Abstract
Background
This study aimed to comprehensively evaluate the accuracy and effect of computed tomography (CT) and magnetic resonance imaging (MRI) based on artificial intelligence (AI) algorithms for predicting lymph node metastasis in breast cancer patients.
Methods
We systematically searched the PubMed, Embase and Cochrane Library databases for literature from inception to June 2023 using keywords that included ‘artificial intelligence’, ‘CT,’ ‘MRI’, ‘breast cancer’ and ‘lymph nodes’. Studies that met the inclusion criteria were screened and their data were extracted for analysis. The main outcome measures included sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and area under the curve (AUC).
Results
A total of 16 studies were included in the final meta-analysis, covering 4,764 breast cancer patients. Among them, 11 studies used the manual algorithm MRI to calculate breast cancer risk, which had a sensitivity of 0.85 (95% confidence interval [CI] 0.79–0.90; p < 0.001; I2 = 75.3%), specificity of 0.81 (95% CI 0.66–0.83; p < 0.001; I2 = 0%), a positive likelihood ratio of 4.6 (95% CI 4.0–4.8), a negative likelihood ratio of 0.18 (95% CI 0.13–0.26) and a diagnostic odds ratio of 25 (95% CI 17–38). Five studies used manual algorithm CT to calculate breast cancer risk, which had a sensitivity of 0.88 (95% CI 0.79–0.94; p < 0.001; I2 = 87.0%), specificity of 0.80 (95% CI 0.69–0.88; p < 0.001; I2 = 91.8%), a positive likelihood ratio of 4.4 (95% CI 2.7–7.0), a negative likelihood ratio of 0.15 (95% CI 0.08–0.27) and a diagnostic odds ratio of 30 (95% CI 12–72). For MRI and CT, the AUC after study pooling was 0.85 (95% CI 0.82–0.88) and 0.91 (95% CI 0.88–0.93), respectively.
Conclusion
Computed tomography and MRI images based on an AI algorithm have good diagnostic accuracy in predicting lymph node metastasis in breast cancer patients and have the potential for clinical application.
Similar content being viewed by others
Introduction
Breast cancer is one of the most common malignant tumours in women and its incidence continues to rise worldwide. According to the World Health Organization, breast cancer has become one of the leading causes of death in women worldwide [1]. Breast cancer not only causes serious physical harm to patients but also places a heavy burden on patients’ mental health and social functioning. Lymph node metastasis plays an important role in the diagnosis and prognosis of breast cancer [2]. Lymph nodes are important body tissue types that function to filter and remove waste products, bacteria and tumour cells from the body [3]. When breast cancer progresses to a certain stage, cancer cells have the potential to metastasise to lymph nodes through the lymphatic system, which is considered a marker of disease progression and metastasis [4]. Therefore, assessing the presence of lymph node metastasis in breast cancer patients is important for guiding treatment decisions and predicting patient prognosis.
Currently, assessment methods for lymph node metastasis in breast cancer include clinical, imaging and invasive examinations. Clinical examination mainly includes physical examination and lymph node palpation, which can provide preliminary diagnostic information [5]. Imaging examinations, such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET-CT) can provide more accurate information on lymph node metastasis [6]. Invasive tests, such as lymph node biopsy and lymphadenectomy, can obtain lymph node tissue samples directly that may help to confirm the diagnosis and staging [7]. However, although these assessment methods are widely used in breast cancer patients, they each have limitations. Clinical examination is limited by physician experience and palpation technique, and lymph nodes may not always be accurately judged for involvement. Imaging studies, while capable of providing detailed structural information, have limitations in detecting micronodal metastases or assessing the extent of metastases. Invasive tests, while providing definitive results, are somewhat limited by their aggressive nature and associated risks [8]. Therefore, to assess lymph node metastasis more accurately in breast cancer patients, it is of great clinical significance that new and more accurate non-invasive assessment methods be developed.
