraoperative blood transfusion, volume of intraoperative blood loss, and resection margin status. The extent of hepatectomy was divided into major (removal of three or more Couinaud segments) and minor (removal of fewer than three Couinaud segments). Types of hepatectomy were divided into anatomical (as per the Brisbane 2000 nomenclature of liver anatomy and resections) and non-anatomical, which includes limited hepatectomy or wedge resection [21].
Follow-up and study endpoints
Following discharge, patients were monitored for tumor recurrence at outpatient clinics according to the relatively uniform scheme. For the initial two years post-surgery, visits were every 2–3 months, with 3–6 month intervals thereafter if recurrence was absent. Monitoring involved routine blood tests, liver function tests, tumor markers, and radiological assessments encompassing lung imaging, abdominal ultrasound, or enhanced computed tomography/magnetic resonance imaging. Therapeutic strategies for recurrent cases were selected based on their general health status, liver functional reserve, and tumor burden at recurrence, and include repeat hepatectomy, radiofrequency ablation, trans-arterial regional therapy, systemic therapy, and supportive care.
Data for this study was reviewed on October 13, 2022. The primary endpoints were overall survival (OS) and recurrence-free survival (RFS). OS was defined as the period from the date of hepatectomy to the date of patient death or last follow-up. RFS was defined as the interval from the date of hepatectomy to tumor recurrence, death, or the last follow-up, whichever occurred first.
Statistics
Continuous variables are presented as mean ± standard deviation and were compared using the Mann-Whitney U-test or Kruskal–Wallis one-way ANOVA as appropriate. Categorical variables are represented as frequency (percentage) and compared using χ2 or Fisher’s exact tests as needed. To enhance clinical applicability, this study used the widely reported cut-off values of 400 ng/mL for AFP and 10 mg/L for CRP [16, 17, 22,23,24,25,26]. Cox proportional hazards regression analysis was used to ascertain the relationship between AFP and AFP with post-hepatectomy outcomes. Variables with a P < 0.05 in the univariable analysis were included in the multivariable analysis. Given the similar hazard ratios of AFP and CRP in the multivariable analysis for OS and RFS, we developed a simple PACE risk score (Prognostic implications of AFP and CRP Elevation). To construct the PACE score, we stratified patients into three distinct risk categories based on their preoperative AFP and CRP levels: low-risk (0 points), intermediate-risk (1 point), and high-risk (2 points). The points for each category were determined by the presence of AFP ≥ 400 ng/mL and/or CRP ≥ 10 mg/L, with the higher scores reflecting a greater risk of adverse postoperative outcomes. Specifically, 0 points were given for AFP ≤ 400 ng/mL and CRP ≤ 10 mg/L, 1 point for either AFP ≥ 400 ng/mL or CRP ≥ 10 mg/L, and 2 points for AFP ≥ 400 ng/mL and CRP ≥ 10 mg/L. The range of the PACE score is 0 to 2, with scores assigned from 0 (indicating low risk) to 2 (indicating high risk). The performance of PACE and PACE combined with BCLC staging system was measured using the concordance index (C-index) and calibration curve used the bootstrap with 1000 resamples. In addition, time-dependent receiver operating characteristic curves were plotted. To evaluate the independent prognostic significance of the PACE score from BCLC staging, we examined its influence on the outcomes after hepatectomy within the BCLC A and B stage cohorts using multivariable Cox regression analysis (analysis was not performed for BCLC 0 stage patients due to sample size limitations). We used the Schoenfeld residual test to verify the assumption of proportional hazards in the Cox analysis.
All statistical analyses for this study were conducted using SPSS version 20 and R version 4.1.1.
Results
Patients characteristics
In total, 2770 patients were enrolled in the study. Patient baseline characteristics are outlined in Table 1. The mean age of patients was 53.2 ± 11.1 years. The predominant liver disease was hepatitis B virus infection (2358, 85.1%). According to BCLC staging, 159 (5.7%), 2158 (77.9%), and 453 (16.4%) patients were identified as stages 0, A, and B, respectively.