In recent years, the rapid development of artificial intelligence (AI) algorithms has brought new opportunities for the imaging diagnosis of breast cancer. Artificial intelligence technology is based on large-scale data training and deep learning algorithms, which can automatically extract features from medical images and perform accurate analysis and judgment [9]. In the diagnosis of breast cancer, artificial intelligence algorithms play an increasingly important role in the imaging field. First, AI algorithms can extract rich information from the image data of breast cancer patients and help doctors perform accurate assessments of tumour development and progression. Through deep learning and neural network technology, AI algorithms can automatically identify breast cancer-related lesion characteristics, such as the shape, size and edge characteristics of the mass, thereby helping doctors to quickly locate and diagnose the patient’s condition [10]. Second, AI algorithms can effectively solve the subjectivity and difference problems present in traditional imaging diagnoses. Because of the complexity of breast cancer imaging characteristics, physicians may interpret the same imaging result differently. Artificial intelligence algorithms, on the other hand, have high consistency and objectivity and can accurately automate judgment according to a large number of training data and algorithm models, reducing the diagnostic differences between doctors [11]. In addition, an AI algorithm has the advantages of processing large-scale data and rapid analysis, which can quickly process complex breast imaging data, reduce the work burden of doctors and improve diagnostic efficiency. Compared with the traditional manual reading method, AI algorithms can realise automatic image analysis and diagnosis, greatly shortening the diagnosis time and improving the early diagnosis rate and treatment effect of breast cancer [12]. Studies have shown that radiomics and AI can ‘see’ features that are generally invisible to the human eye in medical images. These new features have potential value in staging, prognosis and biological evaluation [13].
At present, although some studies have reported the application of AI algorithms in the evaluation of lymph node metastasis in breast cancer, a lack of up-to-date systematic evaluation to comprehensively evaluate the performance of AI algorithms alongside CT and MRI in terms of diagnostic accuracy remains. By performing a systematic review and meta-analysis, this study aims to analyse and summarise the data of existing studies on AI algorithm-assisted CT and MRI in the assessment of breast cancer lymph node metastasis and evaluate its diagnostic accuracy, sensitivity and specificity. This can help clinicians to better understand and apply AI algorithms in the evaluation of breast cancer lymph node metastasis, improve the accuracy of early diagnosis and the reliability of treatment decisions and ultimately improve the treatment outcome and survival rates of breast cancer patients.
Methods
This study reports systematic reviews and meta-analyses of diagnostic test accuracy studies according to the preferred reporting items of the PRISMA-DTA guidelines [14].
Search strategy and literature screening
We performed an extensive literature search to collect as much relevant research data as possible. We searched three electronic databases, PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL), covering the time period from their inception to 18 June 2023. Medical Subject Headings (MeSH)/Emtree vocabulary was combined with free words, and keywords were set as the search mode for titles and abstracts. In addition, we manually searched the reference lists of relevant studies, reviews and meta-analyses for additional papers to ensure that no possible study articles were missed. Two researchers independently performed trial selection according to pre-specified inclusion criteria and imported the literature into Endnote X9.3.3 (Clarivate Analytics, London, UK) for management. Repeated or non-compliant studies were excluded. Eligible studies were identified by screening the titles, abstracts and full texts of all articles. Significant were extracted by two researchers using a pre-created data collection form. During data collection, if there were discrepancies between the two researchers, resolutions were discussed with the assistance of a third researcher.
The search strategy was as follows: (((‘Lymph Nodes’[Mesh]) OR (‘lymph’[Title/Abstract])) AND ((‘Breast Neoplasms’[Mesh]) OR (‘breast cancer’[Title/Abstract]))) AND (((((‘Magnetic Resonance Imaging’[Mesh]) OR (‘magnetic resonance imaging’[Title/Abstract])) OR (‘MRI’[Title/Abstract])) OR (((‘Tomography, X-Ray Computed’[Mesh]) OR (‘computed tomography’[Title/Abstract])) OR (‘CT’[Title/Abstract]))) AND (((‘Artificial Intelligence’[Mesh]) OR (‘Artificial Intelligence’[Title/Abstract])) OR (‘AI’[Title/Abstract]))).
Inclusion and exclusion criteria
This study followed PICOS principles to ensure the reasonable control and comparison of five elements including study participants, interventions, controls, outcome measures and study design. Specifically, we included studies that met the following criteria: (1) breast cancer patients; (2) intervention vs. control based on an AI algorithm for CT or MRI imaging vs. pathologic diagnosis of lymph node metastasis (with pathology as the reference standard); (3) the primary outcome measure was the area under the receiver operating characteristic curve (AUC), and secondary outcomes included sensitivity, specificity, positive and negative likelihood ratios and diagnostic odds ratios; (4) the research design was cohort or case-control studies; and (5) language restriction was English. Concurrently, we excluded studies that met the following criteria: (1) duplicate studies with similar data; (2) unrelated study types such as animal studies, case reports, literature reviews or conference abstracts; and (3) studies with incomplete data or no reported set outcomes. By applying the above inclusion and exclusion criteria, we aimed to ensure the quality and reliability of the study and minimise potential deviations and errors.