Prognostic role of AFP and CRP, and construction of PACE risk score
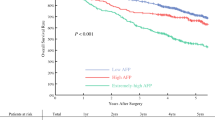
The preoperative AFP level was ≥ 400ng/mL in 894 (32.3%) patients and the preoperative CRP level was ≥ 10 mg/L in 358 (12.9%) patients. Comparisons of baseline characteristics between patients with high and low preoperative AFP or CRP levels are detailed in Table S1. Both elevated preoperative AFP and CRP levels are linked to larger tumor diameter, a higher incidence of multifocal disease, microvascular invasion, and more advanced BCLC staging—all of which are recognized indicators of tumor aggressiveness (all P < 0.05, Table S1). Survival analyses revealed a significant association between elevated levels of AFP and CRP and worse postoperative OS and RFS (all P < 0.001, Fig. 1). Multivariable analyses further identified elevated serum CRP and AFP levels as independent prognostic factors for OS and RFS (all P < 0.05, Tables S2 and S3). Based on these findings, we constructed the PACE score according to whether patients met the criteria of AFP ≥ 400ng/mL and/or CRP ≥ 10 mg/L, designating low-risk (neither criteria met), intermediate-risk (either criteria met), and high-risk (both criteria met) groups.
Comparative analysis of overall (A) and recurrence-free (B) survival between patients with preoperative CRP < 10 mg/L and CRP ≥ 10 mg/L; comparative analysis of overall (C) and recurrence-free (D) survival between patients with preoperative AFP < 400 ng/mL and AFP ≥ 400 ng/mL. Abbreviations: CRP, c-reactive protein; AFP, alpha-fetoprotein
PACE risk score relation to clinicopathological features and outcomes
Increased PACE risk level correlated with more advanced BCLC staging, larger tumor diameter, higher frequency of multiple nodules, satellite lesions, microvascular invasion, and absence or incompleteness of tumor capsule, along with poorer tumor differentiation (all P < 0.05, Table 1).
Kaplan-Meier analyses showed significant prognostic differences among PACE risk groups, as increasing PACE risk levels corresponded to a progressive decline in cumulative OS and RFS rates (all P < 0.001, Fig. 2). Additionally, when compared to the low-risk group, both intermediate and high-risk groups showed discouraging recurrence patterns (Table 2). Notably, extrahepatic recurrence occurred more often in intermediate and high-risk groups (low-risk: n = 25, 3.7% vs. intermediate-risk: n = 40, 8.3% vs. high-risk: n = 7, 6.8%; P < 0.001, Table 2). Furthermore, high-risk patients demonstrated a higher frequency of early recurrence (within 24 months) (low-risk: n = 502, 73.4% vs. intermediate-risk: n = 403, 82.2% vs. high-risk: n = 93, 90.3%, Table 2). Multivariable analyses indicated that intermediate and high-risk PACE scores were independent risks for both OS (HR: 1.413 and 2.425; 95%CI 1.226–1.628 and 1.891–3.109; all P < 0.001) and RFS (HR: 1.433 and 2.517; 95%CI 1.244–1.650 and 1.964–3.225; all P < 0.001) (Tables 3 and 4).
Moreover, we explored the discriminative and calibration capabilities of the PACE score, particularly when combined with the BCLC staging system. As depicted in Table S4, PACE alone provided C-indices of 0.604 for OS and 0.584 for DFS. It is of note that the amalgamation of PACE with BCLC staging improved the predictive accuracy of BCLC staging, with C-indices for OS and DFS at 0.638 and 0.610, respectively. This enhancement was also reflected in the time-dependent ROC curves (Fig. S1), and the calibration curves showed favourable concordance for both PACE and its combination with BCLC staging (Fig. S2).
Subgroup analysis of BCLC A/B cohort: refinement capability of the PACE risk score
Low, intermediate, and high-risk PACE groups represented 60.1%, 34.9%, and 5% of patients at BCLC stage A, and 52.1%, 39.3%, and 8.6% at BCLC stage B, respectively.