Data extraction and risk of bias assessment
Two investigators independently performed data extraction and a third resolved any discrepancies between them. From each included study, the following data were extracted: the first author’s surname, publication year, study design, sample size, lymph node metastasis definition, number of lesions, participant origin, ‘gold standard’ and diagnostic accuracy, specific algorithm model, instrumentation, use of clinical information (e.g. age, tumour stage, biomarker expression) and AUC results. The collected data were fourfold table data (2 × 2) including true positives (TPs), true negatives (TNs), false positives (FPs) and false negatives (FNs). Two researchers independently assessed the methodological quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. The QUADAS-2 tool includes patient selection, index testing, reference standards, processes and timing. Disagreements between any two researchers were resolved by discussion or consultation with a third researcher.
Statistical analysis
This study aimed to investigate the performance of AI-assisted CT and MRI imaging models in the diagnostic accuracy of lymph node metastasis in breast cancer patients by meta-analysis. RevMan 5.4 software and Stata SE 15.0 software were used for data analyses. Sensitivity and specificity were calculated based on FNs, FPs, TNs and TPs and graphically presented with boxes indicating values and horizontal lines indicating confidence intervals (CIs). The total receiver operating characteristic (SROC) curve was used to represent the performance of a diagnostic test. According to the AUC, rough classification accuracy guidelines were established as follows: 0.90–1 (excellent), 0.80–0.90 (good), 0.70–0.80 (fair), 0.60–0.70 (poor) and 0.50–0.60 (unqualified). Summary statistics and their 95% CIs were also calculated for the positive likelihood, negative likelihood and diagnostic odds ratios. Cochran’s Q test, combined with an I2 statistic, was used to assess the heterogeneity of the included study results. According to the degree of heterogeneity, a fixed effect or random effect model was used for meta-analysis. Funnel plots were employed to assess the potential for publication bias, and sensitivity analyses were used to assess the stability of the results. Clinical utility was assessed using Fagan plots, which provided the pretest probability of lymph node metastasis when calculating the post-test probability.
Results
Literature search
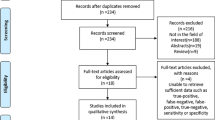
Figure 1 summarises the search and screening results for the relevant studies. Initially, we obtained 126 articles from the database search. By removing duplicate records manually and using software, we removed 44 duplicate articles. Subsequently, we removed 51 articles not related to the research topic by browsing the titles and abstracts and finally selected 31 for full-text reading. During full-text reading, we excluded 13 articles because their outcome measures, comparison strategies or incomplete data were not relevant to our study. Finally, we included 18 articles for systematic review and 16 articles involving 4,764 patients for meta-analysis [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32].
Basic characteristics of included literatures
The characteristics of each eligible study are detailed in Tables 1 and 2. Five studies targeted CT while 11 targeted MRI. Almost all studies used a retrospective design, with most being single-centre studies and only 2 being multi-centre types. In 16 eligible studies, different AI algorithms were adopted for modelling, 5 of which used clinical information in addition to images. In image-omics research, common machine learning classifiers include support vector machines, random forest methods and XGBoost. Of note, only 2 of the 16 studies used independent external validation methods, while others used internal validation methods, including cross-validation, randomly dividing datasets or distinguishing data in chronological order. Among the included studies, there were some differences in the definition of lymph node metastasis, with 14 studies exploring sentinel or axillary lymph node metastases in breast cancer patients, one study investigating the burden of axillary lymph node metastasis and another study considering residual lymph node metastasis. Nearly all studies identified lymph node metastases by pathological examination, including surgical resection or needle biopsy, while one study used 18FDG-PET for indirect assessment.
Risk of bias assessment
The methodological quality of the 16 included studies is shown in Fig. 2. Because the narratives were unclear, 9 studies had an unclear risk of bias in the field of ‘patient selection’ and 1 indicated a high risk; 13 studies had an unclear risk of bias in the field of ‘index test’ because a blinded setting was not accounted for. Only 1 study had an unclear risk of bias score in the field of ‘reference standard’ because the mode of pathological examination was not described in detail. It is important to note that 3 studies may have included high levels of concern regarding patient selection and the ‘index test’ aspect, given their degree of agreement with the questions of this review.
Meta-analysis
The results of a meta-analysis study using AI for lymph node metastasis risk calculation in breast cancer patients are shown in Fig. 3. As indicated, 11 studies used a manual algorithm MRI to calculate breast cancer risk, which had sensitivity of 0.85 (95% CI 0.79–0.90; p < 0.001; I2 = 75.3%), specificity of 0.81 (95% CI 0.66–0.83; p < 0.001; I2 = 0%), a positive likelihood ratio of 4.6 (95% CI 4.0–4.8), a negative likelihood ratio of 0.18 (95% CI 0.13–0.26) and a diagnostic odds ratio of 25 (95% CI 17–38). Five studies used a manual algorithm CT to calculate breast cancer risk, which had sensitivity of 0.88 (95% CI 0.79–0.94; p < 0.001; I2 = 87.0%), specificity of 0.80 (95% CI 0.69–0.88; p < 0.001; I2 = 91.8%), a positive likelihood ratio of 4.4 (95% CI 2.7–7.0), a negative likelihood ratio of 0.15 (95% CI 0.08–0.27) and a diagnostic odds ratio of 30 (95% CI 12–72). In addition, we also used SROC plots to represent the results of a meta-analysis study, based on the use of AI algorithms, to assist MRI or CT in calculating the risk of lymph node metastases in breast cancer patients. As shown in Fig. 4, for MRI or CT, the AUC following the study summary was 0.85 (95% CI 0.82–0.88) and 0.91 (95% CI 0.88–0.93), respectively. These results suggest that the predictive ability of AI algorithms to analyse MRI and CT images for the risk of lymph node metastases in breast cancer patients was classified as good and excellent.
Publication bias and sensitivity analysis
We performed a publication bias analysis of the included studies, as shown in Figs. 5 and 6, and the funnel plot asymmetry test showed no significant publication bias for the included MRI and CT studies (p = 0.82 and 0.85, respectively). While performing the meta-analysis, we also performed a sensitivity analysis. After each exclusion of a single study, there was no large variation in the results, suggesting the stability of the findings; however, heterogeneity between studies remained significant.
Clinical utility
Using an AI-based radiomics MRI model, the post-test probability was increased from 20 to 53% with a positive likelihood ratio of 5 when the pre-test was positive and decreased to 4% with a negative likelihood ratio of 0.18 when the pre-test was negative (Fig. 7). Using the AI-based radiomics CT model, the post-test probability was increased from 20 to 52% with a positive likelihood ratio of 4 when the pretest was positive and a decrease to 4% with a negative likelihood ratio of 0.15 when the pre-test was negative (Fig. 8).
Discussion
The main work of this study comprises a meta-analysis of ultrasound images for predicting lymph node metastasis in breast cancer patients based on AI algorithms. We comprehensively analysed several recent studies and evaluated the application effect of an AI algorithm in this field. The results showed that MRI and CT images based on an AI algorithm showed high predictive accuracy and reliability in the diagnosis of lymph node metastases in breast cancer patients. This is essential for physicians to guide individualised treatment planning, selecting appropriate treatment strategies and reducing the risk of misdiagnosis and missed diagnosis. In addition, imaging analysis using AI algorithms helps to reduce unnecessary needle biopsies and surgical resection, thereby reducing the physical and mental burden on patients and improving treatment outcomes and quality of life. These findings provide important support and guidance for clinical practice and serve as a powerful tool for treatment decision-making and prognostic evaluation of breast cancer patients. Future studies and applications should further explore and optimise the application of AI algorithms in breast cancer diagnosis and treatment to enhance their clinical application value and potential.
An AI algorithm has multiple advantages over traditional examination methods in the evaluation of breast cancer lymph node metastasis. First, AI algorithms can automatically extract features from breast cancer CT and MRI images and perform highly accurate analysis and assessment. Through deep learning and neural network techniques, AI algorithms can identify and localise lymph node metastases in breast cancer, provide more reliable quantitative information and help physicians make accurate diagnostic decisions [33]. Second, an AI algorithm has advantages in the consistency of image interpretation. The image interpretation of breast cancer lymph node metastasis may reflect subjectivity and variation compared with the high consistency and objectivity of an AI algorithm. Through the training of large-scale data and the optimisation of algorithm models, AI algorithms can provide consistent and reliable diagnostic results, reduce diagnostic differences between doctors and improve the diagnostic consistency of breast cancer lymph node metastases [34]. In addition, an AI algorithm can also accelerate the image interpretation speed of breast cancer lymph node metastasis and improve work efficiency. Compared with the traditional manual interpretation method, AI algorithms can automatically analyse and interpret a large number of breast cancer image data, greatly shortening the diagnosis time. This means greater productivity and more timely diagnostic results for clinicians, providing patients with faster and more precise treatment decisions [35]. However, it should be noted that despite the many advantages of AI algorithms in the assessment of breast cancer lymph node metastases, there remain challenges and limitations [36, 37]. For example, training AI algorithms requires a large amount of high-quality labelling data, and the performance of algorithms may be affected by data bias and imbalance. In addition, the application of AI algorithms requires strict validation and regulation to ensure their accuracy, reliability and safety in clinical practice.