As per PACE risk status, a gradual deterioration in cumulative OS was noted across the different groups within BCLC stage A (all P < 0.001, Fig. 3A). Median OS and 5-year OS rates for the low, intermediate, and high-risk groups within BCLC stage A were 67.8 months and 61.2%, 54.6 months and 46.1%, and 24.6 months and 27.3% respectively (P < 0.001). Multivariable analysis revealed that the intermediate and high-risk PACE categories were independent risk factors for postoperative OS in BCLC stage A patients (HR: 1.448 and 2.468; 95% CI 1.228–1.706 and 1.831–3.327; all P < 0.001, Table S5). Similar patterns were discerned in terms of RFS across different risk groups within BCLC stage A, with multivariable analysis further validating that the intermediate and high-risk PACE statuses were independent risk factors for postoperative RFS in BCLC stage A patients (Fig. 3B and Table S6).
Similarly, the prognostic significance of the PACE was assessed in the cohort of BCLC stage B. The median OS and 5-year OS rates in BCLC stage B for the low-risk, intermediate-risk, and high-risk groups were 45.3 months and 40.4%, 27.3 months and 29.7%, and 15.6 months and 5.1% respectively (P < 0.001, Fig. 4A). Multivariable analysis demonstrated that the PACE risk score is an independent determinant of postoperative OS in patients with BCLC stage B (HR: 1.312 and 2.680; 95% CI 0.974–1.766 and 1.675–4.289; P = 0.074 and P < 0.001, Table S7). Correspondingly, a significant difference was observed in the RFS between different risk groups in BCLC stage B, with multivariable analysis further verifying the PACE risk score as an independent risk factor for postoperative RFS in patients with BCLC stage B (Fig. 4B and Table S8).
Discussion
Our multicentre, large-cohort study elucidates that elevated preoperative levels of CRP and AFP are significantly associated with the more aggressive biological tumor behavior and inferior prognosis following hepatectomy. Additionally, a key finding of this research is the development of the PACE risk score for the first time, combining preoperative CRP and AFP levels for patients undergoing hepatectomy for HCC. Notably, subgroup analysis in the BCLC A and B stages cohorts further illustrated the notable clinical relevance of the PACE risk score in guiding prognosis. The PACE risk score, which incorporates variables representative of the patient’s preoperative systemic inflammatory response and tumor biological behavior, may serve as a supplement to the BCLC staging system. The PACE score stands in contrast to more complex models reported in the literature, such as those based on radiomics [27, 28], genomics [29,30,31,32], and deep learning [28, 33]. While these models may exhibit superior predictive performance, their clinical adoption has been limited due to complexities in interpretation, higher costs, and extended processing times, which are less conducive to routine clinical workflows. We recognize the value of simplicity and accessibility in clinical decision-making, as endorsed by many scholars. The BCLC staging system remains in widespread use due to its user-friendly nature. The PACE score is specifically designed to complement the BCLC staging by integrating the systemic inflammatory response and tumor biology, potentially offering enhanced prognostic stratification. We emphasize its utility based on the inclusion of widely available clinical hematological markers—alpha-AFP and CRP—which are already integrated into routine clinical practice. This integration speaks to the practicability and ease of adopting the PACE score without imposing additional burdens on clinicians’ workflows.
The clinical significance of AFP in HCC has been supported by numerous studies and widely implemented in clinical practice [20, 25, 34, 35]. Compared to AFP, CRP’s prognostic value in HCC has been reported in the literature but has not been extensively studied [16,17,18, 36]. CRP is an effective biomarker and is associated with the progression of several malignancies [37,38,39,40]. Elevated CRP is associated with decreased survival rates in both resectable and non-resectable HCC patients [41,42,43]. Our multicenter, large cohort study identified the role of CRP levels as prognostic markers after hepatectomy for HCC, corroborating results from HCC research involving other curative and palliative treatments. For instance, CRP has been reported as a useful marker for assessing the efficacy of sorafenib or lenvatinib treatment [17, 44]. Rekik et al. reported that the STATE score, which incorporates CRP, can effectively predict survival after transarterial chemoembolization for intermediate HCC [43]. However, the mechanism behind the rise of CRP in HCC and its potential prognostic guidance are not fully understood. Inflammatory cytokines IL-1 and IL-6, secreted by tumor and stromal cells, could induce the rise of CRP [45]. This could further stimulate the formation of immunosuppressive immune cells. A study by Wang et al. showed a strong correlation between serum CRP levels and the infiltration of immunosuppressive myeloid cells in HCC tissue, with higher CRP levels typically associated with more infiltrating CD68 + tumor-associated macrophages and CD15 + tumor-associated neutrophils [46]. These studies may provide potential explanations for the prognostic significance of CRP in HCC, but the exact molecular mechanisms of CRP in HCC still require further elucidation.