In conclusion, AI algorithms have shown great potential in the evaluation of breast cancer lymph node metastasis. Its advantages, such as accuracy, consistency and high efficiency, are expected to improve the accuracy of early diagnosis and treatment decisions for breast cancer lymph node metastasis and ultimately improve the prognosis of patients. However, further research and validation are needed to ensure the reliability and validity of AI algorithms in clinical practice and provide better medical services for patients.
This meta-analysis is the first to update the diagnostic value of MRI images in predicting lymph node metastasis in breast cancer patients based on AI algorithms interpreting CT images. In the past, studies explored the use of imaging in breast cancer lymph node metastasis but few systematically integrated and evaluated this area. Existing studies focused on the assessment of breast cancer lymph node metastasis in MRI. As a high-resolution imaging technique, MRI has good soft tissue contrast and spatial resolution and is widely used in the diagnosis and evaluation of breast cancer. Studies analysed lymph node metastases in breast cancer patients by MRI and achieved specific research results [30, 38]. Zhang et al. [39] synthesised 13 articles and concluded that machine-learning-based MRI imageomics has the potential to accurately predict axillary and sentinel lymph node metastasis, with pooled sensitivity, specificity and AUC reaching 0.82, 0.83 and 0.89, respectively. However, Chen et al. [40] found that MRI sequences and algorithms were the main factors affecting the diagnostic accuracy in machine learning-assisted MRI for the judgment of axillary lymph node metastasis. Despite its good sensitivity and negative predictive value, machine learning-assisted MRI still overlooked 20% of patients with axillary lymph node metastases, an undoubtedly fatal result for patients, indicating that it remains non-applicable in daily diagnosis and treatment. Further exploration is needed in the future to improve the accuracy and practical efficiency of AI algorithms.
Compared with conventional mammography and ultrasonography, CT and MRI have unique advantages and differences in the assessment of breast cancer lymph node metastasis. First, CT and MRI have higher resolution and clearer image quality in anatomical structure presentation and can show the location, size and morphological characteristics of lymph nodes. Computed tomography provides high-resolution cross-sectional images, whereas MRI provides more detailed tissue contrast through multiple sequences and contrast enhancement techniques. These characteristics contribute to the qualitative and quantitative analysis of lymph node metastasis [41]. Second, CT and MRI can provide more functional information; CT can be combined with an intravenous contrast agent for lymph node staging and the assessment of its blood supply and haemodynamic characteristics, while MRI can obtain metabolic information about breast lesions through different sequences, such as dynamic contrast-enhanced MRI and magnetic resonance spectroscopy. This functional information can help to assess the activity of the lesion and predict the prognosis of the patient [42]. However, CT and MRI also include limitations and considerations relative to mammography and ultrasonography. First, CT and MRI require patients to enter a device for examination, which is relatively time-consuming and costly. The MRI approach requires a high degree of cooperation from patients and presents limitations for those with, for example, metallic implants or cardiac pacemakers. In contrast, mammography and ultrasonography are more convenient, economical and suitable for most breast cancer patients, especially for women with higher breast density and pregnant women [43]. As previously stated, CT and MRI have higher resolution, clearer image quality and provide more functional information than conventional mammography and ultrasonography in the assessment of breast cancer lymph node metastasis, but the selection of appropriate imaging tools requires comprehensive consideration of patient characteristics, clinical needs feasibility and decision-making.
This study includes some limitations that impacted the interpretation and generalisability of the results. First, the number and quality of studies available for meta-analysis was limited. Although AI algorithms have received much attention related to predicting breast cancer lymph node metastasis, there may still be relatively few alternative studies. This may be because the application of AI algorithms is still in the development stage and related research remains ongoing. In addition, there may be differences between studies, including variations in study design, sample size, data collection and assessment methods, leading to heterogeneity of the results. We included only English literature in the present study, which may have contributed to a language bias. Relevant studies in other languages may not have been included in the analysis, which could have impacted our results. Reporting algorithm performance and the selection of assessment metrics may also differ. Different studies may use different evaluation indicators and thresholds to evaluate the performance of AI algorithms. This variability may lead to heterogeneity in the results and inconsistency in comparisons. In addition, potential data pooling issues may have been present due to heterogeneity of the data and differences in standardisation methods. Furthermore, different data sources, acquisition methods, image quality and feature extraction methods may have been applied in the different studies derived from the literature search. In addition, the predictive performance of the algorithm may vary for different types of breast cancer lymph node metastasis (such as micrometastasis and giant metastasis), which must also be carefully considered.
Conclusion
In summary, the results of this study showed that CT and MRI based on AI algorithm analysis yielded similar and better diagnostic accuracy in predicting lymph node metastasis in breast cancer patients. This indicated that these two imaging modalities have potential clinical application value and provide new ideas and methods for the diagnosis and treatment of breast cancer. Further studies are still needed to validate the results of this study and promote the clinical application of AI and imaging in this field in the future.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Katsura C, Ogunmwonyi I, Kankam HK, Saha S. Breast cancer: presentation, investigation and management. Br J Hosp Med (Lond). 2022;83(2):1–7. https://doi.org/10.12968/hmed.2021.0459
Mikami Y, Yamada A, Suzuki C, et al. Predicting Nonsentinel Lymph Node Metastasis in Breast Cancer: a Multicenter Retrospective Study. J Surg Res. 2021;264:45–50. https://doi.org/10.1016/j.jss.2021.01.047
Han M, Kang R, Zhang C. Lymph node mapping for Tumor Micrometastasis. ACS Biomater Sci Eng. 2022;8(6):2307–20. https://doi.org/10.1021/acsbiomaterials.2c00111
du Bois H, Heim TA, Lund AW. Tumor-draining lymph nodes: at the crossroads of Metastasis and immunity. Sci Immunol. 2021;6(63):eabg3551. https://doi.org/10.1126/sciimmunol.abg3551
Magnoni F, Galimberti V, Corso G, Intra M, Sacchini V, Veronesi P. Axillary Surgery in Breast cancer: an updated historical perspective. Semin Oncol. 2020;47(6):341–52. https://doi.org/10.1053/j.seminoncol.2020.09.001
Chung HL, Le-Petross HT, Leung JWT. Imaging updates to Breast Cancer Lymph Node Management. Radiographics. 2021;41(5):1283–99. https://doi.org/10.1148/rg.2021210053
Balla A, Weaver DL. Pathologic Evaluation of Lymph Nodes in Breast Cancer: contemporary approaches and clinical implications. Surg Pathol Clin. 2022;15(1):15–27. https://doi.org/10.1016/j.path.2021.11.002
Harrison B. Update on sentinel node pathology in Breast cancer. Semin Diagn Pathol. 2022;39(5):355–66. https://doi.org/10.1053/j.semdp.2022.06.016
Lipkova J, Chen RJ, Chen B, et al. Artificial intelligence for multimodal data integration in oncology. Cancer Cell. 2022;40(10):1095–110. https://doi.org/10.1016/j.ccell.2022.09.012
Bitencourt A, Daimiel Naranjo I, Lo Gullo R, Rossi Saccarelli C, Pinker K. AI-enhanced breast imaging: where are we and where are we heading? Eur J Radiol. 2021;142:109882. https://doi.org/10.1016/j.ejrad.2021.109882
Jiang Y, Yang M, Wang S, Li X, Sun Y. Emerging role of deep learning-based artificial intelligence in Tumor pathology. Cancer Commun (Lond). 2020;40(4):154–66. https://doi.org/10.1002/cac2.12012
Meng F, Kottlors J, Shahzad R, et al. AI support for accurate and fast radiological diagnosis of COVID-19: an international multicenter, multivendor CT study. Eur Radiol. 2023;33(6):4280–91. https://doi.org/10.1007/s00330-022-09335-9
Urso L, Manco L, Castello A, Evangelista L, Guidi G, Castellani M, Florimonte L, Cittanti C, Turra A, Panareo S. PET-Derived Radiomics and Artificial intelligence in Breast Cancer: a systematic review. Int J Mol Sci. 2022;23(21):13409. https://doi.org/10.3390/ijms232113409. PMID: 36362190; PMCID: PMC9653918.
McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and Meta-analysis of Diagnostic Test Accuracy studies: the PRISMA-DTA Statement. JAMA. 2018;319(4):388–96. https://doi.org/10.1001/jama.2017.19163
Lee HJ, Nguyen AT, Song MW, et al. Prediction of residual axillary nodal Metastasis following neoadjuvant chemotherapy for Breast Cancer: Radiomics Analysis based on chest computed Tomography. Korean J Radiol. 2023;24(6):498–511. https://doi.org/10.3348/kjr.2022.0731
Li Z, Kitajima K, Hirata K et al. Preliminary study of AI-assisted diagnosis using FDG-PET/CT for axillary lymph node metastasis in patients with breast cancer. EJNMMI Res. 2021;11(1):10. Published 2021 Jan 25. https://doi.org/10.1186/s13550-021-00751-4
Liu Z, Ni S, Yang C, et al. Axillary lymph node Metastasis prediction by contrast-enhanced computed tomography images for Breast cancer patients based on deep learning. Comput Biol Med. 2021;136:104715. https://doi.org/10.1016/j.compbiomed.2021.104715
Park EK, Lee KS, Seo BK, et al. Machine learning approaches to Radiogenomics of Breast Cancer using low-dose perfusion computed Tomography: Predicting Prognostic biomarkers and molecular subtypes. Sci Rep. 2019;9(1):17847. https://doi.org/10.1038/s41598-019-54371-z. Published 2019 Nov 28.
Song BI. A machine learning-based radiomics model for the prediction of axillary lymph-node Metastasis in Breast cancer. Breast Cancer. 2021;28(3):664–71. https://doi.org/10.1007/s12282-020-01202-z
Yang X, Wu L, Ye W, et al. Deep learning signature based on Staging CT for Preoperative Prediction of Sentinel Lymph Node Metastasis in Breast Cancer. Acad Radiol. 2020;27(9):1226–33. https://doi.org/10.1016/j.acra.2019.11.007
Zhang J, Cao G, Pang H, Li J, Yao X. Development and validation of radiomics machine learning model based on contrast-enhanced computed tomography to predict axillary lymph node Metastasis in Breast cancer. Biomol Biomed. 2023;23(2):317–26. https://doi.org/10.17305/bjbms.2022.7853. Published 2023 Mar 16.
Arefan D, Chai R, Sun M, Zuley ML, Wu S. Machine learning prediction of axillary lymph node Metastasis in Breast cancer: 2D versus 3D radiomic features. Med Phys. 2020;47(12):6334–42. https://doi.org/10.1002/mp.14538
Cui X, Wang N, Zhao Y, et al. Preoperative prediction of Axillary Lymph Node Metastasis in Breast Cancer using Radiomics features of DCE-MRI. Sci Rep. 2019;9(1):2240. https://doi.org/10.1038/s41598-019-38502-0. Published 2019 Feb 19.
Fusco R, Sansone M, Granata V et al. Use of Quantitative Morphological and Functional Features for Assessment of Axillary Lymph Node in Breast Dynamic Contrast-Enhanced Magnetic Resonance Imaging. Biomed Res Int. 2018;2018:2610801. Published 2018 May 30. https://doi.org/10.1155/2018/2610801
Han L, Zhu Y, Liu Z, et al. Radiomic nomogram for prediction of axillary lymph node Metastasis in Breast cancer. Eur Radiol. 2019;29(7):3820–9. https://doi.org/10.1007/s00330-018-5981-2
Liu J, Sun D, Chen L, et al. Radiomics Analysis of Dynamic Contrast-Enhanced Magnetic Resonance Imaging for the prediction of Sentinel Lymph Node Metastasis in Breast Cancer. Front Oncol. 2019;9:980. https://doi.org/10.3389/fonc.2019.00980. Published 2019 Sep 30.
Luo J, Ning Z, Zhang S, Feng Q, Zhang Y. Bag of deep features for preoperative prediction of sentinel lymph node metastasis in breast cancer. Phys Med Biol. 2018;63(24):245014. Published 2018 Dec 14. https://doi.org/10.1088/1361-6560/aaf241
Ren T, Cattell R, Duanmu H, et al. Convolutional Neural Network Detection of Axillary Lymph Node Metastasis using standard clinical breast MRI. Clin Breast Cancer. 2020;20(3):e301–8. https://doi.org/10.1016/j.clbc.2019.11.009
Tan H, Gan F, Wu Y, et al. Preoperative prediction of Axillary Lymph Node Metastasis in breast carcinoma using Radiomics features based on the Fat-suppressed T2 sequence. Acad Radiol. 2020;27(9):1217–25. https://doi.org/10.1016/j.acra.2019.11.004
Yu Y, He Z, Ouyang J, et al. Magnetic resonance imaging radiomics predicts preoperative axillary lymph node Metastasis to support surgical decisions and is associated with Tumor microenvironment in invasive Breast cancer: a machine learning, multicenter study. EBioMedicine. 2021;69:103460. https://doi.org/10.1016/j.ebiom.2021.103460
Zhang X, Zhong L, Zhang B, et al. The effects of volume of interest delineation on MRI-based radiomics analysis: evaluation with two Disease groups. Cancer Imaging. 2019;19(1):89. https://doi.org/10.1186/s40644-019-0276-7. Published 2019 Dec 21.