Our study highlights the prognostic significance of the PACE risk score, derived from preoperative serum CRP and AFP levels, in HCC patients undergoing hepatectomy. Prior research has demonstrated the combined utility of AFP and CRP in HCC diagnosis and prognosis [26, 47, 48]. For instance, She et al. suggested that CRP could serve as an auxiliary marker for the HCC diagnosis, particularly AFP-negative HCC [48]. Kornberg et al. introduced a serological risk index based on AFP and CRP to predict liver transplant outcomes in advanced HCC [49]. Additionally, the CRAFITY score, based on these two markers, has been useful in determining HCC patients suitable for anti-PD-1 therapy [26, 47]. Given the above evidence, our research presents the first account of PACE risk score assisting in prognostic stratification following hepatectomy in HCC patients. A higher score suggests a more invasive tumor, poorer oncological outcomes, and more adverse recurrence patterns. This indicates the PACE score’s potential as a reliable prognostic marker for HCC patients after hepatectomy.
Our research found that the PACE risk score is a significant independent predictor for both BCLC A and B-stage cohorts. For BCLC A-stage patients, the BCLC algorithm advises radical treatment with a median OS of roughly five years [8]. In contrast, our data shows the median OS for BCLC A stage patients with low, intermediate, and high risks at 67.8, 54.6, and 24.6 months, respectively. This suggests that for BCLC A-stage patients with high PACE risk, surgical resection alone may not result in significant survival benefits, underscoring the need to consider further adjuvant or neoadjuvant therapy trials for this population. For BCLC B-stage, the standard treatment is TACE with a median OS of about 2.5 years [8]. Some researchers suggest surgical resection as an effective therapy for selected BCLC B-stage patients [50]. Our findings indicate that the PACE risk score can help identify survival subgroups post hepatectomy in BCLC B stage. Within the BCLC B stage cohort, the median OS for the low-risk, intermediate-risk, and high-risk groups are 45.3 months, 27.3 months, and 15.6 months respectively, with the latter two groups’ median OS notably less than the reported 2.5 years. As a high PACE risk score indicates tumor invasiveness, we suggest that low-risk BCLC B-stage patients may derive benefit from surgical resection, while intermediate and high-risk patients might need more integrated strategies to improve their prognosis further. However, further comparative studies are needed to confirm these findings.
Our study has several limitations. Firstly, the retrospective design of the study may be subject to selection bias and residual confounding. Secondly, the predominance of hepatitis B infections in the cohort may introduce observational bias, suggesting the need for further research conducted in other regions. Thirdly, various factors can raise CRP levels, including trauma, infection, inflammation, and tumor stimulation. We excluded patients with concomitant clinical infections and those needing emergency hepatectomy due to ruptured HCC, utilizing only the most recent CRP levels obtained within a week before surgery to minimize possible confounding. However, we accept that our data could be confounded by other factors causing CRP elevation, such as unnoticed chronic inflammatory diseases. Nevertheless, consistent with previous findings linking high CRP with poor outcomes, our data indicate a correlation between elevated CRP levels and aggressive tumor behavior, with independent prognostic relevance. Therefore, despite potential biases, the PACE risk score with CRP is useful for post-surgery prognosis in HCC patients. Last but not least, although our study underwent internal validation via bootstrap resampling, it still lacks validation from an external cohort, particularly one from a Western context with varying etiologies of liver cancer. Further research is necessary to ascertain the applicability of the PACE score in diverse populations.
In conclusion, PACE risk score serves as an effective tool for guiding postoperative risk stratification, potentially acting as a valuable supplement to the BCLC staging system. This amalgamation can offer clinicians a more precise and personalized prognostic evaluation and therapeutic suggestions, enhancing hepatectomy appropriateness assessments, postoperative management, and the effectiveness of the hepatectomy procedure.