Zhang X, Yang Z, Cui W, et al. Preoperative prediction of axillary sentinel lymph node burden with multiparametric MRI-based radiomics nomogram in early-stage Breast cancer. Eur Radiol. 2021;31(8):5924–39. https://doi.org/10.1007/s00330-020-07674-z
Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18(8):500–10. https://doi.org/10.1038/s41568-018-0016-5
Chamberlin J, Kocher MR, Waltz J, et al. Automated detection of lung nodules and coronary artery calcium using artificial intelligence on low-dose CT scans for Lung cancer screening: accuracy and prognostic value. BMC Med. 2021;19(1):55. https://doi.org/10.1186/s12916-021-01928-3. Published 2021 Mar 4.
Rezazade Mehrizi MH, van Ooijen P, Homan M. Applications of artificial intelligence (AI) in diagnostic radiology: a technography study. Eur Radiol. 2021;31(4):1805–11. https://doi.org/10.1007/s00330-020-07230-9
Aktolun C. Artificial intelligence and radiomics in nuclear medicine: potentials and challenges [published correction appears in Eur J Nucl Med Mol Imaging. 2020;47(2):513]. Eur J Nucl Med Mol Imaging. 2019;46(13):2731–6. https://doi.org/10.1007/s00259-019-04593-0
Le EPV, Wang Y, Huang Y, Hickman S, Gilbert FJ. Artificial intelligence in breast imaging. Clin Radiol. 2019;74(5):357–66. https://doi.org/10.1016/j.crad.2019.02.006
Ha R, Chang P, Karcich J, et al. Axillary lymph node evaluation utilizing Convolutional neural networks using MRI dataset. J Digit Imaging. 2018;31(6):851–6. https://doi.org/10.1007/s10278-018-0086-7
Zhang J, Li L, Zhe X, et al. The diagnostic performance of machine learning-based Radiomics of DCE-MRI in Predicting Axillary Lymph Node Metastasis in Breast Cancer: a Meta-analysis. Front Oncol. 2022;12:799209. https://doi.org/10.3389/fonc.2022.799209. Published 2022 Feb 4.
Chen C, Qin Y, Chen H, Zhu D, Gao F, Zhou X. A meta-analysis of the diagnostic performance of machine learning-based MRI in the prediction of axillary lymph node Metastasis in Breast cancer patients. Insights Imaging. 2021;12(1):156. https://doi.org/10.1186/s13244-021-01034-1. Published 2021 Nov 3.
Chen Y, Wen Z, Ma Y, et al. Metastatic lymph node calcification in rectal cancer: comparison of CT and high-resolution MRI. Jpn J Radiol. 2021;39(7):642–51. https://doi.org/10.1007/s11604-021-01108-6
Yu Y, Tan Y, Xie C, et al. Development and validation of a Preoperative Magnetic Resonance Imaging Radiomics-Based Signature to Predict Axillary Lymph Node Metastasis and Disease-Free Survival in patients with early-stage Breast Cancer. JAMA Netw Open. 2020;3(12):e2028086. https://doi.org/10.1001/jamanetworkopen.2020.28086. Published 2020 Dec 1.
Jafari SH, Saadatpour Z, Salmaninejad A, et al. Breast cancer diagnosis: imaging techniques and biochemical markers. J Cell Physiol. 2018;233(7):5200–13. https://doi.org/10.1002/jcp.26379
Acknowledgements
There is no one who has contributed to the manuscript but does not qualify as a collaborator.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Study design: All authors. Data acquisition: Cheng-Jie Liu, Lei Zhang, Yi Sun, Lei Geng, Rui Wang. Data analysis and interpretation: Rui Wang, Kai-Min Shi, Jin-Xin Wan. Manuscript preparation: All authors. Critical revision of the manuscript for intellectual content: Jin-Xin Wan. Manuscript review: All authors. Obtaining financing: None.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of The Second People’s Hospital of Lianyungang. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, CJ., Zhang, L., Sun, Y. et al. Application of CT and MRI images based on an artificial intelligence algorithm for predicting lymph node metastasis in breast cancer patients: a meta-analysis. BMC Cancer 23, 1134 (2023). https://doi.org/10.1186/s12885-023-11638-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-11638-z