Data Availability
All data generated or analyzed during this study are included in this article. Inquiries about further material documents can be directed to the corresponding author.
Abbreviations
- PACE:
-
prognostic implications of alpha-fetoprotein and c-reactive protein Elevation
- AFP:
-
alpha-fetoprotein
- CRP:
-
C-reactive protein
- HBV:
-
hepatitis B virus
- HCV:
-
hepatitis C virus
- PLT:
-
platelet
- MVI:
-
microvascular invasion
- BCLC:
-
Barcelona Clinic Liver Cancer
- OS:
-
overall survival
- RFS:
-
recurrence-free survival
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Huang DQ, Mathurin P, Cortez-Pinto H, Loomba R. Global epidemiology of alcohol-associated Cirrhosis and HCC: trends, projections and risk factors. Nat Rev Gastroenterol Hepatol. 2023;20(1):37–49.
Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver resection Versus local ablation therapies for Hepatocellular Carcinoma within the Milan Criteria: a systematic review and Meta-analysis. Ann Surg. 2021;273(4):656–66.
Zhu P, Liao W, Zhang WG, et al. A prospective study using propensity score matching to compare long-term survival outcomes after Robotic-assisted, laparoscopic, or Open Liver Resection for patients with BCLC stage 0-A Hepatocellular Carcinoma. Ann Surg. 2023;277(1):e103–11.
Lin K, Huang Q, Wang L, et al. Pre- and postoperative models for prediction of recurrence in Non-B, Non-C Hepatocellular Carcinoma. Front Oncol. 2021;11:612588.
Yang P, Qiu J, Li J, et al. Nomograms for pre- and postoperative prediction of long-term survival for patients who underwent Hepatectomy for multiple Hepatocellular Carcinomas. Ann Surg. 2016;263(4):778–86.
Li J, Zhou J, Yang PH, et al. Nomograms for survival prediction in patients undergoing liver resection for Hepatitis B virus related early stage hepatocellular carcinoma. Eur J Cancer. 2016;62:86–95.
Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–93.
Sun HC, Zhou J, Wang Z, et al. Chinese expert consensus on conversion therapy for hepatocellular carcinoma (2021 edition). Hepatobiliary Surg Nutr. 2022;11(2):227–52.
Wei WX, Yang ZS, Lu LH, et al. Long-term survival after partial hepatectomy for sub-stage patients with intermediate stage hepatocellular carcinoma. Int J Surg. 2018;56:256–63.
Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39(11):2008–23.
Wang Y, Peng C, Cheng Z, et al. The prognostic significance of preoperative neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma receiving hepatectomy: a systematic review and meta-analysis. Int J Surg. 2018;55:73–80.
Okamura Y, Sugiura T, Ito T, et al. Neutrophil to lymphocyte ratio as an indicator of the malignant behaviour of hepatocellular carcinoma. Br J Surg. 2016;103(7):891–8.
Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor markers for Hepatocellular Carcinoma: simple and significant predictors of Outcome in patients with HCC. Liver Cancer. 2015;4(2):126–36.
Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137(1):110–8.
Sieghart W, Pinter M, Hucke F, et al. Single determination of C-reactive protein at the time of diagnosis predicts long-term outcome of patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2224–34.
Nakanishi H, Kurosaki M, Tsuchiya K, et al. Novel pretreatment scoring incorporating C-reactive protein to predict overall survival in Advanced Hepatocellular Carcinoma with Sorafenib Treatment. Liver Cancer. 2016;5(4):257–68.
Nagaoka S, Yoshida T, Akiyoshi J, et al. Serum C-reactive protein levels predict survival in hepatocellular carcinoma. Liver Int. 2007;27(8):1091–7.
Kelley RK, Meyer T, Rimassa L, et al. Serum alpha-fetoprotein levels and clinical outcomes in the Phase III CELESTIAL Study of Cabozantinib versus Placebo in patients with Advanced Hepatocellular Carcinoma. Clin Cancer Res. 2020;26(18):4795–804.
Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related Cirrhosis who had received a liver transplant for HCC. J Hepatol. 2017;66(3):552–9.
Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2013;257(3):377–82.
Tsilimigras DI, Hyer JM, Diaz A et al. Synergistic impact of alpha-fetoprotein and Tumor Burden on Long-Term outcomes following curative-intent resection of Hepatocellular Carcinoma. Cancers (Basel). 2021. 13(4).
Tsilimigras DI, Bagante F, Moris D, et al. Recurrence patterns and outcomes after resection of Hepatocellular Carcinoma within and beyond the Barcelona Clinic Liver Cancer Criteria. Ann Surg Oncol. 2020;27(7):2321–31.
Kudo M, Izumi N, Sakamoto M, et al. Survival analysis over 28 years of 173,378 patients with Hepatocellular Carcinoma in Japan. Liver Cancer. 2016;5(3):190–7.
Montal R, Andreu-Oller C, Bassaganyas L, et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br J Cancer. 2019;121(4):340–3.
Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy - development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–63.
Ji GW, Zhu FP, Xu Q, et al. Radiomic features at contrast-enhanced CT predict recurrence in early stage Hepatocellular Carcinoma: a multi-institutional study. Radiology. 2020;294(3):568–79.
Ji GW, Zhu FP, Xu Q, et al. Machine-learning analysis of contrast-enhanced CT radiomics predicts recurrence of hepatocellular carcinoma after resection: a multi-institutional study. EBioMedicine. 2019;50:156–65.
Woo HG, Park ES, Cheon JH, et al. Gene expression-based recurrence prediction of hepatitis B virus-related human hepatocellular carcinoma. Clin Cancer Res. 2008;14(7):2056–64.
Wu C, Lin J, Weng Y, et al. Myeloid signature reveals immune contexture and predicts the prognosis of hepatocellular carcinoma. J Clin Invest. 2020;130(9):4679–93.
Villanueva A, Hoshida Y, Battiston C, et al. Combining clinical, pathology, and gene expression data to predict recurrence of hepatocellular carcinoma. Gastroenterology. 2011;140(5):1501–12e2.
Sun Y, Wu L, Zhong Y, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184(2):404–421e16.
Zeng J, Zeng J, Lin K, et al. Development of a machine learning model to predict early recurrence for hepatocellular carcinoma after curative resection. Hepatobiliary Surg Nutr. 2022;11(2):176–87.
Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127(5 Suppl 1):108–12.
Richardson P, Duan Z, Kramer J, Davila JA, Tyson GL, El-Serag HB. Determinants of serum alpha-fetoprotein levels in hepatitis C-infected patients. Clin Gastroenterol Hepatol. 2012;10(4):428–33.
Jun CH, Ki HS, Lee KH, et al. Impact of serum C-reactive protein level on the prognosis of patients with hepatocellular carcinoma undergoing TACE. Clin Mol Hepatol. 2013;19(1):70–7.
Socha MW, Malinowski B, Puk O, et al. C-reactive protein as a diagnostic and prognostic factor of endometrial cancer. Crit Rev Oncol Hematol. 2021;164:103419.
Dufour JF. C-reactive protein, a prognostic marker in hepatocellular carcinoma. Hepatology. 2013;57(6):2103–5.
Huang J, Baum Y, Alemozaffar M, et al. C-reactive protein in urologic cancers. Mol Aspects Med. 2015;45:28–36.
Mikkelsen MK, Lindblom N, Dyhl-Polk A, Juhl CB, Johansen JS, Nielsen D. Systematic review and meta-analysis of C-reactive protein as a biomarker in Breast cancer. Crit Rev Clin Lab Sci. 2022;59(7):480–500.
Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer. 2005;103(9):1856–64.
An HJ, Jang JW, Bae SH, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2012;18(12):1406–14.
Rekik S, Guyot E, Bhais M, et al. The CRP level and STATE score predict survival in cirrhotic patients with hepatocellular carcinoma treated by transarterial embolization. Dig Liver Dis. 2016;48(9):1088–92.
Tada T, Kumada T, Hiraoka A, et al. C-reactive protein to albumin ratio predicts survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Sci Rep. 2022;12(1):8421.
Ridker PM. From C-Reactive protein to Interleukin-6 to Interleukin-1: moving Upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–56.
Wang Y, Li Z, Huang Z, Yu X, Zheng L, Xu J. C-Reactive protein is an Indicator of the Immunosuppressive Microenvironment fostered by myeloid cells in Hepatocellular Carcinoma. Front Oncol. 2021;11:774823.
Zhang Y, Lu L, He Z, et al. C-Reactive protein levels predict responses to PD-1 inhibitors in Hepatocellular Carcinoma patients. Front Immunol. 2022;13:808101.
She S, Xiang Y, Yang M, et al. C-reactive protein is a biomarker of AFP-negative HBV-related hepatocellular carcinoma. Int J Oncol. 2015;47(2):543–54.
Kornberg A, Schernhammer M, Kornberg J, Friess H, Thrum K. Serological risk index based on alpha-fetoprotein and C-Reactive protein to Indicate Futile Liver Transplantation among patients with Advanced Hepatocellular Carcinoma. Dig Dis Sci. 2019;64(1):269–80.
Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61(1):82–8.
Funding
This study was supported by the Fujian Medical University Qihang Fund Graduate Innovation Project (2022QH2030); National Natural Science Foundation of China (62275050); Science and Technology Innovation Joint Foundation of Fujian Province (2019Y9108); and Major Research Projects for Young and Middle-aged Researchers of Fujian Provincial Health Care Commission (2021ZQNZD013).
Author information
Authors and Affiliations
Contributions
Conception: Kong-Ying Lin, Tian Yang, and Yong-Yi Zeng; Study design: Kong-Ying Lin, Qing-Jing Chen, Tian Yang, and Yong-Yi Zeng; Data collection and acquisition: All authors; Data analysis: Kong-Ying Lin, Qing-Jing Chen, Zhi-Wen Lin, and Shi-Chuan Tang; Manuscript preparation: Kong-Ying Lin, Qing-Jing Chen, Zhi-Wen Lin, and Shi-Chuan Tang; Critical revision: Kong-Ying Lin, Tian Yang, and Yong-Yi Zeng; Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Our study was performed in accordance with the Declaration of Helsinki and was approved by Branch for Medical Research and Clinical Technology Application, Ethics Committee of the First Affiliated Hospital of Fujian Medical University (Approval No.: MTCA, ECFAH of FMU [2015]084 − 2) and/or the institutional /national research committee. Patients were informed that the resected specimens were stored by the hospital and potentially used for scientific research, and that their privacy would be maintained. All patients provided informed consent prior to undergoing surgery.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table S1
. Baseline characteristics of patients with different baseline AFP levels and DCP levels. Table S2. Univariable and multivariable Cox regression analyses on risk factors of overall survival. Table S3. Univariable and multivariable Cox regression analyses on risk factors of recurrence-free survival. Table S4. Predictive performance of PACE, BCLC staging system, and PACE combined with BCLC staging system. Table S5. Univariable and multivariable Cox regression analyses on risk factors of overall survival in BCLC A cohort. Table S6. Univariable and multivariable Cox regression analyses on risk factors of recurrence-free survival in BCLC A cohort. Table S7. Univariable and multivariable Cox regression analyses on risk factors of overall survival in BCLC B cohort. Table S8. Univariable and multivariable Cox regression analyses on risk factors of recurrence-free survival in BCLC B cohort. Figure S1. Time-dependent area under the curve predicting recurrence-free survival (A) and overall survival (B) at various time points. Figure S2. Calibration curves of BCLC (A), PACE (B), BCLC combined with PACE (C) to predict 1-year, 3-year and 5-year RFS; calibration curves of BCLC (D), PACE (E), BCLC combined with PACE (F) to predict 1-year, 3-year and 5-year OS survival.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, KY., Chen, QJ., Tang, SC. et al. Prognostic implications of alpha-fetoprotein and C-reactive protein elevation in hepatocellular carcinoma following resection (PACE): a large cohort study of 2770 patients. BMC Cancer 23, 1190 (2023). https://doi.org/10.1186/s12885-023-11693-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11693-6